Abstract
Background
Clinical symptoms of patients with small intestinal bacterial overgrowth (SIBO) may overlap with symptoms of gastroparesis. Prior studies suggest delayed small intestinal transit is associated with SIBO, but have not shown an association between delayed gastric emptying and SIBO. However, these studies have generally relied on the indirect method of breath testing to diagnose SIBO.
Aims
The aim of this study was to examine the association between a microbiological diagnosis of SIBO and delayed gastric emptying by scintigraphy.
Methods
In a single-center retrospective study of previous research participants who presented for small bowel enteroscopy for diagnostic evaluation of SIBO, we identified 73 participants who underwent gastric emptying study by scintigraphy. A microbiological diagnosis of SIBO was made in patients based on culture results of jejunal aspirates. Clinical symptoms were assessed using the total gastroparesis cardinal symptom index (GCSI) score. We compared delayed gastric emptying, 2- and 4-h gastric retention, and gastroparesis symptoms between patients with and without a microbiological diagnosis of SIBO.
Key Results
Among 29 participants with SIBO and 44 without SIBO, 33 (45%) had evidence of delayed gastric emptying. There was no significant association between a microbiological diagnosis of SIBO and delayed gastric emptying by scintigraphy. Percent retained at 2 and 4 h, and total GCSI scores did not differ significantly between those with and without SIBO.
Conclusions
Although delayed gastric emptying is common in patients with suspected SIBO, gastric emptying is not associated with a microbiological diagnosis of SIBO.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Altered small bowel transit time may predispose individuals to the development of small intestinal bacterial overgrowth (SIBO) [1, 2]. Symptoms of SIBO are wide ranging and can include symptoms such as nausea, abdominal pain, bloating, and early satiety [3] that may overlap with the cardinal symptoms of gastroparesis [4]. The pathophysiological changes that occur in patients with a biochemical or microbiological diagnosis of SIBO remain poorly understood. Existing gaps in our understanding of the pathophysiological mechanisms of SIBO may, in part, explain the poorly defined clinical profile of patients with a diagnosis of SIBO [5,6,7].
Several studies have suggested that delayed orocecal and small intestinal transit may predispose patients to the development of SIBO [1, 2, 8,9,10,11]. However, the role of delayed gastric emptying in SIBO is less clear. High rates of SIBO by glucose and lactulose breath test in patients with gastroparesis have been reported by some [12, 13]. However, studies have not demonstrated a clear association between gastric emptying rates and a diagnosis of SIBO based on breath testing [1, 12, 13]. There are limited data describing the association between gastric emptying and SIBO diagnosis based on culture results of small bowel aspirates. In one study involving 30 patients, prolonged gastric emptying time was more common in aspirate positive patients compared to aspirate negative patients [2].
It is conceivable that individuals with abnormal small intestinal motility who are at risk of bacterial overgrowth may further be at risk of generalized disorders of gastrointestinal motility and gastroparesis. Alternatively, individuals who may be at risk of gastroparesis due to comorbid conditions such as diabetes may also be at risk of associated conditions such as chronic pancreatitis that may be complicated by SIBO [14]. Further study of the relationship between a microbiological diagnosis of SIBO and gastric emptying measured through validated techniques may serve several purposes: to identify pathophysiologic mechanisms of SIBO that could contribute to symptom generation in SIBO, to appraise the relative validity of small bowel aspirates as a clinically useful diagnostic test, and to provide a rationale for considering alternative strategies for the evaluation and management of patients with gastroparesis. The aim of this study was to examine the association between SIBO diagnosed through culture of small bowel aspirates and delayed gastric emptying measured by scintigraphy.
Materials and Methods
Participants and Study Design
We performed a single-center retrospective study of previous research participants who presented for small bowel enteroscopy for diagnostic evaluation of SIBO at Indiana University from March 2013 to June 2019. All patients who underwent enteroscopy for suspected SIBO based on clinical presentation were eligible for inclusion regardless of etiology. We excluded patients with intake of prebiotics, probiotics, antibiotics, or bowel cleansers within 30 days prior to enteroscopy and patients who were pregnant or unable to provide informed consent at the time of enteroscopy. Medical records were reviewed to identify all participants who had undergone a gastric emptying study by scintigraphy within 12 months (either before or after) of the diagnostic enteroscopy. The study was approved by the Institutional Review Board at Indiana University School of Medicine. Informed consent for study participation was obtained at the time of the upper enteroscopy.
Demographics and Baseline Clinical Variables
Demographic and clinical data including age, gender, body mass index (BMI), proton-pump inhibitor use, history of surgeries associated with decreased acid production (e.g., Billroth I or II, vagotomy, gastric bypass for obesity), use of daily opiates, the presence of diabetes, and clinical symptoms assessed using the gastroparesis cardinal symptom index (GCSI) total score were collected at the time of study enrollment. All demographic and clinical data were collected prior to the determination of SIBO status to minimize potential for information bias.
Diagnostic Assessment for SIBO
Participants underwent diagnostic assessment of suspected SIBO with upper enteroscopy using a pediatric colonoscope or a small-caliber upper enteroscope for collection of luminal aspirate from the proximal jejunum using a standardized protocol as previously described [15]. Aspirate samples were collected into a sterile syringe and sent to the microbiology laboratory for aerobic and anaerobic cultures. Samples were inoculated onto blood agar, MacConkey agar, chocolate agar, and colistin and nalidixic acid (CNA) agar plates and incubated for a minimum of 48 h. Bacterial growth was quantified by counting total colony-forming units (CFU) per mL. A microbiological diagnosis of SIBO was made in patients with either colonic-type or upper aerodigestive tract (UAT) SIBO. Colonic-type SIBO was defined as > 104 CFU/mL of colonic-type bacteria (e.g., Escherichia coli spp, Klebsiella spp, Proteus mirabilis, Acinetobacter spp, Enterobacter spp, Citrobacter spp, Bacteroides spp or Clostridium spp). UAT SIBO was defined as > 105 CFU/mL of oropharyngeal or aerodigestive tract bacteria (e.g., Streptococcus spp, Staphylococcus spp, Enterococcus spp, Lactobacillus spp, Fusobacterium spp, or Peptostreptococcus spp). Participants with both colonic-type and UAT SIBO were classified as having colonic-type SIBO.
Assessment of Gastric Emptying
Medical records were reviewed to abstract results of gastric scintigraphy performed within 12 months of small bowel enteroscopy. All gastric emptying studies were performed for a minimum duration of 4 h following the ingestion of a low-fat solid test meal. Delayed gastric emptying was defined as greater than 60% retention at 2 h or greater than 10% retention at 4 h.
Study Endpoints
The primary endpoint was the presence or absence of delayed gastric emptying. Secondary endpoints were percent retention at 2 h and 4 h and total GCSI score.
Statistical Analyses
Data are reported as mean (± SD), mean (IQR), or percentages where appropriate. In the primary analysis, we compared the proportion of individuals with evidence of delayed gastric emptying between patients with and without a microbiological diagnosis of SIBO using Fischer’s exact test. Secondary analyses included comparisons of 2 and 4 h gastric retention values and total GCSI scores by SIBO diagnosis using t test for normally distributed continuous variables and Wilcoxon rank-sum test for skewed continuous variables. Subgroup analyses were conducted to compare endpoints of interest among patient groups based on type of bacterial overgrowth (no SIBO, colonic-type SIBO, and UAT SIBO) using the Fisher’s exact test for categorical variables, ANOVA F test for normally distributed continuous variables and Kruskal–Wallis test for skewed continuous variables. Logistic regression was also used to examine the association between delayed gastric emptying and SIBO diagnosis after adjusting for relevant covariates of interest including age, gender, and BMI. All statistical analyses were 2-sided at the 5% significance level. Participants with missing data were excluded.
Results
Participants Characteristics
Among 167 patients who underwent small bowel enteroscopy for diagnostic evaluation of SIBO, we identified 75 patients who had gastric emptying scintigraphy performed within 12 months of enteroscopy. There were two patients in whom complete gastric emptying reports were not available. Among the remaining 73 participants, 29 had SIBO and 44 had no SIBO (Fig. 1). Most participants (93%) were female. Mean age (± SD) and BMI (± SD) for the overall cohort were 51.3 (± 14.0) years and 25.7 (± 6.6) kg/m2. In the overall cohort, 22% had a diagnosis of diabetes, 25% used daily opioids, 58% used proton-pump inhibitors, and 7% had a prior history of surgery associated with decreased gastric acid production (Table 1). Comparison of the no SIBO and SIBO groups revealed that only prior history of surgery associated with decreased gastric acid production was associated with a microbiological SIBO diagnosis (p = 0.008).
Associations of Gastric Emptying, Symptoms, and SIBO
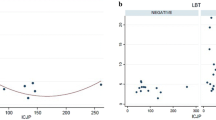
Data on delayed gastric emptying, percent retention at 2 and 4 h, and clinical symptoms are summarized in Table 1 and Fig. 2. Overall, there were 33 individuals with evidence of delayed gastric emptying (45%) by scintigraphy. There was no significant association (odds ratio [OR], 95% confidence interval [CI]) between a microbiological diagnosis of SIBO and delayed gastric emptying before adjusting for age, gender, and BMI (OR = 1.55, 95% CI [0.60–3.98]) or after (OR = 1.72, 95% CI [0.64–4.62]). Percent retained at 2 and 4 h did not differ significantly between the SIBO and no SIBO groups (Fig. 2). Total GSCI scores were not significantly associated with SIBO status (Table 1). Subscale scores for nausea and vomiting, fullness and early satiety, and bloating were not associated with SIBO status. Exploratory comparisons of total GCSI and subscale scores between patients with and without delayed gastric emptying showed no significant associations (all p = ns).
Analysis by Type of Bacterial Overgrowth
Analyses of study endpoints by SIBO subgroup (no SIBO, colonic-type SIBO, and UAT SIBO) are shown in Table 2 and Fig. 2. Only surgery associated with decreased gastric acid production was associated with subgroup status (p < 0.01). There were no significant overall associations between delayed gastric emptying, percent retained at 2 or 4 h, or total GCSI score and SIBO subgroup.
Discussion
In this study, we found that delayed gastric emptying was common in patients undergoing diagnostic evaluation of suspected SIBO, occurring in 45% of patients overall. However, among patients with suspected SIBO, there was no significant association between a confirmed microbiological diagnosis of SIBO and study endpoints of delayed gastric emptying, 2-h meal retention, and 4-h meal retention. Findings suggest that although there may be significant clinical overlap between patients with suspected SIBO and patients with suspected gastroparesis, it does not appear that delayed gastric emptying increases the risk of culture-confirmed SIBO or that SIBO promotes delays in gastric emptying. Others have also reported that gastric emptying times are similar among patients with and without SIBO [1, 12, 13]. However, these studies have relied mainly on breath testing to diagnose SIBO, and the accuracy of these tests has been called into question due to concerns that they may reflect other parameters apart from SIBO such bacterial fermentation of substrate that has reached the cecum [16, 17], carbohydrate malabsorption, or small bowel dysfunction [18].
We further assessed associations of gastric emptying with type of bacterial overgrowth and found no significant differences between microbiologically defined subgroups. Suri et al. [8] recently examined patients undergoing lactulose breath testing to find that methane-producing SIBO was associated with greater small intestinal delay than hydrogen-producing SIBO. Although we did not specifically examine the role of methanogens in our study, methanogens such as Methanobrevibacter smithii are most commonly found in the distal small intestine [19]. Thus, it is possible that those with an overgrowth of distal small bowel microbes could also be at increased risk of overgrowth by colonic-type bacteria. In our study, patients with colonic-type overgrowth did not exhibit greater severity of delay in gastric emptying. However, the specific role of methanogens will require further study.
The lack of an association between gastroparesis symptoms and a culture-based SIBO diagnosis is not surprising given that gastric emptying did not differ by SIBO status. In general, identification of a consistent symptom phenotype in SIBO has been lacking. George et al. [13] previously described higher bloating, early satiety, and postprandial fullness in patients with positive lactulose breath testing based on a single hydrogen peak of > 20 ppm above baseline and Rao et al. recently described an association between SIBO and clinical symptoms of brain fog [11]. However, others have failed to demonstrate clear differences in symptoms between patients with and without SIBO [2, 8, 15] or consistent associations between SIBO and functional gastrointestinal disorders such as functional dyspepsia [20] and irritable bowel syndrome [7]. Much of the uncertainty may be attributable to the imprecise definition of SIBO, the lack of appropriately validated diagnostic tests, and variability in test methodology. Guidelines on best practices for breath tests, published in the North American Consensus document [21], have helped standardize the approach to breath testing for the diagnosis of SIBO. Yet, there remain significant knowledge gaps in breath testing, which include the lack of a validated gold standard test for diagnosing SIBO. The importance of establishing and using validated diagnostic testing methods was carefully illustrated by Vijayvargiya et al. [22, 23] who showed that there are significant associations between upper gastrointestinal symptoms and gastric emptying only when using optimal testing methods to assess gastric emptying. Although gastroparesis symptoms were not associated with delayed gastric emptying defined using optimal methods in our study, it is important to note that symptom assessments took place at the time of enteroscopy rather than at the time of the gastric emptying test.
Our study has several limitations including the retrospective study design, possible tertiary referral bias, and limitations of culture-based approaches including the potential for contamination and inaccessibility of the distal small bowel. Results of gastric emptying studies were not available in all patients who underwent small bowel enteroscopy. Furthermore, we were unable to determine the duration or cause of gastroparesis or of SIBO in our cohort given the retrospective nature of the study. Duration of gastroparesis may play an important role on SIBO development [12], as long-standing delays may be associated with more severe intestinal pathology that could further promote bacterial colonization of the small intestine. Finally, findings could have been influenced by the presence of potential confounders such as comorbid diabetes [24], proton-pump inhibitor use [25,26,27], prior surgical history [4, 15], prokinetic use, and opioid use [14, 28]. In our cohort, 22% of our patients had diabetes, 25% reported daily opiates use, and 58% reported daily proton-pump inhibitor use. However, in our study cohort, no significant differences in the frequency of these potential confounders were observed between SIBO and non-SIBO groups.
In summary, delayed gastric emptying is common in patients with SIBO. However, delay in gastric emptying and gastroparesis symptoms are not associated with a culture-confirmed diagnosis of SIBO and it is not clear that SIBO predisposes individuals to gastroparesis or vice versa. Future prospective studies that take into account disease duration and application of newer techniques to enable detailed characterization of the small intestinal microbiota will be required to unravel the pathophysiological mechanisms that contribute to the relationship between gastroparesis and SIBO.
References
Roland BC, Ciarleglio MM, Clarke JO, et al. Small intestinal transit time is delayed in small intestinal bacterial overgrowth. J Clin Gastroenterol. 2015;49:571–576. https://doi.org/10.1097/MCG.0000000000000257.
Chander Roland B, Mullin GE, Passi M, et al. A prospective evaluation of ileocecal valve dysfunction and intestinal motility derangements in small intestinal bacterial overgrowth. Dig Dis Sci. 2017;62:3525–3535. https://doi.org/10.1007/s10620-017-4726-4.
Bohm M, Siwiec RM, Wo JM. Diagnosis and management of small intestinal bacterial overgrowth. Nutr Clin Pract. 2013;28:289–299. https://doi.org/10.1177/0884533613485882.
Camilleri M, Chedid V, Ford AC, et al. Gastroparesis. Nat Rev Dis Primers. 2018;4:41. https://doi.org/10.1038/s41572-018-0038-z.
Walters B, Vanner SJ. Detection of bacterial overgrowth in IBS using the lactulose H2 breath test: comparison with 14C-D-xylose and healthy controls. Am J Gastroenterol. 2005;100:1566–1570. https://doi.org/10.1111/j.1572-0241.2005.40795.x.
Saffouri GB, Shields-Cutler RR, Chen J, et al. Small intestinal microbial dysbiosis underlies symptoms associated with functional gastrointestinal disorders. Nat Commun. 2019;10:2012. https://doi.org/10.1038/s41467-019-09964-7.
Posserud I, Stotzer PO, Bjornsson ES, Abrahamsson H, Simren M. Small intestinal bacterial overgrowth in patients with irritable bowel syndrome. Gut. 2007;56:802–808. https://doi.org/10.1136/gut.2006.108712.
Suri J, Kataria R, Malik Z, Parkman HP, Schey R. Elevated methane levels in small intestinal bacterial overgrowth suggests delayed small bowel and colonic transit. Med (Baltimore). 2018;97:e10554. https://doi.org/10.1097/MD.0000000000010554.
Rana SV, Malik A, Bhadada SK, Sachdeva N, Morya RK, Sharma G. Malabsorption, orocecal transit time and small intestinal bacterial overgrowth in type 2 diabetic patients: a connection. Indian J Clin Biochem. 2017;32:84–89. https://doi.org/10.1007/s12291-016-0569-6.
Kaur J, Rana SV, Gupta R, Gupta V, Sharma SK, Dhawan DK. Prolonged orocecal transit time enhances serum bile acids through bacterial overgrowth, contributing factor to gallstone disease. J Clin Gastroenterol. 2014;48:365–369. https://doi.org/10.1097/MCG.0b013e3182a14fba.
Rao SSC, Rehman A, Yu S, Andino NM. Brain fogginess, gas and bloating: a link between SIBO, probiotics and metabolic acidosis. Clin Transl Gastroenterol. 2018;9:162. https://doi.org/10.1038/s41424-018-0030-7.
Reddymasu SC, McCallum RW. Small intestinal bacterial overgrowth in gastroparesis: are there any predictors? J Clin Gastroenterol. 2010;44:e8–e13. https://doi.org/10.1097/MCG.0b013e3181aec746.
George NS, Sankineni A, Parkman HP. Small intestinal bacterial overgrowth in gastroparesis. Dig Dis Sci. 2014;59:645–652. https://doi.org/10.1007/s10620-012-2426-7.
Lee AA, Baker JR, Wamsteker EJ, Saad R, DiMagno MJ. Small intestinal bacterial overgrowth is common in chronic pancreatitis and associates with diabetes, chronic pancreatitis severity, low zinc levels, and opiate use. Am J Gastroenterol. 2019;114:1163–1171. https://doi.org/10.14309/ajg.0000000000000200.
Bohm M, Shin A, Teagarden S, et al. Risk factors associated with upper aerodigestive tract or coliform bacterial overgrowth of the small intestine in symptomatic patients. J Clin Gastroenterol. 2018;. https://doi.org/10.1097/MCG.0000000000001150.
Lin EC, Massey BT. Scintigraphy demonstrates high rate of false-positive results from glucose breath tests for small bowel bacterial overgrowth. Clin Gastroenterol Hepatol. 2016;14:203–208. https://doi.org/10.1016/j.cgh.2015.07.032.
Paterson W, Camilleri M, Simren M, Boeckxstaens G, Vanner SJ. Breath testing consensus guidelines for SIBO: RES IPSA LOCQUITOR. Am J Gastroenterol. 2017;112:1888–1889. https://doi.org/10.1038/ajg.2017.233.
Sundin OH, Mendoza-Ladd A, Morales E, et al. Does a glucose-based hydrogen and methane breath test detect bacterial overgrowth in the jejunum? Neurogastroenterol Motil. 2018;30:e13350. https://doi.org/10.1111/nmo.13350.
Triantafyllou K, Chang C, Pimentel M. Methanogens, methane and gastrointestinal motility. J Neurogastroenterol Motil. 2014;20:31–40. https://doi.org/10.5056/jnm.2014.20.1.31.
Shimura S, Ishimura N, Mikami H, et al. Small intestinal bacterial overgrowth in patients with refractory functional gastrointestinal disorders. J Neurogastroenterol Motil. 2016;22:60–68. https://doi.org/10.5056/jnm15116.
Rezaie A, Buresi M, Lembo A, et al. Hydrogen and methane-based breath testing in gastrointestinal disorders: the North American consensus. Am J Gastroenterol. 2017;112:775–784. https://doi.org/10.1038/ajg.2017.46.
Vijayvargiya P, Jameie-Oskooei S, Camilleri M, Chedid V, Erwin PJ, Murad MH. Association between delayed gastric emptying and upper gastrointestinal symptoms: a systematic review and meta-analysis. Gut. 2019;68:804–813. https://doi.org/10.1136/gutjnl-2018-316405.
Vijayvargiya P, Camilleri M, Chedid V, Mandawat A, Erwin PJ, Murad MH. Effects of promotility agents on gastric emptying and symptoms: a systematic review and meta-analysis. Gastroenterology. 2019;156:1650–1660. https://doi.org/10.1053/j.gastro.2019.01.249.
Bharucha AE. Epidemiology and natural history of gastroparesis. Gastroenterol Clin North Am. 2015;44:9–19. https://doi.org/10.1016/j.gtc.2014.11.002.
Dukowicz AC, Lacy BE, Levine GM. Small intestinal bacterial overgrowth: a comprehensive review. Gastroenterol Hepatol (N Y). 2007;3:112–122.
Lo WK, Chan WW. Proton pump inhibitor use and the risk of small intestinal bacterial overgrowth: a meta-analysis. Clin Gastroenterol Hepatol. 2013;11:483–490. https://doi.org/10.1016/j.cgh.2012.12.011.
Sanaka M, Yamamoto T, Kuyama Y. Effects of proton pump inhibitors on gastric emptying: a systematic review. Dig Dis Sci. 2010;55:2431–2440. https://doi.org/10.1007/s10620-009-1076-x.
Hasler WL, Wilson LA, Nguyen LA, et al. Opioid use and potency are associated with clinical features, quality of life, and use of resources in patients with gastroparesis. Clin Gastroenterol Hepatol. 2019;17:1285–1294. https://doi.org/10.1016/j.cgh.2018.10.013.
Funding
AS is supported by NIDDK K23DK122015.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
They authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Calderon, G., Siwiec, R.M., Bohm, M.E. et al. Delayed Gastric Emptying Is Not Associated with a Microbiological Diagnosis of Small Intestinal Bacterial Overgrowth. Dig Dis Sci 66, 160–166 (2021). https://doi.org/10.1007/s10620-020-06153-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-020-06153-1