Abstract
Background
We previously reported that preoperative chemolipiodolization of the whole liver is effective for reducing the incidence of postoperative recurrence and prolonging survival in patients with resectable hepatocellular carcinoma (HCC). The present randomized controlled trial was performed to evaluate the influence of preoperative transcatheter arterial chemoembolization (TACE) on survival after the resection of HCC.
Methods
Operative results and long-term outcome were prospectively compared among 42 patients who received only selective TACE targeting the tumor (selective group), 39 patients who received TACE targeting the tumor plus chemolipiodolization of the whole liver (whole-liver group), and 43 patients without preoperative TACE or chemolipiodolization (control group).
Results
There were no serious side effects of TACE or chemolipiodolization and the operative outcomes did not differ among the three groups. Even though preoperative TACE induced complete tumor necrosis, there were no significant differences in the pattern of intrahepatic recurrence or the time until recurrence among the three groups. There were also no significant differences in disease-free survival or overall survival among the three groups, even among patients with larger tumor size.
Conclusion
These results indicate that preoperative selective TACE and whole-liver chemolipiodolization plus TACE do not reduce the incidence of postoperative recurrence or prolong survival in patients with resectable HCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide [1]. Although the majority of patients are still found in Asia and Africa, recent studies have shown that the incidence and mortality rate of HCC are rising in North America and Europe [2, 3]. There has been an increase in reports of non-surgical therapeutic options for small HCC, such as percutaneous ethanol injection therapy [4], microwave coagulation therapy [5], and percutaneous radiofrequency ablation (RFA) [6], but there is ongoing controversy regarding the best method of treating small tumors. In Japan, liver transplantation is not a practical option for most HCC patients, because the national health insurance scheme only covers transplantation for patients with decompensated cirrhosis whose tumors fit the Milan criteria. Resection is, therefore, generally the first-line treatment for patients with small tumors and underlying chronic liver disease, but the long-term survival rate after potentially curative resection of HCC is still unsatisfactory because of the high rate of recurrence [7]. To improve prognosis, it is important to prevent the recurrence of HCC after its initial resection, but standard therapy for intrahepatic metastasis has not yet been developed.
With various improvements in interventional radiology, transcatheter arterial chemoembolization (TACE) has become an increasingly important palliative treatment for HCC. Initially, TACE was only performed for unresectable HCC, as well as for some early tumors that were extremely difficult to resect. More recently, TACE has been used as preoperative adjuvant therapy in patients who have resectable HCC with the hope that it may improve survival [8–13]. Based on the current evidence, however, preoperative TACE is not routinely recommended for patients undergoing hepatectomy to treat resectable HCC [14–16], and TACE may be contraindicated in patients with cirrhosis because it can lead to the progressive deterioration of liver function [14]. Whether preoperative TACE can improve the long-term survival of HCC patients is still unclear, and there have been only three randomized controlled trials evaluating the influence of preoperative TACE on survival [15, 17, 18]. We previously reported that preoperative chemolipiodolization of the entire liver is effective for reducing the incidence of postoperative recurrence and for prolonging survival in patients with resectable HCC [19]. Accordingly, the present randomized controlled trial was conducted to better assess the influence of preoperative TACE combined with whole-liver chemolipiodolization on survival after the resection of HCC.
Patients and Methods
Patients
Between January 2004 and June 2007, 124 patients with HCC underwent curative hepatic resection at our institution. A curative operation was defined as the resection of all detectable tumors. The eligibility criteria for inclusion in this study were as follows: (1) age 20–80 years; (2) a preoperative diagnosis of HCC with no previous treatment; (3) no other malignancies; (4) Child–Pugh score A or B; (5) leukocyte count ≥3,000/mm3; (6) hemoglobin level ≥9.5 g/dl; (7) platelet count ≥50,000/mm3; (8) serum creatinine level <1.2 mg/dl; (9) total bilirubin <2.0 mg/dl; (10) local nodular disease without extrahepatic metastasis; and (11) Eastern Cooperative Oncology Group (ECOG) performance status 0–1 [20]. The etiology of HCC (HCV-related or other [HBV-related or non-B, non-C-related]) and the size of the tumor on imaging were taken into consideration when dividing patients into the three groups. The sample size was estimated based on our previously reported 3-year disease-free survival rates in selective and whole-liver groups, being 25 and 60%, respectively [19]. We needed 37 patients in each group for a type I error rate of 5% and a type II error rate of 20% with a two-tailed test. Among the 124 patients, TACE was performed preoperatively in 81. Patients were randomized to receive chemolipiodolization with gelatin sponge (equal to TACE) targeting the tumor (selective group, n = 42), chemolipiodolization with gelatin sponge (equal to TACE) targeting the tumor plus chemolipiodolization without gelatin sponge for the non-cancerous liver (whole-liver group, n = 39), or no preoperative TACE (control group, n = 43). The study protocol was explained to all patients, and they understood that they would be randomly selected for one of the above three groups. All patients gave written informed consent to participation in the trial. They were randomized by the envelope method and were informed of the result of the randomization before angiography. All operations were performed by the same surgeon, who had experience of over 700 hepatic resections. The protocol for this study was approved by the ethics committee of Kansai Medical University. The primary outcome measures were disease-free survival rate and overall survival rate. Secondary outcome measures included procedure-related complications and hospital mortality (Fig. 1).
Study design. We randomly divided patients into three groups: chemolipiodolization with gelatin sponge (equal to transcatheter arterial chemoembolization [TACE]) targeting the tumor (selective group, n = 42), chemolipiodolization with gelatin sponge (equal to TACE) targeting the tumor plus chemolipiodolization without gelatin sponge for the non-cancerous liver (whole-liver group, n = 39), or no preoperative TACE (control group, n = 43)
Chemolipiodolization
A catheter was selectively inserted into the right or left hepatic artery, a segmental artery, or a subsegmental artery by Seldinger’s method. In the selective group, TACE was performed via the right hepatic artery in 16 patients, the left hepatic artery in 10 patients, a segmental artery in 9 patients, and a subsegmental artery in 7 patients. In the whole-liver group, TACE (i.e., chemolipiodolization with gelatin sponge) was performed via the right hepatic artery in 18 patients and the left hepatic artery in 13 patients to target the tumor, while chemolipiodolization alone was performed on the non-cancerous side via the left or right hepatic artery. In a further 8 patients, TACE was performed via a right or left subsegmental artery to target the tumor and chemolipiodolization of the non-cancerous liver was performed via the right and left hepatic arteries as the catheter was withdrawn. The selective group was treated with epirubicin (Farmorubicin) at a mean (± standard deviation [SD]) dose of 47.0 ± 17.8 mg, iodized oil (Lipiodol) at a mean volume of 3.8 ± 2.1 ml, and gelatin sponge particles. In the whole-liver group, epirubicin (28.1 ± 5.5 mg), Lipiodol (2.9 ± 1.4 ml), and gelatin sponge particles were used to treat the tumor, while only epirubicin (22.2 ± 6.2 mg) and Lipiodol (1.9 ± 0.8 ml) were infused into the non-cancerous liver. In the control group, only angiography was performed.
Clinicopathologic Variables and Surgery
Before randomization, each patient underwent conventional liver function tests, measurement of the indocyanine green retention rate at 15 min (ICGR15), and technetium-99m-diethylenetriamine-pentaacetic acid-galactosyl human serum albumin (99mTc-GSA) liver scintigraphy [21]. Hepatitis screening was undertaken by testing for hepatitis B surface antigen (HBsAg) and hepatitis C antibody (HCVAb). The levels of α-fetoprotein (AFP) and protein induced by vitamin K absence or antagonist-II (PIVKA-II) were also measured. Surgical procedures were classified according to the Brisbane terminology proposed by Strasberg et al. [22]. In brief, anatomic resection was defined as resection of the tumor together with the related portal vein branches and the corresponding hepatic territory, and was classified as hemihepatectomy (resection of half of the liver), extended hemihepatectomy (hemihepatectomy plus removal of additional contiguous segments), sectionectomy (resection of two Couinaud subsegments [23]), or segmentectomy (resection of one Couinaud subsegment). All of the other procedures were non-anatomic and were classified as limited resection. Peripheral tumors and those with extrahepatic growth were managed by limited resection because this achieved adequate surgical margins. Central tumors located near the hepatic hilum or major vessels were treated by enucleation because it was too difficult or dangerous to remove enough of the liver to obtain an adequate margin. One senior pathologist reviewed all the specimens for histologic confirmation of the diagnosis. The width of the surgical margin was measured from the tumor border to the resection line. We evaluated the extent of necrosis on the largest tumor at its greatest diameter, even in cases with multiple tumors. The tumor stage was defined according to the TNM classification [24].
Follow-Up
Patients who survived were followed up after discharge, with physical examination, liver function tests, and ultrasound, computed tomography (CT), or magnetic resonance imaging being performed at least every 3 months to detect intrahepatic recurrence. Chest radiographs were also obtained to detect pulmonary metastases and chest CT was performed if the plain radiograph showed any abnormalities. Bone metastases were diagnosed by bone scintigraphy.
If the recurrence of HCC was detected by changes in the levels of tumor markers or by imaging, recurrence limited to the remnant liver was treated by TACE, lipiodolization, re-resection, or percutaneous local ablation therapy, such as RFA. If extrahepatic metastases were detected, active treatment was undertaken in patients with good hepatic functional reserve (Child–Pugh class A or B) and good performance status (0 or 1) who had a solitary extrahepatic metastasis and no evidence of intrahepatic recurrence, while other patients were treated only with radiation therapy to control symptoms caused by bone metastases.
Statistical Analysis
The results were expressed as the mean ± SD. Continuous variables were evaluated with the Mann–Whitney U-test or the Kruskal–Wallis test, as appropriate. Categorical data were compared with the Chi-square test or Fisher’s exact test. The Kaplan–Meier method was used to calculate the disease-free survival rate and the overall survival rate as of June 2010, and the significance of differences in survival rates was assessed with the generalized log-rank test. In all analyses, P < 0.05 was considered to indicate statistical significance.
Results
There were no serious side effects of selective TACE or whole-liver chemolipiodolization. The interval between selective TACE, whole-liver chemolipiodolization, or angiography and hepatic resection was 21.2 ± 10.8, 23.0 ± 13.2, and 20.0 ± 13.2 days, respectively. Table 1 shows the preoperative characteristics of the patients in the three groups. There were no significant differences among the groups with respect to gender, age, Child–Pugh class, etiology of hepatitis or cirrhosis, alcohol abuse, preoperative liver function, or serum AFP and PIVKA-II levels. The operative results and pathologic findings in each group are listed in Table 2. The operating time, blood loss, requirement for transfusion, and operative procedures did not differ significantly among the three groups, nor did the rates of postoperative complications and hospital deaths. There were no significant differences in tumor size or the number of tumors detected on imaging before randomization among the groups. Although the tumor sizes measured in the surgical specimens were smaller in the selective group and the whole-liver group compared with the control group, the differences were not significant. In the selective, whole-liver, and control groups, complete tumor necrosis was confirmed in 9/42 patients (21%), 8/39 patients (21%), and 0/43 patients (0%), respectively. The other pathological characteristics of the tumors were comparable among the three groups.
Recurrence and Survival
The pattern of recurrence and time to recurrence in the three groups are shown in Table 3. A total of 27 patients in the selective group, 28 patients in the whole-liver group, and 26 patients in the control group developed recurrence of HCC. Extrahepatic recurrence was significantly less common in the selective and whole-liver groups compared with the control group. However, the percentage of intrahepatic recurrences due to multinodular/diffuse tumors and the incidence of recurrence within 6 months or 1 year following curative resection were not significantly different among the three groups.
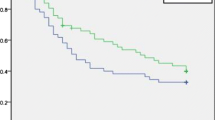
The disease-free survival rates of the entire TACE group (selective and whole-liver groups) and the control group were 65 and 53% at 1 year, and 27 and 32% at 3 years, respectively (Fig. 2a). The overall survival rates of the entire TACE group and the control group were 88 and 83% at 1 year, 75 and 60% at 3 years, and 47 and 56% at 5 years, respectively (Fig. 2b). There were no significant differences in disease-free survival (P = 0.6603) or overall survival (P = 0.4115) between the two groups. Comparing the three groups, the disease-free survival rates of the selective group, whole-liver group, and control group were 67, 63, and 53% at 1 year, and 29, 27, and 32% at 3 years, respectively (Fig. 3a). The overall survival rates of the selective, whole-liver, and control groups were 91, 84, and 83% at 1 year, and 80, 70, and 60% at 3 years, respectively (Fig. 3b). There were no significant differences in disease-free survival (P = 0.8303) or overall survival (P = 0.7126) among the three groups.
a Comparison of disease-free survival after the resection of hepatocellular carcinoma (HCC) between patients receiving preoperative selective TACE and patients receiving preoperative TACE plus whole-liver chemolipiodolization (entire TACE group, n = 81, solid line) and patients not receiving preoperative TACE (control group, n = 43, dotted line). There were no significant differences in disease-free survival between the two groups (P = 0.6603). b Comparison of overall survival after the resection of HCC between patients receiving preoperative selective TACE and patients receiving preoperative TACE plus whole-liver chemolipiodolization (entire TACE group, n = 81, solid line) and patients not receiving preoperative TACE (control group, n = 43, dotted line). There were no significant differences in overall survival between the two groups (P = 0.4115)
a Comparison of disease-free survival after the resection of HCC among patients receiving preoperative selective TACE (selective group, n = 42, thin solid line), patients receiving preoperative TACE plus whole-liver chemolipiodolization (whole-liver group, n = 39, thick solid line), and patients not receiving preoperative TACE (control group, n = 43, dotted line). There were no significant differences in disease-free survival among the three groups (P = 0.8303). b Comparison of overall survival after the resection of HCC among the selective group (n = 42, thin solid line), the whole-liver group (n = 39, thick solid line), and the control group (n = 43, dotted line). There were no significant differences in overall survival among the three groups (P = 0.7126)
When only patients with a solitary tumor measuring ≥5 cm in the greatest diameter were analyzed, the disease-free survival rates of the selective, whole-liver, and control groups were 50, 34, and 44% at 1 year, and 10, 11, and 9% at 3 years, respectively (P = 0.8650) (Fig. 4a). Among these patients, there were also no differences in the overall survival rate between the selective, whole-liver, and control groups, with survival rates of 82, 79, and 67% at 1 year, and 53, 68, and 47% at 3 years, respectively (P = 0.7264) (Fig. 4b).
a Comparison of disease-free survival after resection of a solitary HCC ≥5 cm in the greatest diameter among patients receiving preoperative selective TACE (selective group, n = 11, thin solid line), patients receiving preoperative TACE plus whole-liver chemolipiodolization (whole-liver group, n = 10, thick solid line), and patients without preoperative TACE (control group, n = 16, dotted line). There were no significant differences in disease-free survival among the three groups (P = 0.8650). b Comparison of overall survival after resection of a solitary HCC ≥5 cm in the greatest diameter among the selective group (n = 11, thin solid line), the whole-liver group (n = 10, thick solid line), and the control group (n = 16, dotted line). There were no significant differences in overall survival among the three groups (P = 0.7264)
Discussion
In our previous retrospective study, we found that preoperative chemolipiodolization of the whole liver achieved significant prolongation of both disease-free survival and overall survival for HCC patients [19]. The precise mechanism remains unclear, but some possible explanations are: (1) subclinical micrometastases due to portal vein dissemination or multicentric primary tumors are eliminated by whole-liver therapy and (2) reducing the tumor burden before surgery may lessen the chance of developing resistance to chemotherapy. TACE is a well-recognized treatment for HCC, either as adjuvant therapy or as a definitive procedure in patients whose tumors are considered to be unresectable [25, 26]. Preoperative TACE is not only intended to prevent recurrence by controlling intrahepatic spread via the portal system, but also to facilitate surgery by reducing tumor bulk. In particular, minimizing resection of the non-tumorous liver is vital in patients with cirrhosis to avoid postoperative hepatic failure. Uchida et al. [14] reported a lower survival rate among cirrhosis patients who underwent TACE prior to the resection of HCC compared with patients who did not undergo TACE, and they recommended against preoperative TACE for patients with cirrhosis because the procedure could accelerate the deterioration of liver function. Lu et al. [11] performed a retrospective analysis of 120 HCC patients and concluded that preoperative TACE might benefit those with tumors >8 cm in diameter, but not those with tumors 2–8 cm in diameter. In contrast, it was reported that downstaging or total necrosis of the tumor was achieved by preoperative TACE in 62% of 103 HCC patients with cirrhosis, leading to an improvement of disease-free survival after liver resection and liver transplantation [13]. Thus, the value of preoperative TACE is still controversial.
A meta-analysis including seven randomized clinical trials was undertaken in the late 1990s to investigate the usefulness of TACE for treating unresectable HCC, which demonstrated an improvement in 2-year survival (odds ratio 0.53, P = 0.017) compared with control patients who were treated conservatively or received suboptimal management [27]. This established the role of TACE as the standard care for unresectable HCC, whether as palliative therapy or to improve resectability [27]. Subsequent investigations were directed towards the preoperative use of TACE as neoadjuvant therapy to prevent recurrence. To assess the clinical efficacy of preoperative TACE for resectable HCC, two randomized trials were conducted in 1995 and 1996 [15, 17] (Table 4). Both of these trials found no improvement in disease-free survival following neoadjuvant TACE, and Wu et al. [17] reported worse overall survival in the TACE group. In 2009, a randomized trial of neoadjuvant TACE for large resectable HCC was reported [18]. The results were similar, with no difference in disease-free survival or overall survival between the groups with or without TACE (Table 4). The present study is the fourth randomized trial to compare the long-term prognosis after the resection of HCC in patients with or without preoperative TACE. However, it is difficult to simply compare these trials. Zhou et al. [18] and Wu et al. [17] enrolled patients with large HCCs, whereas Yamasaki et al. [15] and the current trial enrolled patients with smaller HCCs. In the trial reported by Wu et al. [17], patients who received TACE underwent surgery a mean of 17.9 weeks after the detection of HCC, which was significantly longer than those not receiving TACE, who underwent resection 2.3 weeks after the detection of HCC (P = 0.009). In this study, patients in all groups underwent surgery in 20–23 days. Differences in the conclusions of the different trials could be attributed to the differences in the study designs or background characteristics.
We found no significant differences in disease-free survival or overall survival between the entire TACE group (selective and whole-liver groups) and the control group, or among the whole-liver, selective, and control groups, even among patients with tumor size >5 cm (Figs. 2, 3, and 4). The extrahepatic recurrence rate was significantly lower in the selective and whole-liver groups compared with the control group. However, even though preoperative TACE induced complete tumor necrosis, there were no significant differences in the pattern of intrahepatic recurrence or the time until recurrence among the three groups.
In conclusion, preoperative selective TACE or TACE plus whole-liver chemolipiodolization neither reduced the incidence of postoperative recurrence nor prolonged survival in patients with resectable HCC. Thus, despite its safety and feasibility, we cannot recommend preoperative TACE as a routine procedure before hepatectomy in patients with resectable HCC.
References
Bosch FX, Ribes J, Borràs J. Epidemiology of primary liver cancer. Semin Liver Dis. 1999;19:271–285.
Taylor-Robinson SD, Foster GR, Arora S, Hargreaves S, Thomas HC. Increase in primary liver cancer in the UK, 1979–94. Lancet. 1997;350:1142–1143.
El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–750.
Kotoh K, Sakai H, Sakamoto S, et al. The effect of percutaneous ethanol injection therapy on small solitary hepatocellular carcinoma is comparable to that of hepatectomy. Am J Gastroenterol. 1994;89:194–198.
Seki T, Wakabayashi M, Nakagawa T, et al. Ultrasonically guided percutaneous microwave coagulation therapy for small hepatocellular carcinoma. Cancer. 1994;74:817–825.
Chen MS, Li JQ, Zheng Y, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321–328.
Tung-Ping Poon R, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg. 2000;232:10–24.
Nakamura H, Tanaka T, Hori S, et al. Transcatheter embolization of hepatocellular carcinoma: assessment of efficacy in cases of resection following embolization. Radiology. 1983;147:401–405.
Sakurai M, Okamura J, Kuroda C. Transcatheter chemo-embolization effective for treating hepatocellular carcinoma. A histopathologic study. Cancer. 1984;54:387–392.
Harada T, Matsuo K, Inoue T, et al. Is preoperative hepatic arterial chemoembolization safe and effective for hepatocellular carcinoma? Ann Surg. 1996;224:4–9.
Lu CD, Peng SY, Jiang XC, Chiba Y, Tanigawa N. Preoperative transcatheter arterial chemoembolization and prognosis of patients with hepatocellular carcinomas: retrospective analysis of 120 cases. World J Surg. 1999;23:293–300.
Sugo H, Futagawa S, Beppu T, Fukasawa M, Kojima K. Role of preoperative transcatheter arterial chemoembolization for resectable hepatocellular carcinoma: relation between postoperative course and the pattern of tumor recurrence. World J Surg. 2003;27:1295–1299.
Majno PE, Adam R, Bismuth H, et al. Influence of preoperative transarterial lipiodol chemoembolization on resection and transplantation for hepatocellular carcinoma in patients with cirrhosis. Ann Surg. 1997;226:688–703.
Uchida M, Kohno H, Kubota H, et al. Role of preoperative transcatheter arterial oily chemoembolization for resectable hepatocellular carcinoma. World J Surg. 1996;20:326–331.
Yamasaki S, Hasegawa H, Kinoshita H, et al. A prospective randomized trial of the preventive effect of pre-operative transcatheter arterial embolization against recurrence of hepatocellular carcinoma. Jpn J Cancer Res. 1996;87:206–211.
Nagasue N, Kohno H, Tachibana M, Yamanoi A, Ohmori H, El-Assal ON. Prognostic factors after hepatic resection for hepatocellular carcinoma associated with child-turcotte class B and C cirrhosis. Ann Surg. 1999;229:84–90.
Wu CC, Ho YZ, Ho WL, Wu TC, Liu TJ, P’eng FK. Preoperative transcatheter arterial chemoembolization for resectable large hepatocellular carcinoma: a reappraisal. Br J Surg. 1995;82:122–126.
Zhou WP, Lai EC, Li AJ, et al. A prospective, randomized, controlled trial of preoperative transarterial chemoembolization for resectable large hepatocellular carcinoma. Ann Surg. 2009;249:195–202.
Kaibori M, Tanigawa N, Matsui Y, Kwon AH, Sawada S, Kamiyama Y. Preoperative chemolipiodolization of the whole liver for hepatocellular carcinoma. Anticancer Res. 2004;24:1929–1933.
Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655.
Kwon AH, Ha-Kawa SK, Uetsuji S, Inoue T, Matsui Y, Kamiyama Y. Preoperative determination of the surgical procedure for hepatectomy using technetium-99m-galactosyl human serum albumin (99mTc-GSA) liver scintigraphy. Hepatology. 1997;25:426–429.
Strasberg SM, Belghiti J, Clavn P-A, et al. The Brisbane 2000 terminology of liver anatomy and resection. Terminology Committee of the International Hepato-Pancreato-Biliary Association. HPB. 2000;2:333–339.
Couinaud C, ed. Le Foie: Études Anatomiques et Chirurgicales. Paris: Masson; 1957.
Sobin LH, Wittekind C, eds. TNM Classification of Malignant Tumours. 5th ed. New York: Wiley; 1997.
Yamada R, Sato M, Kawabata M, Nakatsuka H, Nakamura K, Takashima S. Hepatic artery embolization in 120 patients with unresectable hepatoma. Radiology. 1983;148:397–401.
Sato Y, Fujiwara K, Ogata I, et al. Transcatheter arterial embolization for hepatocellular carcinoma. Benefits and limitations for unresectable cases with liver cirrhosis evaluated by comparison with other conservative treatments. Cancer. 1985;55:2822–2825.
Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37:429–442.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaibori, M., Tanigawa, N., Kariya, S. et al. A Prospective Randomized Controlled Trial of Preoperative Whole-Liver Chemolipiodolization for Hepatocellular Carcinoma. Dig Dis Sci 57, 1404–1412 (2012). https://doi.org/10.1007/s10620-012-2029-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-012-2029-3