Abstract
Objective
Gut microbe-derived methane may slow colon transit causing chronic constipation (CC). Effect of rifaximin on breath methane and slow-transit CC was evaluated.
Method
Bristol stool form, frequency, colon transit time (CTT), and breath methane were evaluated in 23 patients with CC (10 patients with constipation-predominant irritable bowel syndrome [IBS-C], 13 functional constipation, Rome III) and m-ethane production compared with 68 non-constipating IBS. Methane-producing CC (basal ≥ 10 PPM and/or post-lactulose rise by > 10 PPM) was randomized (double-blind) to rifaximin (400-mg thrice/day, 2-weeks) or placebo. Stool forms, frequency, breath methane, and CTT were recorded afterward.
Results
CC patients tended to be methane producer more often (13/23 [56.5%] vs. 25/68 [36.5%], p = 0.07) and had greater area under curve (AUC) for methane (2415 [435–23,580] vs. 1335 [0–6562.5], p = 0.02) than non-constipating IBS. Methane producers (8/13 [61.5%]) and 5/10 (50%) non-producers had abnormal CTT (marker retention: 36-h, 53 [0–60] vs. 19 [8–56], p = 0.06; 60-h, 16 [0–57] vs. 13 [3–56], p = 0.877). Six and 7/13 methane producers were randomized to rifaximin and placebo, respectively. Rifaximin reduced AUC for methane more (6697.5 [1777.5–23,580] vs. 2617.5 [562.5–19,867.5], p = 0.005) than placebo (3945 [2415–12,952.5] vs. 3720 [502.5–9210], p = 0.118) at 1 month. CTT normalized in 4/6 (66.7%) on rifaximin (36-h retention, 54 [44–57] vs. 36 [23–60], p = 0.05; 60-h, 45 [3–57] vs. 14 [11–51], p = 0.09) but none on placebo (p = 0.02) (36-h, 31 [0–60] vs. 25 [0–45], p = 0.078; 60-h, 6 [0–54] vs. 12 [0–28], p = 0.2). Weekly stool frequency (3 [1–9] and 7 [1–14], p = 0.05) and forms improved with rifaximin than placebo.
Conclusion
Rifaximin improves CC by altering methane production and colon transit.
Trial registration
Clinical Trial Registry, India: REF/2012/01/003216

ᅟ
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic constipation (CC) is a common problem in gastroenterology practice and in the community [1]. It may result from slow colon transit [1, 2]. There are limited therapeutic options for slow transit CC. Excessive methane production due to the presence of methanogenic flora in the gut may cause slow gut transit [3]. Methane is produced as a result of anaerobic fermentation of both endogenous and exogenous carbohydrates by enteric microflora in humans [3]. It is not utilized by humans, and analysis of breath methane may serve as an indirect measure of methane production in the gut. Recent literature suggests that gases such as hydrogen sulfide and methane may have active effects on gut function [3,4,5,6]. Excess methane production is often associated with CC [3, 7]. Reduction in methane production with antibiotic treatment directed against methanogenic microflora in the gut may accelerate colonic transit resulting in improvement in constipation [8]. We previously reported a patient from our centre with slow transit CC associated with excess methane production; her stool form and frequency improved after reduction of methane production using gut-specific antibiotic (rifaximin), which also accelerated colonic transit [8, 9]. However, there is no randomized placebo-controlled trial to evaluate efficacy of rifaximin to treat patients with slow-transit CC with excess methane production. We, therefore, studied the effect of treatment with a non-absorbable antibiotic, rifaximin, on patients with slow transit CC associated with high methane production. The objectives of our study were the followings: (i) to evaluate the frequency of abnormally high breath methane among patients with CC and compare it with controls with non-constipating irritable bowel syndrome (IBS), (ii) to evaluate the colon transit time (CTT) in patients with CC, (iii) to study the effect of rifaximin on the breath methane in patients with CC associated with excess methane production, (iv) to study the effect of reduction of breath methane production, if any, on CTT, and (v) to study the stool characteristics of patients with CC before and after treatment with rifaximin.
Method
Subjects
Outline of the study protocol is summarized in Fig. 1. Twenty-three patients with CC (both IBS-C and functional constipation, FC; Rome III criteria) [10] and 68 controls with non-constipating IBS (Rome III) attending the gastroenterology outpatient of a multi-level teaching hospital in northern India were included in the study after obtaining informed consent (Fig. 1). Patients who received antibiotics or probiotics within 12 weeks previously were excluded.
Each patient was subjected to routine investigations as indicated such as stool microscopy, occult blood, hemogram, anorectal manometry, balloon expulsion test, defecography, and flexible proctosigmoidoscopy using standard techniques [1]. No patient received any drug that could alter gastrointestinal (GI) motility or predispose to development of small intestinal bacterial overgrowth such as prokinetics, antisecretory drugs, or narcotics within 12 weeks before the study. The study protocol was approved by the Institutional Ethics Committee. The trial was registered in a clinical trial registry (registration number in Clinical Trial Registry, India: REF/2012/01/003216).
Clinical evaluation
Each patient was interviewed using a standard questionnaire to record demographic and clinical symptoms of constipation. Predominant stool form was recorded using Bristol stool form chart with pictorial representation and descriptor [11] over a 1-week period before and after treatment [10].
Evaluation of breath methane by LHBT
Lactulose hydrogen breath test (LHBT) was performed using breath gas analyzer according to a standard protocol [12]. Basal breath specimens were obtained after a 12-h fast; the patients avoided slowly absorbed carbohydrates (lentils, bread, potato, corn) and fiber the previous evening to avoid delayed excretion of hydrogen and methane in the breath. Cigarette smoking and strenuous physical activity were not permitted for 2-h before and during the test to prevent hyperventilation and consequent changes in breath hydrogen and methane content. The patients were asked to brush teeth, and rinse mouths with an antiseptic wash and water, to eliminate an early peak due to the action of oral bacteria on test sugars. An average of three values was taken as the basal breath gas level. The patients were then asked to take 15 mL lactulose solution containing 10-g lactulose. Thereafter, breath gas was estimated every 15 min for 4 h. A positive methane breath test was defined in two ways: either if the breath methane level ≥ 10 PPM at baseline or if there was an increase in breath methane ≥ 10 PPM above baseline after ingestion of lactulose [12].
Study of CTT
The test for CTT was performed according to a standard protocol [13] using a radio-opaque marker. Subjects were asked to ingest 2 capsules at a time (10 markers in each capsule) at 0, 12, and 24 h. Subsequently, abdominal radiographs were obtained in erect posture at 36 and 60 h and the total markers retained were counted. The subjects were on a normal diet and did normal physical activities during the period of the study. No patient took any drug that could alter GI motility within 7 days before and during the study period. Laxatives and enemas were also avoided during the study period. As validated previously, retention of more than 30 markers at 36-h radiograph and 14 markers at 60-h radiograph was considered abnormal [13].
Study of stool characteristics
Each patient was asked to fill in a stool diary (depicting both the Bristol stool form pictures as well as descriptors) [11] for 7 days which recorded their stool characteristics such as frequency, forms (according to Bristol stool scale), and subjective feeling about the bowel movement.
Randomization and treatment allocation
Patients with high breath methane were randomized using a computer-generated random number table to receive rifaximin 400 mg, or placebo, thrice daily for 14 days. Commercially available tablet of rifaximin was removed from its packing and inserted inside empty capsules by a person who was not involved in the randomization, dispensing the drug, breath tests, or patient evaluation. Similar looking empty capsules were used as a placebo, which contained glucose powder. Both the study drug and placebo capsules were coded and the patients, as well as the investigators, were blinded during the study. Moreover, a closed envelop technique was used for allocation concealment. Adverse event, if any, was also recorded.
Eradication
Eradication of methane was documented after treatment using LHBT. CTT and stool characteristics (over 1 week) were also recorded and compared after treatment.
Result
Demographic and clinical characteristics of patients and controls
Twenty-three patients (50 years [18–72], 11 [47.8%] male) with CC and 68 controls (36 years [17–67], 55 [80.9%] male) with non-constipating IBS were included in the study. Though patients with CC were more often female (p = 0.003), difference in age was non-significant. Table 1 summarizes the demographic and clinical parameters of all the patients with constipation. Patients (10/23 (43.5%)) had constipation-predominant IBS and the others had FC by Rome III criteria [10].
Breath test
Patients with CC more often tended to be methane producers (13/23 [56.5%] vs. 25/68 [36.5%], p = 0.07) and had higher area under curve (AUC) for methane (2415 [435–23,580] vs. 1335 [0–6562.5], p = 0.021) than non-constipating IBS (Fig. 2).
There was no relationship between methane production as binary variable and symptoms (Table 1).
Colonic transit time
Methane producers (8/13 [61.5%]) and 5/10 (50%) methane non-producers had abnormal CTT (markers retained at 36-h, 53 [0–60] vs. 19 [8–56], p = 0.062; at 60-h, 16 [0–57] vs. 13 [3–56], p = 0.877). There was no relationship between slow or normal colon transit as binary variable and symptoms such as abdominal pain or discomfort, bloating, mucus, incomplete evacuation, and straining though less than three stools/week was commoner among methane producers than non-producers (3/13, 23% vs. 3/10, 30%, p = 0.03).
Effect of rifaximin on stool characteristics
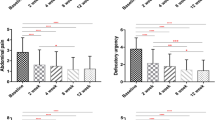
Weekly stool frequency (before treatment, 3 [1–9] vs. after treatment, 7 [1–14], p = 0.05) and forms (type 1, 2/6 [33.3%]; type 2, 1/6 [16.6%]; type 3, 3/6 [50%] vs. type 1, 1/6 [16.6%]; type 3, 3/6 [50%]; type 4, 1/6 [16.6%]; type 5, 1/6 [16.6%]) improved with rifaximin than with placebo (7 [3–21] vs. 7 [1–14], p = 0.08 and type 1, 1/7 [14.3%]; type 2, 1/7 [14.3%]; type 3, 1/7 [14.3%]; type 4, 4/7 [57.1%] vs. type 1, 1/7 [14.3%]; type 2, 2/7 [28.6%]; type 3, 1/7 [14.3%]; type 4, 3/7 [42.8%]) (Fig. 3).
Effect of rifaximin on methane production
Of 13 methane producers, 6 (46.2%) were randomized to receive rifaximin and 7 (53.8%) to placebo (Fig. 1). After 1 month, AUC for methane was lower among patients on rifaximin (6697.5 [1777.5–23,580] vs. 2617.5 [562.5–19,867.5], p = 0.005) than those on placebo (3945 [2415–12,952.5] vs. 3720 [502.5–9210, p = 0.118) (Fig. 4).
Effect of rifaximin on CTT
CTT normalized in 4/6 (66.7%) on rifaximin (markers retained at 36-h, 54 [44–57] vs. 36 [23–60], p = 0.05; at 60-h, 45 [3–57] vs. 14 [11–51], p = 0.09) but did not normalize in anyone on placebo (p = 0.02) (markers retained at 36-h, 31 [0–60] vs. 25 [0–45], p = 0.078; at 60-h, 6 [0–54] vs. 12 [0–28], p = 0.2) (Fig. 5). There was a correlation between the AUC of breath methane after treatment with rifaximin and number of markers retained in abdomen in 60-h radiograph following treatment (r = 0.65, p = 0.017) (Fig. 6).
Colon transit study using radio-opaque markers in a patient with chronic constipation with high breath methane at 36 h before (a) and after (b) treatment with rifaximin. Though before treatment 53 radio-opaque markers were retained in the abdomen diffusely (normal < 30 markers), after treatment it reduced to 18 markers (normal < 30 markers). The patient had the disappearance of breath methane on lactulose hydrogen breath test and improvement in constipation
Improvement in stool frequency, CTT, and breath methane was comparable among patients with FC (n = 6) and IBS-C (n = 7) though the number of patients for such subgroup analysis was small.
Adverse event
No patient developed any adverse event either during ingestion of the radio-opaque markers or during rifaximin administration, except one reporting transient nausea during rifaximin treatment.
Discussion
The present randomized controlled trial showed that (i) patients with CC tended to be methane producer more often and had greater AUC for methane than controls, (ii) rifaximin reduced AUC for methane more than placebo, (iii) CTT normalized more often with rifaximin than placebo, and (iv) weekly stool frequency and forms tended to improve with rifaximin as compared to placebo.
Constipation is a common problem in gastroenterology practice [1]. According to pathophysiology, most patients with CC have either slow colonic transit, fecal evacuation disorder, or a combination of both these factors [1]. Slow colonic transit in patients with CC may be related to excess methane production by methanogenic bacteria such as Methanobrevibacter smithii and Methanobrevibacter stadmanii [14]. A few studies revealed that excess breath methane on LHBT is associated with CC [3, 5, 7, 15,16,17,18]. This is related to the effect of methane gas on enteric neurons, pacemaker and muscle cells, ileal motor function, and on gut serotonin [4, 19,20,21]. Since serotonin is a molecule involved in colonic motility, its reduction is associated with constipation [21]. Hence, it is quite expected that suppression of methanogenic flora by antibiotics such as rifaximin would result in improvement in colonic transit and, therefore, in constipation.
Earlier, we reported a patient in whom treatment with rifaximin for 10 days resulted in a reduction in breath methane on LHBT, improved colon transit, and constipation as evidenced by improvement in stool form and frequency [8]. In an earlier retrospective chart review by Pimentel's group, of 74 patients with IBS-C (Rome I) and high breath methane (≥ 3 PPM) on LHBT, receiving the treatment with rifaximin and neomycin (n = 27), 85% had a clinical response, compared with 63% of subjects who were treated with neomycin only (n = 8) (p = 0.15) and 56% of subjects treated with rifaximin only (n = 39) (p = 0.01) [22]. In another study by the same group, 31 subjects with IBS-C (Rome II) and breath methane on LHBT > 3 PPM were randomized to neomycin and placebo or neomycin and rifaximin for 14 days [9]; authors found that though rifaximin improved constipation and reduced breath methane, rifaximin with neomycin had the highest efficacy [9]. However, only patients with IBS-C and not FC were included in both these studies and colonic transit study was not evaluated. Furthermore, that study did not have a subgroup treated with placebo alone. Moreover, the cutoff of methane to classify subjects as methane producer was quite low in this study (≥ 3 PPM). In our study, however, we assessed colon transit time both before and after treatment with rifaximin and the cutoff to classify subjects as methane producer was higher. Moreover, both patients with IBS-C and FC were included. Therefore, this is perhaps the first randomized placebo-controlled trial to prove that rifaximin improves constipation both among IBS-C and FC patients with slow colon transit associated with excess methane production.
Our study, however, had a few limitations. This includes small sample size, particularly for the randomized controlled trial. The dose of rifaximin was also lower than used currently in practice and in randomized controlled trials on IBS [23, 24]. However, since we found a significant effect even with a lower dose of rifaximin, this might not be a major limitation. Moreover, patients’ satisfaction, which is an important self-reported outcome measure, was not recorded. Patients with CC were more often female than controls; however, gender is not expected to influence methane production. Diet and gut microbiota were not evaluated in this study, which are its limitations. Studies are needed in future overcoming these limitations on a larger number of patients to confirm these findings and to evaluate the duration of the effect of such intervention.
We conclude that rifaximin improves constipation both among patients with IBS-C and FC by reducing breath methane and colon transit time. More studies, on a larger sample of patients, are needed to make the conclusion of this study robust and for its widespread clinical application.
References
Ghoshal UC. Chronic constipation in Rome IV era: the Indian perspective. Indian J Gastroenterol. 2017;36:163–73.
Ghoshal UC, Singh R. Frequency and risk factors of functional gastro-intestinal disorders in a rural Indian population. J Gastroenterol Hepatol. 2017;32:378–87.
Parthasarathy G, Chen J, Chen X, et al. Relationship between microbiota of the colonic mucosa vs feces and symptoms, colonic transit, and methane production in female patients with chronic constipation. Gastroenterology. 2016;150:367–79 e1.
Triantafyllou K, Chang C, Pimentel M. Methanogens, methane and gastrointestinal motility. J Neurogastroenterol Motil. 2014;20:31–40.
Attaluri A, Jackson M, Valestin J, Rao SSC. Methanogenic flora is associated with altered colonic transit but not stool characteristics in constipation without IBS. Am J Gastroenterol. 2010;105:1407–11.
Matsunami M, Tarui T, Mitani K, et al. Luminal hydrogen sulfide plays a pronociceptive role in mouse colon. Gut. 2009;58:751–61.
Kunkel D, Basseri RJ, Makhani MD, Chong K, Chang C, Pimentel M. Methane on breath testing is associated with constipation: a systematic review and meta-analysis. Dig Dis Sci. 2011;56:1612–8.
Ghoshal UC, Srivastava D, Verma A, Misra A. Slow transit constipation associated with excess methane production and its improvement following rifaximin therapy: a case report. J Neurogastroenterol Motil. 2011;17:185–8.
Pimentel M, Chang C, Chua KS, et al. Antibiotic treatment of constipation-predominant irritable bowel syndrome. Dig Dis Sci. 2014;59:1278–85.
Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–91.
Ghoshal UC, Gwee KA, Chen M, et al. Development, translation and validation of enhanced Asian Rome III questionnaires for diagnosis of functional bowel diseases in major Asian languages: a Rome Foundation-Asian Neurogastroenterology and Motility Association Working Team report. J Neurogastroenterol Motil. 2015;21:83–92.
Ghoshal UC. How to interpret hydrogen breath tests. J Neurogastroenterol Motil. 2011;17:312–7.
Ghoshal UC, Gupta D, Kumar A, Misra A. Colonic transit study by radio-opaque markers to investigate constipation: validation of a new protocol for a population with rapid gut transit. Natl Med J India. 2007;20:225–9.
Ghoshal U, Shukla R, Srivastava D, Ghoshal UC. Irritable bowel syndrome, particularly the constipation-predominant form, involves an increase in methanobrevibacter smithii, which is associated with higher methane production. Gut Liver. 2016;10:932–8.
Lee KM, Paik CN, Chung WC, Yang JM, Choi MG. Breath methane positivity is more common and higher in patients with objectively proven delayed transit constipation. Eur J Gastroenterol Hepatol. 2013;25:726–32.
Kim G, Deepinder F, Morales W, et al. Methanogen in patients with constipation-predominant IBS and methane on breath. Dig Dis Sci. 2012;57:3213–8.
Makhani M, Yang J, Mirocha J, Low K, Pimentel M. Factor analysis demonstrates a symptom cluster related to methane and non-methane production in irritable bowel syndrome. J Clin Gastroenterol. 2011;45:40–4.
Chatterjee S, Park S, Low K, Kong Y, Pimentel M. The degree of breath methane production in IBS correlates with the severity of constipation. Am J Gastroenterol. 2007;102:837–41.
Park YM, Lee YJ, Hussain Z, Lee YH, Park H. The effects and mechanism of action of methane on ileal motor function. Neurogastroenterol Motil. 2017;29. https://doi.org/10.1111/nmo.13077.
Pimentel M, Lin HC, Enayati P, et al. Methane, a gas produced by enteric bacteria, slows intestinal transit and augments small intestinal contractile activity. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1089–95.
Barbara G, Feinle-Bisset C, Ghoshal UC, et al. The intestinal microenvironment and functional gastrointestinal disorders. Gastroenterology. 2016;150:1305–1318.e8. https://doi.org/10.1053/j.gastro.2016.02.028.
Low K, Hwang L, Hua J, Zhu A, Morales W, Pimentel M. A combination of rifaximin and neomycin is most effective in treating irritable bowel syndrome patients with methane on lactulose breath test. J Clin Gastroenterol. 2010;44:547–50.
Pimentel M, Lembo A, Chey WD, et al. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011;364:22–32.
Lembo A, Pimentel M, Rao SS, et al. Repeat treatment with rifaximin is safe and effective in patients with diarrhea-predominant irritable bowel syndrome. Gastroenterology. 2016;151:1113–21.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study protocol was approved by the Institutional Ethics Committee.
Conflict of interest
UCG, DS, and AM declare that they have no conflict of interest.
Ethical clearance
The authors declare that the study was performed in a manner conforming to the Helsinki declaration of 1975, as revised in 2000 and 2008 concerning human and animal rights, and the authors followed the policy concerning informed consent as shown on Springer.com.
Disclaimer
The authors are solely responsible for the data and the content of the paper. In no way, the Honorary Editor-in-Chief, Editorial Board Members, or the printer/publishers are responsible for the results/findings and content of this article.
Rights and permissions
About this article
Cite this article
Ghoshal, U.C., Srivastava, D. & Misra, A. A randomized double-blind placebo-controlled trial showing rifaximin to improve constipation by reducing methane production and accelerating colon transit: A pilot study. Indian J Gastroenterol 37, 416–423 (2018). https://doi.org/10.1007/s12664-018-0901-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12664-018-0901-6