Abstract
Background
The antibiotic rifaximin is used to treat non-constipated irritable bowel syndrome (IBS). Methane production is associated with constipation and its severity in constipation-predominant IBS (C-IBS). A previous retrospective study suggested that rifaximin and neomycin was superior to neomycin alone in improving symptoms in methane-positive subjects.
Aims
To determine the effectiveness of neomycin alone or with rifaximin in improving symptoms in methane-positive C-IBS subjects.
Methods
A double-blind, randomized, placebo-controlled trial was performed from 2010 to 2013 at three tertiary care centers. Subjects aged 18–65 with C-IBS (Rome II criteria) and breath methane (>3 ppm) meeting the inclusion and exclusion criteria were recruited. Subjects completed a baseline symptom questionnaire rating the severity of abdominal and bowel symptoms on a visual analog scale and were randomized to receive neomycin and placebo or neomycin and rifaximin for 14 days. Symptom severity was assessed by weekly questionnaire for 2 weeks of therapy and 4 additional weeks of follow-up.
Results
Thirty-one subjects (16 neomycin and placebo, 15 neomycin and rifaximin) were included in the intention-to-treat analysis. Constipation severity was significantly lower in the neomycin and rifaximin group (28.6 ± 30.8) compared to neomycin alone (61.2 ± 24.1) (P = 0.0042), with greater improvement in constipation (P = 0.007), straining (P = 0.017) and bloating (P = 0.020), but not abdominal pain. In the neomycin and rifaximin group, subjects with methane <3 ppm after treatment reported significantly lower constipation severity (30.5 ± 21.8) than subjects with persistent methane (67.2 ± 32.1) (P = 0.020).
Conclusions
Rifaximin plus neomycin is superior to neomycin alone in improving multiple C-IBS symptoms. This effect is predicted by a reduction in breath methane.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Irritable bowel syndrome (IBS) is the most common gastrointestinal disorder accounting for significant health care and economic costs in gastroenterology [1]. Traditionally, IBS is divided into three main subgroups including diarrhea-predominant IBS (D-IBS), mixed IBS (M-IBS) and constipation-predominant IBS (C-IBS) [2]. Despite the enormous burden, this disorder has suffered from poor funding. This has led to a slow progression of understanding the causes of this condition.
In the last decade, studies have begun to focus on gut microbes in the pathophysiology of IBS. One hypothesis incriminates acute gastroenteritis in the precipitation of IBS. Based on two meta-analyses, IBS develops in approximately 10 % of subjects after acute gastroenteritis [3, 4]. Another hypothesis finds that small intestinal bacterial overgrowth (SIBO) contributes to the pathogenesis of IBS based upon breath testing [5], small bowel culture [6, 7], small bowel qPCR [8] and response to antibiotics [9] in non-C-IBS subjects.
Breath test studies suggest that methane production is associated with constipation and C-IBS [10, 11]. It has subsequently been determined that methane gas slows intestinal transit [12], and furthermore, constipation severity appears proportional to the degree of methane production [13]. The organism believed to be largely responsible for methane production in humans is Methanobrevibacter smithii [16]. This archaeon is resistant to many antibiotics and does not appear to respond readily to single antibiotic therapy. In a post hoc analysis of a neomycin trial [14] and a retrospective study [15], eradication of methane was found to be associated with an improvement in constipation.
We hypothesized that dual antibiotic therapy with rifaximin plus neomycin could provide greater improvement in symptoms in C-IBS subjects than neomycin alone. Thus, our aim was to examine the role of single antibiotic (neomycin) compared to dual antibiotic (rifaximin plus neomycin) therapy in improving symptoms in subjects with C-IBS who produced methane.
Methods
This study was an investigator-initiated trial supported by Salix Pharmaceuticals. Salix provided the placebo and rifaximin as described below but were not involved in any other aspect of the study including implementation, recruitment, analysis or construction of the manuscript.
Study Subjects
Consecutive patients (18–65 years of age) who fulfilled the Rome II criteria for C-IBS [2] were recruited. Patients were included if they reported <3 complete and spontaneous bowel movements (CSBMs) per week and had breath methane >3 ppm. Subjects were excluded if they had a history of diabetes, HIV, D-IBS, known renal disease, hearing difficulty, previous intestinal surgery (except appendectomy or cholecystectomy), current pregnancy or other known gastrointestinal disorder. Subjects were also excluded if they had taken an antibiotic or probiotic in the previous 30 days or were currently taking narcotics, proton pump inhibitors, tricyclic antidepressants or medications known to alter intestinal motility. All subjects provided informed written consent, and the study was approved by the institutional review board of all three study sites. Subjects who completed the study received a stipend. The study was registered with clinicaltrials.gov (NCT00945334).
Screening Phase
All subjects with a diagnosis of C-IBS presented for a fasting breath test after a 12-h fast. Only subjects with methane level of >3 ppm on a single breath sample were enrolled. Subjects then completed a baseline study questionnaire and a baseline audiogram. As there was a possibility of ototoxicity with neomycin, a baseline audiogram was performed; this was only repeated if subjects reported a subsequent change in hearing. Subjects were then asked to complete a daily stool diary for 2 weeks with weekly questionnaires for overall symptom severity. Subjects were eligible for randomization only if they were confirmed to have <3 CSBMs per week during stool diary.
Treatment Phase
Subjects who successfully completed the screening phase were then randomized in a double-blind manner to receive identical tablets of either neomycin (500 mg twice daily) and placebo (three times daily) or neomycin twice daily and rifaximin (550 mg three times daily) for 14 days. The randomization scheme was set in blocks of 4, and each site received batches of drug in multiples of 4 to balance each study site into blocks. The allocation was concealed. The randomization was done by the drug company with a tracking list provided to the study site pharmacy in a concealed envelope. The pharmacy, investigators and patients were blinded. During the 14 days of treatment, subjects completed weekly symptom questionnaires (two questionnaires over 2 weeks).
Follow-Up Phase
Following completion of therapy, subjects were asked to continue with the weekly symptom questionnaires for an additional 4 weeks. During the final week of follow-up, subjects were asked to repeat the 7-day stool diary followed by the final weekly questionnaire. Finally, subjects were asked to repeat a single fasting breath sample to examine for the presence of methane.
Study Questionnaires
The baseline study questionnaire included subject demographics as well as symptom severity information. Follow-up questionnaires included a repeat evaluation of symptom severity. The following were assessed: abdominal pain, constipation, bloating, urgency, incomplete evacuation, straining and diarrhea. Severity was rated using a visual analog scale (VAS) from 0 to 100 mm (with 0 = no symptom and 100 = severe symptoms).
Breath Testing
Subjects were asked to fast for 12 h prior to a breath sample being obtained. Breath samples were collected via a Quintron dual bag collecting system (Quintron Instrument Company, Milwaukee, WI). The sampled gas was analyzed using a BreathTracker SC (Quintron Instrument Company, Milwaukee, WI). Output was reported as methane in parts per million (ppm) after correction for alveolar sample quality using breath CO2 concentration.
Outcome Measures
The primary outcome measure of this study was constipation severity as assessed on a VAS score from the first week following completion of the treatment phase. Secondary outcome measures included the severity of constipation, abdominal pain, urgency, bloating and straining over the entire study period. In addition, the eradication of methane was evaluated as a determinant of improvement at 4 weeks post-therapy.
Statistical Analyses Plan
The intention-to-treat (ITT) population included all individuals who had a complete baseline assessment and received at least a single dose of therapy in the treatment phase. Successful completion of treatment was considered if subjects took more than 75 % of the study medication. Based on a power calculation, the study was intended to recruit a total of 88 subjects; however, due to slow enrollment, the study was ended early.
Baseline qualitative data and side effect rates were compared by Fisher’s exact test. Baseline quantitative data were normal so were compared by t test. Data were expressed as mean ± SD. The primary endpoint was also normally distributed and compared by t test.
To examine the effect of each group over the duration of the study, a mixed longitudinal model was used. Because the VAS severity varied widely across weeks for most individuals, we considered week to be a categorical variable in the mixed model. Within-patient correlation across time was addressed using an autoregressive (first-order) model for the covariance structure. Missing data were mostly intermittent, and we assumed them to be missing at random. The normality assumption was satisfied for both groups most weeks for constipation, straining and bloating data; however, normality was not satisfied for abdominal pain. The models were analyzed with a single covariate (baseline diarrhea, constipation, abdominal pain or bloating severity score). The covariate models did not improve the fit and did not change the results substantively. Hence, we presented the simpler (no covariate) model results. All statistical analyses were conducted using SAS, version 9.1 (SAS Institute, Cary, North Carolina). A P value <0.05 was considered statistically significant.
Results
Study Population
In this study, 37 C-IBS methane-positive subjects were enrolled (36 from Cedars-Sinai and 1 from Mayo Clinic) (Fig. 1). Of these 37 subjects, five failed the screen phase and one subject did not have a baseline questionnaire for comparison. This left 32 subjects who entered the treatment phase and were considered part of the ITT group. These were randomized to two treatment groups (16 neomycin and placebo and 16 neomycin and rifaximin). The demographics and baseline characteristics were similar between these two groups (Table 1). One subject in the neomycin and rifaximin group withdrew from the study (see below under “Side Effects”), leaving 31 subjects which were included in the final ITT analysis. Of the remaining subjects, 20 returned their pill containers. All subjects took more than 75 % of their medication, and all but one took more than 90 % of the assigned pills.
Primary Outcome Measure
The primary endpoint was the severity of constipation in each arm at 1 week after completion of therapy. A lower severity score was observed for the group receiving rifaximin with neomycin. The VAS score for constipation among subjects receiving both drugs was 28.6 ± 30.8 compared to 61.2 ± 24.1 for neomycin alone (P = 0.0020) (Fig. 2). After adjusting for baseline severity of constipation, the findings were still significant (P = 0.0042).
Secondary Outcome Measures
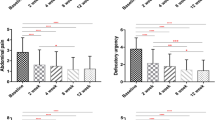
Secondary outcome measured analyses included evaluating bowel symptoms as a function of group, week and group-by-week interaction. Figures 3 and 4 present the profiles of symptom scores by treatment arm. The profiles were essentially parallel across weeks (no interaction effect), and the mean values in the rifaximin and neomycin group were improved compared with those in the neomycin alone group, indicating the superiority of combined antibiotics across the 6 weeks of study. After treatment with rifaximin and neomycin, subjects demonstrated a sustained benefit from the treatment compared to neomycin alone for constipation (Fig. 3a), bloating (Fig. 3b) and straining (Fig. 4a), but not abdominal pain (Fig. 4b).
a Constipation severity throughout the whole study. P = 0.0007 for the longitudinal comparison of groups by week in favor of neomycin and rifaximin using a linear mixed model analysis. b Bloating severity through the whole study. P = 0.020 for the longitudinal comparison of groups by week in favor of neomycin/rifaximin using a linear mixed model analysis
a Straining severity through the whole study. P = 0.017 for the longitudinal comparison of groups by week in favor of neomycin/rifaximin using a linear mixed model analysis. b Abdominal pain severity through the whole study. P = 0.53 for the longitudinal comparison of groups by week with no benefit of neomycin/rifaximin versus neomycin alone using a linear mixed model analysis
Methane As a Determinant of Outcome
Among the 15 subjects receiving rifaximin plus neomycin, 10 subjects had methane levels ≤3 ppm following treatment. Subjects with undetectable methane based on this threshold had a lower constipation severity on the final visit than subjects who did not (Fig. 5). In the neomycin group, methane was ≤3 ppm in the final visit for 11 out of 16 subjects, but there was no significant difference in constipation severity between those that did and did not have this lower methane level. The reduction in methane from baseline was greater but not significantly different between neomycin plus rifaximin (median = 15 ppm drop) and neomycin alone (median = 7.5 ppm drop) (P = 0.45) due to small number of subjects in the study.
Side Effects
Overall, both treatments were well tolerated by most study subjects, and no side effects were reported after cessation of therapy, although nausea was reported quite commonly from neomycin (Table 2). No difference was seen in terms of side effects between groups. Two adverse events were reported during the study. One male patient who received neomycin and rifaximin reported tinnitus. The subject stopped therapy and underwent a repeat audiogram. The second audiogram demonstrated better auditory acuity than the first. Shortly after the tinnitus, the subject developed an upper respiratory tract infection (URTI). The tinnitus resolved with resolution of the URTI. The second patient was a female subject in the neomycin/placebo group who felt “unwell” and stopped drug on day 12. She had begun a self-initiated weight loss program during treatment. Since she completed 75 % of drug, she continued in the follow-up portion of the study and completed the trial.
Discussion
In this study, rifaximin complimented neomycin in significantly improving constipation and other symptoms of C-IBS. Importantly, we found a sustained benefit for 4 weeks following therapy including improvements in constipation, bloating and straining. Finally, subjects receiving rifaximin plus neomycin in whom the methane biomarker was absent at 4 weeks after therapy had a greater improvement than subjects in whom methane remained present (>3 ppm).
There have been a number of studies examining antibiotic therapy in the treatment of IBS. In a non-selected population of IBS, neomycin was successful in improving IBS compared to placebo [14]. However, neomycin had a poor rate of normalizing the breath test. Rifaximin, a non-absorbable antibiotic, has been found in multiple published randomized trials to demonstrate superiority over placebo in the treatment of IBS [17]. The largest of these trials (TARGET 1 and TARGET 2) was specifically designed for non-constipated IBS subjects [9]. In these two trials, the efficacy of rifaximin was seen after 14 days of rifaximin therapy and included significant improvements in global symptom rating, bloating, stool consistency and abdominal pain. More importantly, the treatment had a sustained effect such that these benefits lasted for 3 months following cessation of therapy [9].
While the basis of antibiotic therapy in IBS during the last decade mainly originates from the finding of an increased prevalence of SIBO in IBS, this derangement of gut microbiota is conventionally considered only in the differential diagnosis of subjects with diarrhea and not constipation. While it is difficult to assess the case for SIBO in C-IBS, breath test studies suggest that when methane is present, subjects tend to be constipated [10, 11]. It appears that the microbial organism responsible for methane production in humans is Methanobrevibacter smithii, since the level of this organism in stool is proportional to the level of methane on breath test and the degree of constipation both subjectively and objectively [16]. More importantly, a cause-and-effect relationship between methane and transit was suggested based on animal studies [12]. Together, these findings suggest that elimination of methane could benefit C-IBS and maybe even constipation, particularly if methane is present [11].
Existing data support the prospect that antibiotics may improve C-IBS on the basis of eliminating methane. In a post hoc analysis of a neomycin double-blind study in treating IBS, the subset of C-IBS subjects with methane appeared to respond to neomycin with eradication of methane thereby favorably predicting improvement [14]. Since neomycin alone appears effective in C-IBS, it was important in this study to have this as the active control group. In a more recent retrospective study, subjects receiving neomycin alone or rifaximin alone did not have a substantial benefit. However, a combination of neomycin and rifaximin appeared to eliminate methane in more than 80 % of subjects with similar outcome in constipation symptoms [15]. The interesting aspect of our current study is that despite having a non-placebo active control arm, there was still and benefit with combined therapy. Perhaps the differences would have been even greater if one arm of the study was double placebo.
In this randomized double-blind placebo-controlled study, we evaluated the effect of rifaximin and neomycin in the treatment of C-IBS subjects with methane present in their breath. As was seen with rifaximin use in non-C-IBS, antibiotic therapy appears to be both beneficial regarding symptom improvement and have some degree of sustained response even after cessation of therapy. Interestingly, the response to antibiotic therapy in the case of C-IBS appears more robust in that with only a small number of subjects, there are marked differences. This may be due to a number of reasons. First and foremost, methane appears to be a marker for C-IBS [13]. Having a marker may narrow the population only to those subjects with the abnormal microbes for the treatment. Secondly, combined antibiotics appear to have a more potent effect resulting in greater efficacy. Interestingly, methane is also a marker for success since eradication of methane predicted a favorable clinical response. In this study, unlike the previous retrospective response of >80 % with rifaximin and neomycin [15], only half of subjects eradicated methane on breath test.
There are some limitations with the study. The study only included a small number of subjects, but the effect in this group is strong. However, durability in the follow-up phase lacked power. Whether this is a short-term benefit or whether the effects are sustained for a longer duration merits further study. The abdominal pain scores fluctuated significantly, and we did not have the power to detect a significant difference. In addition, while there appeared to be a greater reduction in methane in the combined drug group, this was underpowered to reach significance. This is a problem with the study design. Previous studies have shown that methane production is proportional to constipation severity [13]. However, these studies were based on the area-under-the-curve for a full lactulose breath test. This was not done here. In this study, a single fasting breath sample was used. A full lactulose breath test and a larger number of subjects could determine the extent of such an effect on methane production and its association with post-treatment outcomes.
In conclusion, in this first randomized controlled trial of methane-positive C-IBS subjects, we found the combination of neomycin and rifaximin was superior to neomycin alone. This study should provide a catalyst for a larger scale, multicenter trial for treatment of C-IBS using antibiotics and the development of approaches that may eradicate methane and thereby improve symptoms.
References
Sandler RS, Everhart JE, Donowitz M, et al. The burden of selected digestive diseases in the Unites States. Gastroenterology. 2002;122:1500–1511.
Thompson WG, Longstreth GF, Drossman DA, Heaton KW, Irvine EJ, Muller-Lissner SA. Functional bowel disorders and functional abdominal pain. Gut. 1999;45:II43–II47.
Halvorson HA, Schlett CD, Riddle MS. Post-infectious irritable bowel syndrome: a meta-analysis. Am J Gastroenterol. 2006;101:1894–1899.
Thabane M, Kottachchi DT, Marshall JK. Systematic review and meta-analysis: the incidence and prognosis of post-infectious irritable bowel syndrome. Aliment Pharmacol Ther. 2007;26:535–544.
Shah ED, Basseri RJ, Chong K, et al. Abnormal breath testing in IBS: a meta-analysis. Dig Dis Sci. 2010;55:2441–2449.
Posserud I, Stotzer PO, Björnsson ES, et al. Small intestinal bacterial overgrowth in patients with irritable bowel syndrome. Gut. 2007;56:802–808.
Pyleris E, Giamarellos-Bourboulis EJ, Tzivras D, et al. The prevalence of overgrowth by aerobic bacteria in the small intestine by small bowel culture: relationship with irritable bowel syndrome. Dig Dis Sci. 2012;57:1321–1329.
Chang C, Funari V, Giamarellos-Bourboulis EJ, et al. Deep sequencing reveals that the microbiome of the human duodenum is unique and unrelated to stool bacterial profiling. Gastroenterology. 2013;144:S908.
Pimentel M, Lembo A, Chey WD, et al. Rifaximin therapy for the treatment of irritable bowel syndrome without constipation. N Engl J Med. 2011;364:22–32.
Kunkel D, Basseri RJ, Makhani MD, et al. Methane on breath test is associated with constipation: a systematic review and meta-analysis. Dig Dis Sci. 2011;56:1612–1618.
Attaluri A, Jackson M, Valestin J, et al. Methanogenic flora is associated with altered colonic transit but not stool characteristics in constipation without IBS. Am J Gastroenterol. 2010;105:1407–1411.
Pimentel M, Lin HC, Enayati P, et al. Methane, a gas produced by enteric bacteria, slows intestinal transit, an augments small intestinal contractile activity. Am J Physiol. 2006;290:G1089–G1095.
Chatterjee S, Park S, Low K, et al. The degree of methane production in IBS correlates with the severity of constipation. Am J Gastroenterol. 2007;102:837–841.
Pimentel M, Chatterjee S, Chow EJ, et al. Neomycin improves constipation predominant irritable bowel syndrome in a fashion that is dependent on the presence of methane gas: sub-analysis of a double blind randomized controlled study. Dig Dis Sci. 2006;51:1297–1301.
Low K, Hwang L, Hua J, et al. A combination of rifaximin and neomycin is most effective in treating irritable bowel syndrome patients with methane on lactulose breath test. J Clin Gastroenterol. 2010;44:547–550.
Kim G, Deepinder F, Morales W, et al. Methanobrevibacter smithii is the predominant methanogen in patients with constipation predominant IBS and methane on breath. Dig Dis Sci. 2012;57:3213–3218.
Menees SB, Maneerattannaporn M, Kim HM, et al. The effect and safety of rifaximin for the irritable bowel syndrome: a systematic review and meta-analysis. Am J Gastroenterol. 2012;107:28–35.
Acknowledgments
This study was supported by funding from Salix Pharmaceuticals. Salix provided the placebo and rifaximin for this study and was responsible for the randomization of study subjects and blinding of investigators to the study drugs during the conduct of the study, but were not involved in any other aspect of the study including implementation, recruitment, analysis or construction of the manuscript.
Conflict of interest
Dr. Pimentel has received grants from and is a consultant for Salix Pharmaceuticals, with whom Cedars-Sinai has a licensing agreement. The remaining authors have nothing to disclose. This study was supported by funding from Salix Pharmaceuticals.
Author information
Authors and Affiliations
Corresponding author
Additional information
Trial Registration: The study was registered with clinicaltrials.gov (NCT00945334).
Rights and permissions
About this article
Cite this article
Pimentel, M., Chang, C., Chua, K.S. et al. Antibiotic Treatment of Constipation-Predominant Irritable Bowel Syndrome. Dig Dis Sci 59, 1278–1285 (2014). https://doi.org/10.1007/s10620-014-3157-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-014-3157-8