Abstract
Goals and Background
Simple benign strictures may be relieved with one to three dilation sessions. Resistant benign strictures are anatomically complex and resistant to therapy. We sought to determine the efficacy and safety of esophageal self-dilation with bougie dilators in the largest series to date.
Study
A retrospective chart review was performed to identify patients who underwent esophageal self-dilation at two tertiary referral centers (Mayo Clinic, Scottsdale, Arizona and Mayo Clinic Rochester, Minnesota) between January 1, 2003 and June 30, 2012. Demographic details and clinical information regarding relief of dysphagia, complications, and frequency of endoscopic and self-dilation were abstracted.
Results
Of the 32 patients who began self-dilation for nonmalignant strictures, 30 [22 men; median (range) age, 62 years (22–86 years)] were included in the study. Median (range) follow-up was 37 months (14–281 months). Stricture etiology included radiation therapy (n = 8), anastomotic stricture (n = 9), eosinophilic esophagitis (n = 4), caustic ingestion (n = 3), photodynamic therapy (n = 2), granulation tissue (n = 2), peptic stricture (n = 1) and one patient had radiation therapy and peptic stricture. The average number (range) of physician performed dilations before self-dilation was 12 (4–55). Esophageal self-dilation was successful in treating 90 % of patients. Dysphagia score (2 vs. 1; P < 0.001), stricture diameter (median; 5 vs. 12 mm; P < 0.001) and weight (median; 73 vs. 77 kg; P < 0.001) were significantly different between EDG dilation versus self-dilation.
Conclusions
Esophageal self-dilation is a safe, effective treatment for resistant, benign esophageal strictures. This management strategy should be strongly considered in this patient population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Benign esophageal strictures are widespread in gastroenterology practice. Causes include peptic strictures, eosinophilic esophagitis, pill esophagitis, caustic ingestions, radiation injuries, anastomotic strictures, photodynamic therapy-induced strictures, lichen planus, rings and webs [1, 2]. The primary treatment for esophageal strictures has been physician-performed dilation therapy. Although dilation frequently relieves symptoms of dysphagia, recurrent dysphagia can arise from subsequent narrowing of the stricture.
Resistant, benign esophageal strictures (RBES) may be difficult to treat. They are generally longer than 2 cm, irregular and with a narrow diameter; they can also be angulated [3]. They may require multiple physician-performed dilations and corticosteroid injections [4], incisional therapy [5], or temporary stent placement [6–8]. If these measures prove unsuccessful or are not appropriate, self-dilation can be proposed to the patient as the next step or as an alternative therapy [9, 10].
Several small studies, including one from our institution [10], have reported excellent results with home Maloney dilator esophageal self-dilation therapy [10–13]. However, outcomes for only a small numbers of patients have been reported in the current medical literature. We therefore sought to evaluate the efficacy and safety of esophageal self-dilation with bougie dilators and to update our previously published experience into the largest series in the Western world with long-term follow-up.
Methods
All patients who underwent esophageal self-dilation for RBES between January 1, 2003 and June 30, 2012 at Mayo Clinic, Rochester, Minnesota, or Mayo Clinic, Scottsdale, Arizona, were identified through the esophageal clinic registered nurse database. This retrospective study was approved by the Mayo Clinic Institutional Review Board.
Demographic and clinical characteristics were abstracted, including age, sex, dysphagia symptoms, diet type, stricture etiology and anatomy, history of gastroesophageal reflux disease (GERD) prior to symptoms of dysphagia, subjective symptoms of acid reflux, food regurgitation, and hiatal hernia. Weight change was calculated by subtracting the closest measured patient’s weight at the last report visit to the closest measured weight at initial self-dilation teaching session visit. Procedure-related characteristics included number of esophagogastroduodenoscopies (EGDs) before and after initiation of self-dilation, use of stricture-directed corticosteroid injections, needle knife therapy, stent placement, and self-dilation (duration, frequency, bougie dilator diameter). No angulated strictures were present, and stricture biopsies were performed in all patients to exclude malignancy before initiation of self-dilation. Stricture diameter was measured during the index EGD procedure by comparing the outer diameter of the upper endoscope to the stricture diameter. In some cases the 8-mm open biopsy forceps was used as a reference. Adverse events were recorded, and use of antireflux therapy was noted. Success of self-dilation was defined as improvement in dysphagia score of ≥1 on a 0–4 point scale after initiation of the procedure following the completion of the teaching sessions.
Dysphagia was rated retrospectively according to diet consistency, on a scale from 0 to 4, whereby 0 = no dysphagia (normal diet), 1 = inability to swallow some solids, 2 = inability to swallow all solids, 3 = inability to swallow semisolids, and 4 = inability to swallow liquids. Symptoms of dysphagia, diet, and stricture anatomy before initiation of self-dilation were compared with those at the time of the last report visit.
The method of esophageal self-dilation has been described previously [9, 14]. Briefly, self-dilation teaching consists of patients: (1) viewing the patient-directed self-dilation teaching video [14]; (2) meeting with other patients currently performing self-dilation; (3) undergoing physician-performed endoscopic dilation for stricture specifications; and (4) participating in three self-dilation practice sessions supervised by a physician or a registered nurse. The first teaching session is typically the day after the physician-performed dilation. The diameter of the bougie dilator is never larger than the largest dilator effectively passed by a physician. The dilator is marked with a tape to indicate the needed depth of insertion in order to achieve successful dilation beyond the stricture. After elective lidocaine gargle, the distal 10 cm of the dilator is lubricated (water or gel). Dilator tip is placed over the tongue, advanced to the back of the pharynx and once it reaches the cricopharynx the patient swallows while advancing the dilator; (5) patients are subsequently given a bougie dilator (usually a Maloney) to take home with instructions on dilator care (after each self-dilation the dilator should be washed with soap and water and placed down on a flat surface for tip protection) and daily self-dilation, usually in the morning; (6) Intermittent telephone calls and/or patient visits are performed to monitor patient symptoms, technique, and compliance. Initial self-dilation and subsequent frequency of self-dilation is variable and tailored to each patient.
Statistical Analysis
Data were assessed statistically using SPSS version 15.0 (IBM SPSS Inc). Frequency distributions were evaluated for all categorical variables (e.g., sex). The Wilcoxon signed rank test was used to compare patients’ symptoms before and after self-dilation. Results were expressed as median (range), as appropriate. A P value of <0.05 was considered statistically significant.
Results
In total, we identified 32 patients [22 men; median (range) age 62 years (22–86 years)] who initiated esophageal self-dilation for nonmalignant RBES. Two patients failed self-dilation because of anxiety. Four patients had more than one dominant stricture; of these four patients, one patient had two different etiologies. Baseline patient and stricture characteristics are summarized in Table 1. Median (range) follow-up was 37 months (14–281 months). Hiatal hernia was present in 12/30 (40 %) patients, and one patient had a minute preanastomotic diverticulum.
The average number of EGD dilations before initiation of self-dilation was 12 (range 4–55), with 13 (43 %) patients undergoing more than 15 dilations. The frequency of EGD dilations was weekly (n = 9 [30 %]), biweekly (n = 9 [30 %]), monthly (n = 6 [20 %]), bimonthly (n = 2 [7 %]), and every 4 months (n = 4 [13 %]). During serial physician-performed dilations, 14 (47 %) patients had one or more additional therapies: triamcinolone acetate injections (n = 13 [43 %]), stent placement (n = 8 [27 %]), or needle knife therapy (n = 4 [13 %]). A rendezvous procedure was required in two (6 %) patients to establish esophageal luminal patency before serial dilation. Tube feeding was required for nutritional support in 11 (37 %) patients (gastrostomy tube [n = 8]; jejunostomy tube [n = 3]). Twelve (40 %) patients reported regurgitation of food, and 6 (20 %) reported symptoms of acid reflux.
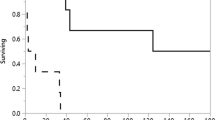
The median (range) duration of dysphagia symptoms before initiation of self-dilation was 12 months (range 2–120 months). Initially, self-dilation was performed daily by 28 patients and twice daily by two patients, whose attempts at once-daily self-dilation proved less beneficial. Twenty-nine patients used a Maloney dilator, and one patient used a Hurst dilator (Fig. 1.) The initial median (range) dilator diameter was 14 mm (range 11–15 mm); at follow-up, it was 14 mm (12–16 mm).
The median (range) follow-up after initiation of self-dilation was 32 months (range 2–245 months). At follow-up, 37 % of patients were able to stop (n = 3) or decrease (n = 8) the frequency of self-dilation as their symptoms of dysphagia improved or resolved; five patients were able to decrease their frequency of self-dilation to twice weekly, two to once weekly and one to once monthly. Three patients stopped self-dilation therapy because of intolerance (throat and/or chest pain); none of the three had any clinical evidence of esophageal perforation. One patient stopped dilation after undergoing esophagectomy for high-grade dysplasia identified during Barrett’s esophagus surveillance.
The average number (range) of EGD dilations performed for symptoms of dysphagia before and after initiation of self-dilation per year was 21.7 (0.6–64) and 1.0 (0.2–6), respectively (P < 0.0001).
Acid reflux symptoms were reported by 23 (77 %) patients, which was four times higher than that reported before self-dilation. All patients with symptoms of acid reflux were on daily proton pump inhibitor therapy. During the period when patients were performing self-dilation, no EGD was required in 12 (40 %) patients; one to two EGDs were required in 12 patients (40 %); and more than two EGDs were required in six (20 %) patients. Reasons for EGD included symptoms of dysphagia in 12 (40 %) patients, dilator size increase in three (10 %), surveillance in two (7 %), and bleeding unrelated to self-dilation in one (3 %). Symptoms of dysphagia were due to persistence of stricture (n = 5), noncompliance (n = 3), development of a new stricture (n = 1), esophageal spasms (n = 2), and Candida esophagitis (n = 1).
During EGD dilations, two (7 %) patients were on some solids, 19 (63 %) patients were on semisolids, six (20 %) patients were on liquids and three (10 %) patients were on strict NPO diets. During self-dilation, 15 (50 %) patients were on regular diet, 12 (40 %) were on some solids and three (10 %) were on semisolids. No patients required continuation of tube feedings for supplemental nutrition.
Results showed a statistically significant (P < 0.001) difference in symptoms of dysphagia, stricture diameter, weight at follow-up visit, and total number of EGDs before and after initiation of self-dilation (Table 2). Median (range) improvement in the dysphagia score before and after initiation of self-dilation was 1 (0–4). One patient had a total of 23 physician-performed dilations after initiation of self-dilation for incidental caustic ingestion. She has been performing self-dilation intermittently for ten years; therefore, during the pauses she was receiving physician-performed dilations.
No adverse events (e.g., perforation, bleeding, aspiration or clinically evident bacteremia) were observed in any patient. During the study period, two patients died after initiation of self-dilation: one each from recurrent lung carcinoma and natural causes. Neither death was related to self-dilation or esophageal strictures.
Discussion
There are various options for management of RBES after physician-performed esophageal dilation therapy becomes ineffective. These include a combination of stricture-directed steroid injections, stent placement, needle knife intervention, surgery, conservative management with a feeding tube to supplement nutrition, and, importantly, self-dilation. Although self-dilation is not commonly used, it is a safe and effective treatment alternative in motivated patients [10–12]. Adequate teaching of the correct way to conduct self-dilation is essential, as is close monitoring of the first self-dilation attempts.
Previous small-case series using esophageal self-dilation have shown it to be an effective, relatively safe treatment option for refractory esophageal strictures [10–12, 15–17]. This report describes what we believe to be the largest series to date of patients performing esophageal self-dilation with mechanical dilators (Maloney or Hurst) that includes extended longitudinal follow-up. Larger studies on this topic have been completed in India and China using other dilating instruments, including Foley catheters [13, 18], self-made rubber [19] and mechanical dilators [15], and “endless string dilators” [20] with similar outcomes.
We observed that self-dilation was successful in 90 % of the patients who were trained to use it. Intolerance, including throat/chest pain was the main reason for discontinuation of therapy (n = 3). Although there is considerable trepidation in learning self-dilation, we found that only 6 % of patients who agreed to try the technique failed to master it. This study did not evaluate how many patients will not even agree to attempt to learn the technique. Therefore, our 94 % successful training rate may be an overestimate of overall patient success at mastering self-dilation. Favorable outcomes in our study support the results of previously published reports on this topic [10–12, 15–17].
Our results suggest that esophageal self-dilation can provide long-term relief of dysphagia (32 months) without adverse events in carefully selected and very motivated patients; 27 (90 %) patients were on a normal diet or one with some solids. We did not observe any previously reported adverse events, including perforation [21], pneumomediastinum [22], bougie mis-swallowing [23], and bougie right main-stem bronchus intubation [24]. Moreover, we did not detect any other complications, such as bleeding and clinically evident bacteremia. Lack of complications in our study should be viewed with caution because of the relatively small sample size studied. In addition, none of the patients in our study had angulated strictures; possibly the lack of angulation had positive impact on our procedure safety outcomes.
During self-dilation, most patients experienced subjective symptoms of acid reflux, which suggests that the stricture causing the dysphagia may have also been a barrier to reflux. Therefore, antireflux therapy in this patient group is essential. Acid reflux symptoms were well controlled with medications and/or were short lived in most patients. Lack of objective diagnosis of acid reflux (i.e., ambulatory esophageal pH monitoring) represents a limitation in our study. However, even though the diagnosis was based on clinical symptoms alone, the fact that symptoms improved with antireflux medications favors acid reflux diagnosis.
We believe that self-dilation may be underutilized in clinical practice. One reason for this may be that the training of gastroenterologists with blind bougie (Maloney, Hurst) dilation has essentially stopped since the 1980s incorporation of over-the-wire bougie dilators into mainstream gastroenterology practice. Esophageal stenting has also become a common practice for management of refractory esophageal strictures. However, esophageal stenting with plastic, fully covered metal, or biodegradable stents is no more than 50 % effective [25–27]. Moreover, stenting of benign strictures may have serious complications, including stent associated esophagorespiratory fistulas, which developed in 14 % of patients stented for benign strictures. Patients with this fistula had significant morbidity and mortality with a mean survival of 7.5 months [28]. Anastomotic and radiation strictures, which make up more that half of the study patients, were at the greatest risk of fistula formation in this study [28]. At our institution, self-dilation is used before stenting in most cases. We have developed a teaching video for patients (and physicians) which we believe is beneficial in helping patients considering self-dilation appreciate that others like themselves have mastered the technique [14].
Limitations of our study include its retrospective nature, the selection bias inherent in studying patients only from a tertiary care center and a small sample size, which prevented us from performing a subgroup analysis of variables possibly affecting the effectiveness of self-dilation. The low incidence of RBES patients at our institution precludes the use of a randomized controlled trial. However, because this is the largest study with extended follow-up on this topic to date, we believe it does add valuable information to the current knowledge of this treatment choice.
In conclusion, our study suggests that esophageal self-dilation appears to be a safe and effective treatment option for RBES, especially in patients who are motivated. We believe that this technique is being underused by physicians caring for patients with RBES and that it should be strongly considered in this patient population as an alternative treatment method.
References
Spechler SJ. Aga technical review on treatment of patients with dysphagia caused by benign disorders of the distal esophagus. Gastroenterology. 1999;117:233–254.
Siersema PD, de Wijkerslooth LR. Dilation of refractory benign esophageal strictures. Gastrointest Endosc. 2009;70:1000–1012.
Lew RJ, Kochman ML. A review of endoscopic methods of esophageal dilation. J Clin Gastroenterol. 2002;35:117–126.
Ramage JI Jr, Rumalla A, Baron TH, et al. A prospective, randomized, double-blind, placebo-controlled trial of endoscopic steroid injection therapy for recalcitrant esophageal peptic strictures. Am J Gastroenterol. 2005;100:2419–2425.
Hordijk ML, Siersema PD, Tilanus HW, Kuipers EJ. Electrocautery therapy for refractory anastomotic strictures of the esophagus. Gastrointest Endosc. 2006;63:157–163.
Eloubeidi MA, Lopes TL. Novel removable internally fully covered self-expanding metal esophageal stent: feasibility, technique of removal, and tissue response in humans. Am J Gastroenterol. 2009;104:1374–1381.
Repici A, Hassan C, Sharma P, Conio M, Siersema P. Systematic review: the role of self-expanding plastic stents for benign oesophageal strictures. Aliment Pharmacol Ther. 2010;31:1268–1275.
Repici A, Vleggaar FP, Hassan C, et al. Efficacy and safety of biodegradable stents for refractory benign esophageal strictures: the best (biodegradable esophageal stent) study. Gastrointest Endosc. 2010;72:927–934.
Dzeletovic I, Fleischer DE. Self-dilation for resistant, benign esophageal strictures. Am J Gastroenterol. 2010;105:2142–2143.
Dzeletovic I, Fleischer DE, Crowell MD, et al. Self dilation as a treatment for resistant benign esophageal strictures: outcome, technique, and quality of life assessment. Dig Dis Sci. 2011;56:435–440.
Kim CH, Groskreutz JL, Gehrking SJ. Recurrent benign esophageal strictures treated with self-bougienage: report of seven cases. Mayo Clin Proc. 1990;65:799–803.
Grobe JL, Kozarek RA, Sanowski RA. Self-bougienage in the treatment of benign esophageal stricture. J Clin Gastroenterol. 1984;6:109–112.
Shad SK, Gupta S, Chattopadhyay TK. Self-dilatation of cervical oesophagogastric anastomotic stricture: a simple and effective technique. Br J Surg. 1991;78:1254–1255.
Dzeletovic I, Fleischer DE. Esophageal Self Dilation: A Teaching Guide for Physicians. ASGE Endoscopic Learning Library; 2011.
Sullivan S, Corke M, Watson W. Self dilation of esophageal strictures. Can J Gastroenterol. 1991;5:49–50.
Moody GA, Mayberry JF, Probert CS. Mercury bougie self-dilation of the esophagus in the 1990s. J Clin Gastroenterol. 1992;15:264.
Gilmore IT, Sheers R. Oesophageal self-bougienage. Lancet. 1982;1:620–621.
Manjunath S, Ramachandra C, Veerendra Kumar KV, Prabhakaran PS. Simple dilatation of anastomotic strictures following oesophagectomy in unsedated patients. Eur J Surg Oncol. 2006;32:1015–1017.
Deng Y, Wang H, Li Z, et al. Application of portable esophageal dilator in treatment of esophageal stenosis at home. Lin Chuang Er Bi Yan Hou Ke Za Zhi. 2006;20:457–459.
Bapat RD, Bakhshi GD, Kantharia CV, Shirodkar SS, Iyer AP, Ranka S. Self-bougienage: long-term relief of corrosive esophageal strictures. Indian J Gastroenterol. 2001;20:180–182.
Palmer E. The requirement for esophageal bougienage. GP. 1966;33:97–101.
Noppen MM, Corne L, Peters O, Smekens L, Musch W, Vincken W. Pneumomediastinum after self-dilation of the esophagus. Chest. 1987;92:757–758.
Luo SD, Hsu RF. A rare but life-threatening complication of self-bougienage: iatrogenic esophageal foreign body. Endoscopy. 2008;40:E17–E18.
Kashima ML, Eisele DW. Complication of esophageal self-dilation for radiation-induced hypopharyngeal stenosis. Dysphagia. 2003;18:92–95.
Bakken JC, Wong Kee Song LM, de Groen PC, Baron TH. Use of a fully covered self-expandable metal stent for the treatment of benign esophageal diseases. Gastrointest Endosc. 2010;72:712–720.
Sharma P, Kozarek R. Role of esophageal stents in benign and malignant diseases. Am J Gastroenterol. 2010;105:258–273; quiz 274.
van Boeckel PG, Vleggaar FP, Siersema PD. A comparison of temporary self-expanding plastic and biodegradable stents for refractory benign esophageal strictures. Clin Gastroenterol Hepatol. 2011;9:653–659.
Bick BL, Wong Kee Song LM, Buttar NS, et al. Stent-associated esophagorespiratory fistulas: incidence and risk factors. Gastrointest Endosc. 2013;77:181–189.
Conflict of interests
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dzeletovic, I., Fleischer, D.E., Crowell, M.D. et al. Self-Dilation as a Treatment for Resistant, Benign Esophageal Strictures. Dig Dis Sci 58, 3218–3223 (2013). https://doi.org/10.1007/s10620-013-2822-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-013-2822-7