Abstract
Background
Self-expandable metal stents (SEMS) are widely utilized to relieve symptoms of malignant gastric outlet obstruction (GOO), but GOO is frequently complicated by nonresectable distal biliary obstruction. The optimal endoscopic approach to biliary drainage in this setting remains controversial and has yet to be resolved.
Aims
To compare the safety and efficacy of endoscopic ultrasound-guided transmural biliary drainage (EUS-BD) and transpapillary drainage in patients with an indwelling duodenal SEMS.
Methods
Patients who underwent EUS-BD or transpapillary drainage for distal malignant biliary obstruction with an indwelling duodenal SEMS between June 2007 and August 2012 at three Japanese tertiary referral centers were identified retrospectively. We compared times to stent dysfunction, causes of dysfunction, and procedural related complications between these two groups.
Results
Twenty patients were included in the study (7 EUS-BD and 13 transpapillary drainage). EUS-BD was performed via hepaticogastrostomy using a SEMS in three patients and via choledochoduodenostomy using a SEMS or a plastic stent in two patients each. Transpapillary drainage was performed using a SEMS in all patients. The stent patency rate in the EUS-BD group was higher than that in the transpapillary drainage group (100 vs. 71 % at 1 month and 83 vs. 29 % at 3 months, respectively). The rate of stent dysfunction in the EUS-BD group tended to be lower than that in the transpapillary group (14 vs. 54 %; P = 0.157). Complication rates were similar between the groups (P = 1.000), with moderate bleeding in one patient in the EUS-BD group and mild pancreatitis in one patient in the transpapillary group.
Conclusion
Endoscopic ultrasound-guided transmural biliary drainage is an alternative to transpapillary drainage in patients with an indwelling duodenal SEMS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endoscopic biliary drainage is the mainstay of palliative management of nonresectable distal malignant biliary obstruction (MBO). Distal MBO is occasionally complicated by malignant gastric outlet obstruction (GOO) [1–3] for which placement of a self-expandable metal stent (SEMS) is widely accepted as the appropriate nonsurgical palliative treatment, particularly in cases with a poor prognosis due to underlying malignancy [4–7]. Endoscopic biliary drainage in patients with combined biliary and duodenal obstructions poses a major challenge for endoscopists due to deformity of the duodenum, and an indwelling duodenal SEMS, if present, hinders transpapillary biliary drainage.
The feasibility and effectiveness of transpapillary biliary SEMS combined with a duodenal SEMS have been reported in several case series [1, 8, 9], but early dysfunction of biliary SEMS placed across the papilla is often encountered due to duodenobiliary reflux enhanced by duodenal stenosis and reduced duodenal peristalsis [10]. The number of reports on the effectiveness of endoscopic ultrasound-guided biliary drainage (EUS-BD), such as hepaticogastrostomy and choledochoduodenostomy have been increasing [11–15]. This procedure provides a biliary drainage route away from a duodenal SEMS and, thus, may be expected to prolong the time to dysfunction of a biliary stent even in cases with an indwelling duodenal SEMS. However, the appropriate strategy for endoscopic biliary drainage remains controversial in patients with an indwelling duodenal SEMS.
In this study, we evaluated the feasibility and effectiveness of EUS-BD compared with transpapillary SEMS in cases with an indwelling duodenal SEMS.
Methods
Study Design
This was a multicenter retrospective study which compared the outcomes of EUS-BD with those of transpapillary SEMS in patients with a duodenal SEMS at three Japanese referral centers. We identified patients who met the enrollment criteria based on our prospective database of biliary interventions and reviewed charts to evaluate the outcomes of biliary drainage. This study was approved by the Ethics Committee of each participating hospital.
Patients
We enrolled consecutive patients with nonresectable distal MBO who underwent endoscopic placement of a biliary plastic stent or a SEMS in the presence of a duodenal SEMS at the University of Tokyo and two affiliated hospitals between June 2007 and August 2012. EUS-BD was introduced into our clinical practice in patients with difficult/impossible endoscopic retrograde cholangiopancreatography (ERCP) around 2009. The final diagnosis of primary malignancy was confirmed by either pathological or typical radiological findings with compatible clinical courses. Data on patient baseline characteristics, survival, placement of duodenal and biliary stents, outcomes of biliary drainage, and re-interventions were studied retrospectively. Written informed consent was obtained from each enrolled patient prior to the procedure.
EUS-BD in the Presence of a Duodenal SEMS
A linear array echoendoscope (model EG-530UT2, Fuji Film Corp., Kanagawa, Japan or model GF-UCT240, Olympus Optical, Tokyo, Japan) was inserted with the patient under moderate sedation using diazepam and pethidine hydrochloride. The tip of the echoendoscope was positioned in the gastric fundus or duodenal bulb when accessing the intrahepatic and extrahepatic bile ducts, respectively. Biliary access was obtained using a 19-gauge needle (Expect Flex, Boston Scientific, Natick, MA or EchoTip Ultra, Cook Medical, Winston-Salem, NC) and a 0.025-inch guidewire (RevoWave; Piolax Medical Devices, Kanagawa, Japan) or 0.035-inch, 400-cm-long hydrophilic guidewire (Radifocus; Terumo Co., Tokyo, Japan). After the guidewire had been sufficiently advanced within the bile duct, the puncture tract was dilated using an ERCP cannula (MTW; Endoscopie Inc., Wesel, Germany), a 6-F electrocautery (Cysto-Gastro-Set; Endo-Flex, Voerde, Germany), and a 4-mm dilation balloon (Eliminator; Bard Interventional Products, Billerica, MA), as appropriate. Subsequently, a covered SEMS was deployed during a hepaticogastrostomy or a covered SEMS, or plastic stent was deployed during a choledochoduodenostomy (Fig. 1). Our strategy of EUS-BD was as follows: as first-choice procedure we attempted to perform choledochoduodenostomy in light of its potentially lower complication rate relative to hepaticogastrostomy [16, 17]; as alternative when the transduodenal approach to the biliary system was hindered by the duodenal tumor invasion, we attempted to perform hepaticogastrostomy.
Transpapillary SEMS Placement in the Presence of a Duodenal SEMS
A side-viewing duodenoscope (JF-260V; Olympus Optical) was inserted with the patient under moderate sedation and passed through an indwelling duodenal SEMS in cases of a duodenal SEMS proximal to the papilla. Biliary access was obtained using the wire-guided cannulation technique [18] with an ERCP cannula (MTW; Endoscopie Inc.) and a 0.035-inch guidewire (Jagwire; Boston Scientific or Radifocus). In cases with a duodenal SEMS placed across the papilla, the bile duct was cannulated through the mesh of the duodenal SEMS. A biliary SEMS was subseqeuntly deployed with its distal end inside the duodenal SEMS (Fig. 2).
Definitions
Distal MBO was defined as a biliary stricture located ≥2 cm from the hepatic hilum. Biliary stent dysfunction was defined as stent occlusion, stent migration, or nonocclusion cholangitis. Stent occlusion was defined as biochemical evidence of cholestasis with biliary dilation on imaging studies or when endoscopic findings suggested occlusion at re-intervention. The causes of stent occlusion were determined based on endoscopic findings and biopsy results at re-intervention. Stent migration was diagnosed when re-intervention for biliary stent dysfunction revealed a completely or partially migrated SEMS. Nonocclusion cholangitis was defined as cholangitis requiring a re-intervention or hospitalization without obvious evidence of SEMS occlusion in cases with fever and elevated liver enzymes. Procedure-related complications were graded according to consensus guidelines [19]. Types of duodenal stenosis were classified according to the location of the stenosis in relation to the major papilla: type I, proximal to and no involvement of the papilla; type II, affecting the second portion of the duodenum and the papilla or type III, affecting the third portion of the duodenum without involvement of the major papilla [9].
Statistical Analysis
Results are expressed as the number and percentage of patients. Survival time was the period between biliary stent placement and death. Time to dysfunction of a biliary stent was the period between biliary stent placement and dysfunction or death, if dysfunction was not observed until death. Survival time and time to dysfunction were estimated by the Kaplan–Meier method and the estimates compared with the log-rank test. A P value <0.05 was considered to indicate significance. All analyses were performed using JMP 9.0.3 (SAS Institute, Cary, NC).
Results
Patients’ Characteristics
Twenty consecutive patients who underwent endoscopic biliary drainage for nonresectable distal MBO in the presence of a duodenal SEMS were identified. The patients’ characteristics are summarized in Table 1. The underlying malignancies were mainly pancreatic cancer (75 %). The causes of distal MBO in four patients with gastric cancer were lymph node metastasis of the primary cancer (3 patients) and tumor invasion (1 patient). Among the 20 patients enrolled in the study, EUS-BD and transpapillary biliary drainage were carried out in seven and 13 patients, respectively. Five and two patients in the transmural drainage group had type II and III duodenal stenosis, respectively, and nine and four patients in the transpapillary drainage group had type I and III duodenal stenosis, respectively. One uncovered and six covered duodenal SEMS were placed in the transmural drainage group; in the transpapillary drainage group, these numbers were five and nine, respectively. EUS-BD was performed concurrently with duodenal SEMS placement in five patients (71 %), and 20 and 14 days after duodenal SEMS placement in one patient each. The transpapillary biliary drainage was performed concurrently with duodenal SEMS placement in 11 patients (85 %), and 105 and 49 days after duodenal SEMS placement in one patient each.
Outcomes of EUS-BD (EUS-BD Group)
Endoscopic ultrasound-guided biliary drainage was performed via hepaticogastrostomy and choledochoduodenostomy in three and four patients, respectively (Fig. 1). A covered SEMS was placed in all cases with hepaticogastrostomy, and plastic stents and covered SEMSs were placed in two patients each with choledochoduodenostomy. The first SEMS via hepaticogastrostomy was misplaced in the patient (patient no. 5), with its distal end in the peritoneal cavity, and was subsequently managed by placing another SEMS in a tandem fashion [20]. Moderate bleeding was observed in one patient as a procedure-related complication. The bleeding occurred at the puncture site of EUS-guided hepaticogastrostomy and required a two-unit blood transfusion, but no endoscopic intervention was performed. The median survival time was 112 days, and six patients (86 %) died during the follow-up period.
A biliary stent dysfunction was observed in one patient (14 %) who developed cholangitis caused by occlusion of a plastic stent due to sludge at 32 days after EUS-BD. In this case, bile duct cannulation was achieved alongside the plastic stent in situ followed by placement of another plastic stent, and the cholangitis subsided.
Outcomes of Transpapillary Biliary Drainage (Transpapillary Group)
Transpapillary biliary drainage was performed in 13 patients using a SEMS (Fig. 2). Covered- and uncovered-type SEMS were placed in 11 and two patients, respectively. In three patients, a biliary SEMS was placed through the mesh of a duodenal SEMS which had been placed across the papilla to secure a sufficient margin from the duodenal obstruction despite there being no tumor involvement of the papilla. One patient (8 %) developed mild pancreatitis, which subsided only with conservative treatment. The median survival time was 164 days, and 11 patients (85 %) died during the follow-up period.
Biliary stent dysfunction was observed in seven patients (54 %), with a median time to dysfunction of 53 days. The causes of stent dysfunction included nonocclusion cholangitis (3 patients), occlusion sludge (2 patients), occlusion due to food impaction (1 patient), and an unknown cause (1 patient). Among those with stent dysfunction, endoscopic transpapillary and percutaneous re-interventions were performed in two patients each.
Comparison Between EUS-BD and Transpapillary Drainage Groups
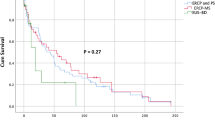
Survival times did not differ significantly between the groups (P = 0.854). The rate of dysfunction tended to be lower in the EUS-BD group than in the transpapillary group (14 vs. 54 % respectively; P = 0.157), and time to dysfunction was significantly longer in the EUS-BD group than in the transpapillary group [median (not available) vs. 53 days, respectively; P = 0.048; Fig. 3]. The stent patency rate was with EUS-BD group than with transpapillary drainage (100 vs. 71 %, respectively, at 1 month; 83 vs. 29 % at 3 months; 83 vs. 29 % at 6 months). Complication rates did not differ significantly between the groups (P = 1.000). Percutaneous transhepatic biliary drainage was required as a re-intervention for biliary stent dysfunction only in the transpapillary group (15 %).
Discussion
The results of this multicenter retrospective study of 20 patients who underwent endoscopic biliary drainage in the presence of a duodenal SEMS demonstrate that EUS-BD was feasible and effective in this patient group and that the time to EUS-BD dysfunction was significantly longer with EUS-BD than with transpapillary drainage. The feasibility of EUS-BD in the presence of a duodenal SEMS has been reported in several studies [21–23]. However, distal MBO usually precedes malignant GOO for anatomical reasons [9, 10], and thus no previous study has compared transmural and transpapillary biliary drainage in the presence of a duodenal SEMS (Table 2).
Endoscopic SEMS placement has become the mainstream of biliary drainage in cases of nonresectable distal MBO [24–26], and a SEMS is mostly placed across the papilla of Vater. In this setting, SEMS is predisposed to reflux of duodenal contents [27], sometimes leading to stent occlusion or ascending cholangitis [28]. We reported previously that duodenal tumor invasion is a risk factor for early biliary SEMS dysfunction (<3 months) and that duodenobiliary reflux enhanced by tumor invasion is a key contributor to this complication [29]. In addition, an indwelling duodenal SEMS is an even stronger risk factor for transpapillary SEMS dysfunction due to the further increased duodenobiliary reflux via reduced duodenal peristalsis that might not be sufficiently resolved by a duodenal SEMS [10].
The effectiveness of transpapillary SEMS combined with duodenal SEMS, so-called “double-stenting”, has been reported [1, 8, 9]. However, biliary SEMS is predisposed to enhanced duodenobiliary reflux, and long-term outcomes are disappointing [10]. In our transpapillary group, SEMS dysfunction was observed in over one-half of the patients, with a median time of <2 months. Notably, the vast majority of SEMS dysfunctions may have been associated with duodenobiliary reflux (nonocclusion cholangitis, sludge, and food impaction). Furthermore, an indwelling duodenal SEMS makes it difficult to endoscopically manage dysfunction of a biliary SEMS. Indeed, percutaneous transhepatic biliary drainage was required as a re-intervention in 15 % of patients in the transpapillary drainage group, leading to deterioration in the quality of life of these patients. One patient in the transmural drainage group with dysfunction of a plastic stent was successfully managed by endoscopic intervention. Given these worse outcomes of transpapillary biliary drainage, the indications for biliary drainage in the presence of duodenal SEMS should be further considered.
EUS-BD has emerged as an alternative method in cases of failed ERCP [11, 30], for which malignant GOO is one of the most common reasons. EUS-BD is theoretically less susceptible to stagnation of duodenal contents and is expected to have a longer patency rate due to less duodenobiliary reflux. In the present study, EUS-BD was feasible and effective in the presence of a duodenal SEMS. EUS-BD—hepaticogastrostomy in particular—can be carried out whether a duodenal SEMS is present or not, and its safety and effectiveness have been reported [12–14]. No dysfunction of a biliary SEMS due to duodeobiliary reflux occurred in our EUS-BD group, whereas the causes of stent dysfunction in our transpapillary group were mostly attributable to the reflux of duodenal contents. Considering its technical feasibility and potentially prolonged time to biliary stent dysfunction, EUS-BD can be an alternative to transpapillary biliary drainage in patients with an indwelling duodenal SEMS, and a randomized controlled trial that includes a sufficient number of patients is desired to confirm the superiority of transmural over transpapillary biliary drainage in the presence of duodenal SEMS. Another advantage of EUS-BD is insusceptibility to post-ERCP pancreatitis, one of the most serious complications of ERCP [31]. In contrast, a bile leak after EUS-BD is a potential complication that is not seen in transpapillary biliary drainage, and should be overcome. In the present study, type II duodenal stenosis was more frequently observed in the EUS-BD group, inferring a treatment selection bias because this type of duodenal stenosis involves the papilla and inhibits transpapillary bililary drainage. A prospective study with adjustment for the type of duodenal stenosis would facilitate a comparison of EUS-BD with transpapillary biliary drainage.
Some limitations of this study should be discussed. This study was based on a nonrandomized retrospective design. EUS-guided antegrade placement of a biliary stent [23] is an alternative method, particularly in patients with GOO proximal to the ampulla. Finally, the follow-up time was relatively short, as patients with duodenal obstruction are generally associated with a poor prognosis.
Based on the results of our study, we conclude that EUS-guided transmural biliary drainage is an alternative to transpapillary biliary drainage in cases with an indwelling duodenal SEMS.
References
Maire F, Hammel P, Ponsot P, et al. Long-term outcome of biliary and duodenal stents in palliative treatment of patients with unresectable adenocarcinoma of the head of pancreas. Am J Gastroenterol. 2006;101:735–742.
Flamm CR, Mark DH, Aronson N. Evidence-based assessment of ERCP approaches to managing pancreaticobiliary malignancies. Gastrointest Endosc. 2002;56:S218–S225.
Wong YT, Brams DM, Munson L, et al. Gastric outlet obstruction secondary to pancreatic cancer: surgical vs endoscopic palliation. Surg Endosc. 2002;16:310–312.
Baron TH. Expandable metal stents for the treatment of cancerous obstruction of the gastrointestinal tract. N Engl J Med. 2001;344:1681–1687.
Dormann A, Meisner S, Verin N, Wenk Lang A. Self-expanding metal stents for gastroduodenal malignancies: systematic review of their clinical effectiveness. Endoscopy. 2004;36:543–550.
Sasaki T, Isayama H, Maetani I, et al. Japanese multicenter estimation of WallFlex duodenal stent for unresectable malignant gastric outlet obstruction. Dig Endosc. 2013;25:1–6.
Isayama H, Sasaki T, Nakai Y, et al. Management of malignant gastric outlet obstruction with a modified triple-layer covered metal stent. Gastrointest Endosc. 2012;75:757–763.
Kaw M, Singh S, Gagneja H. Clinical outcome of simultaneous self-expandable metal stents for palliation of malignant biliary and duodenal obstruction. Surg Endosc. 2003;17:457–461.
Mutignani M, Tringali A, Shah SG, et al. Combined endoscopic stent insertion in malignant biliary and duodenal obstruction. Endoscopy. 2007;39:440–447.
Hamada T, Nakai Y, Isayama H, et al. Duodenal metal stent placement is a risk factor for biliary metal stent dysfunction: an analysis using a time-dependent covariate. Surg Endosc. 2013;27:1243–1248.
Kahaleh M, Hernandez AJ, Tokar J, Adams RB, Shami VM, Yeaton P. Interventional EUS-guided cholangiography: evaluation of a technique in evolution. Gastrointest Endosc. 2006;64:52–59.
Horaguchi J, Fujita N, Noda Y, et al. Endosonography-guided biliary drainage in cases with difficult transpapillary endoscopic biliary drainage. Dig Endosc. 2009;21:239–244.
Park do H, Song TJ, Eum J, et al. EUS-guided hepaticogastrostomy with a fully covered metal stent as the biliary diversion technique for an occluded biliary metal stent after a failed ERCP (with videos). Gastrointest Endosc. 2010;71:413–419.
Itoi T, Isayama H, Sofuni A, et al. Stent selection and tips on placement technique of EUS-guided biliary drainage: transduodenal and transgastric stenting. J Hepatobiliary Pancreat Sci. 2011;18:664–672.
Kawakubo K, Isayama H, Nakai Y, et al. Simultaneous duodenal metal stent placement and EUS-guided choledochoduodenostomy for unresectable pancreatic cancer. Gut Liver. 2012;6:399–402.
Dhir V, Artifon EL, Gupta K, et al. Multicenter study on endoscopic ultrasound-guided expandable biliary metal stent placement: Choice of access route, direction of stent insertion, and drainage route. Dig Endosc. 2014;26:430–435.
Kawakubo K, Isayama H, Kato H, et al. Multicenter retrospective study of endoscopic ultrasound-guided biliary drainage for malignant biliary obstruction in Japan. J Hepatobiliary Pancreat Sci. 2014;21:328–334.
Nakai Y, Isayama H, Tsujino T, et al. Impact of introduction of wire-guided cannulation in therapeutic biliary endoscopic retrograde cholangiopancreatography. J Gastroenterol Hepatol. 2011;26:1552–1558.
Cotton P, Lehman G, Vennes J, et al. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991;37:383–393.
Hamada T, Nakai Y, Isayama H, Koike K. Tandem stent placement as a rescue for stent misplacement in endoscopic ultrasonography-guided hepaticogastrostomy. Dig Endosc. 2013; 25:340–341.
Belletrutti PJ, Gerdes H, Schattner MA. Successful endoscopic ultrasound-guided transduodenal biliary drainage through a pre-existing duodenal stent. JOP. 2010;11:234–236.
Iwamuro M, Kawamoto H, Harada R, et al. Combined duodenal stent placement and endoscopic ultrasonography-guided biliary drainage for malignant duodenal obstruction with biliary stricture. Dig Endosc. 2010;22:236–240.
Khashab MA, Fujii LL, Baron TH, et al. EUS-guided biliary drainage for patients with malignant biliary obstruction with an indwelling duodenal stent (with videos). Gastrointest Endosc. 2012;76:209–213.
Davids P, Groen A, Rauws E, Tytgat G, Huibregtse K. Randomised trial of self-expanding metal stents versus polyethylene stents for distal malignant biliary obstruction. Lancet. 1992;340:1488–1492.
Knyrim K, Wagner H, Pausch J, Vakil N. A prospective, randomized, controlled trial of metal stents for malignant obstruction of the common bile duct. Endoscopy. 1993;25:207–212.
Isayama H, Yasuda I, Ryozawa S, et al. Results of a Japanese multicenter, randomized trial of endoscopic stenting for non-resectable pancreatic head cancer (Jm-test): covered Wallstent versus doublelayer Stent. Dig Endosc. 2011;23:310–315.
Misra S, Dwivedi M. Reflux of duodenal contents and cholangitis in patients undergoing self-expanding metal stent placement. Gastrointest Endosc. 2009;70:317–321.
Okamoto T, Fujioka S, Yanagisawa S, et al. Placement of a metallic stent across the main duodenal papilla may predispose to cholangitis. Gastrointest Endosc. 2006;63:792–796.
Hamada T, Isayama H, Nakai Y, et al. Duodenal invasion is a risk factor for the early dysfunction of biliary metal stents in unresectable pancreatic cancer. Gastrointest Endosc. 2011;74:548–555.
Giovannini M, Moutardier V, Pesenti C, Bories E, Lelong B, Delpero J. Endoscopic ultrasound-guided bilioduodenal anastomosis: a new technique for biliary drainage. Endoscopy. 2001;33:898–900.
Kawakubo K, Isayama H, Nakai Y, et al. Risk factors for pancreatitis following transpapillary self-expandable metal stent placement. Surg Endosc. 2012;26:771–776.
Maetani I, Isayama H, Mizumoto Y. Palliation in patients with malignant gastric outlet obstruction with a newly designed enteral stent: a multicenter study. Gastrointest Endosc. 2007;66:355–360.
Kim YW, Choi CW, Kang DH, et al. A double-layered (ComVi) self-expandable metal stent for malignant gastroduodenal obstruction: a prospective multicenter study. Dig Dis Sci. 2011;56:2030–2036.
Nakai Y, Isayama H, Komatsu Y, et al. Efficacy and safety of the covered Wallstent in patients with distal malignant biliary obstruction. Gastrointest Endosc. 2005;62:742–748.
Isayama H, Mukai T, Itoi T, et al. Comparison of partially covered nitinol stents with partially covered stainless stents as a historical control in a multicenter study of distal malignant biliary obstruction: the WATCH study. Gastrointest Endosc. 2012;76:84–92.
Isayama H, Kawabe T, Nakai Y, et al. Management of distal malignant biliary obstruction with the ComVi stent, a new covered metallic stent. Surg Endosc. 2010;24:131–137.
Acknowledgments
We gratefully acknowledge the assistance of Dr. Keiji Ogura, Tokyo Metropolitan Police Hospital, and Dr. Ryou Nakata, Japanese Red Cross Medical Center.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hamada, T., Isayama, H., Nakai, Y. et al. Transmural Biliary Drainage Can Be an Alternative to Transpapillary Drainage in Patients with an Indwelling Duodenal Stent. Dig Dis Sci 59, 1931–1938 (2014). https://doi.org/10.1007/s10620-014-3062-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-014-3062-1