Abstract
Background
This study aimed to assess the prognostic impact of preoperative transcatheter arterial chemoembolization (TACE) on long-term survival outcomes in patients undergoing resection of small solitary hepatocellular carcinoma (HCC).
Methods
Enrolled patients had undergone macroscopic curative resection of solitary 2–5 cm HCC with (n = 105) or without (n = 830; control group) preoperative TACE.
Results
TACE group was divided into subgroups A (n = 68, 1–2 TACEs within 12 months), B (n = 23, ≥3 TACEs within 12 months), and C (n = 14, TACE prior to 12 months). The number of TACE sessions was 1.8 ± 1.6. In TACE A-C subgroups, pathological response of tumor necrosis >50 % at median post-TACE period after final TACE was observed in 41 (60.3 %) at 1.9 months, 10 (43.5 %) at 2.1 months, and 2 (14.3 %) at 18.9 months, respectively. The 5-year tumor recurrence and patient survival rates were 62.8 and 70.4 % in TACE group and 51.4 and 83.4 % in control group, respectively (p ≤ 0.003). Median periods of postoperative tumor recurrence in TACE A-C subgroups and control group were 35, 13, 14, and 55 months, respectively (p < 0.001); and postoperative survival periods at 75 % survival rate were 51, 38, 51, and 98 months, respectively (p = 0.003). TACE-induced extensive tumor necrosis did not improve postoperative prognosis in TACE A subgroup (p ≥ 0.053). Postoperative prognosis after preoperative sequential TACE and portal vein embolization was comparable to that of the control group (p ≥ 0.052).
Conclusions
Preoperative TACE for small solitary HCCs may adversely affect post-resection prognosis, irrespective of pathological responses. Preoperative TACE should be avoided for patients with resectable small HCCs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common malignancy worldwide and is one of the leading causes of cancer-related deaths [1, 2]. Hepatic resection (HR) is considered to be the preferred treatment method for HCC, but is also considered to be a challenging surgical procedure in the presence of liver cirrhosis. Transcatheter arterial chemoembolization (TACE) represents one of the locoregional therapies for HCC. TACE often improves long‐term outcomes in patients with unresectable HCCs, thus it is considered to be an acceptably effective treatment for inoperable patients with large or multifocal HCCs [3–5]. By contrast, TACE has also been performed as preoperative adjuvant chemotherapy in patients with resectable HCC with an expectation of improvement in post-resection survival. However, whether preoperative TACE actually improves long‐term outcomes in patients with initially resectable HCC has become the subject of debate [6–10].
The purpose of preoperative TACE as a neoadjuvant therapy is to reduce tumor volume, to induce tumor necrosis, and to prevent cancer cell dissemination during the surgical procedure [6, 7, 11]. To date, four randomized controlled trials (RCTs) have assessed the prognostic effects of preoperative TACE [6, 7, 12, 13]. However, it has been difficult to compare the results of these RCTs and objectively assess the prognostic effects of neoadjuvant TACE because of differences in baseline clinical characteristics, such as tumor size, the cause of liver disease, and the chemotherapeutic agents used for TACE. Hence, the postoperative survival benefit from preoperative TACE for HCC remains unclear.
TACE has been frequently performed with an intention of definitive treatment instead of neoadjuvant intention. Surgical HR is also considered after tumor recurrence or suboptimal TACE responses. Such clinical situations are different from the neoadjuvant setting adopted in the abovementioned RCTs.
This study primarily aimed to assess the prognostic impact of preoperative TACE on long-term survival outcomes in patients undergoing resection of small solitary HCCs.
Patients and methods
Patients
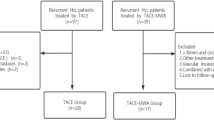
The HCC database at our institution was searched to identify patients who underwent primary HR for HCC over a 10-year period from January 2002 to December 2011; 3702 patients were initially identified. Detailed profiles of patients with solitary HCCs were previously presented [14]. To avoid potential bias from other important prognostic factors, patients were primarily screened according to the following inclusion criteria: solitary HCC between 2.0 and 5.0 cm in diameter, anatomical resection, macroscopic curative resection with tumor-free surgical margins, typical HCC pathology with exclusion of combined HCC-cholangiocarcinoma tumor, no macroscopic vascular invasion, no extrahepatic metastasis, no preoperative HCC treatment other than TACE, and patient survival ≥3 months after HR. Through this screening process, 935 patients (25.3 %) were identified. Next, patients were divided into two groups according to preoperative TACE as the TACE [n = 105 (11.2 %)] and control [n = 830 (88.8 %)] groups. Further artificial selection of control group patients based on propensity score matching was not performed because the screening process was carried out by strict selection criteria, in which a typical propensity score matching was already achieved. Comparison of the parameters usable for propensity score matching are summarized at Table 1.
The TACE group was further divided into three subgroups according to the timing and number of TACE sessions: the first group (TACE A subgroup) was a TACE subgroup (n = 68) in whom one or two sessions of TACE were performed within 12 months (mostly <3 months) before HR; the second group (TACE B subgroup) was a TACE subgroup in whom the last TACE was performed within 12 months before HR and the total number of TACE sessions was three or more (n = 23); and the third group (TACE C subgroup) was a TACE subgroup in whom the last TACE was performed prior to ≥12 months before HR, irrespective of the number of TACE sessions (n = 14).
The medical records of these patients were reviewed retrospectively after receiving approval by the Institutional Review Board of our institution. Patients were followed until July 2014 through a medical records review, so the patient follow-up period was greater than 30 months or until patient death. All patients were completely followed up to identify survival status with the assistance of the National Health Insurance Service.
Preoperative evaluation and surgical procedures
Korean individuals with chronic liver disease have been regularly followed up for detection of HCC according to the guidelines of the Korean Association for the Study of the Liver [15, 16]. Routine preoperative evaluation for HCC included abdomen and chest computed tomography (CT), magnetic resonance imaging (MRI), 2-18F-fluoro-2-deoxy-d-glucose positron emission tomography (FDG-PET), and upper gastrointestinal endoscopy. TACE was routinely performed 2–8 weeks before preoperative portal vein embolization (PVE) for major hepatectomy [17]. The details of this preoperative evaluation process have been previously described [14].
The extent of HR was primarily determined based on the future remnant liver volume with consideration for tumor-free resection margins and hepatic functional reserves [18]. Anatomical hepatectomy included resection of one or more adjacent hepatic segments along the hepatic vasculature. Perioperative mortality was defined as death from any cause within 90 days of surgery, so such patients were excluded in the screening process of this study.
Preoperative TACE and response assessment
Irrespective of performing TACE at our institution or before referral to our institution, TACE was generally performed in accordance with the Korean guidelines [19]. The TACE response of solitary HCC was initially assessed using the modified Response Evaluation Criteria in Solid Tumors (mRECIST) [20], which includes the following four response categories for target lesions: complete response (100 % reduction), partial response (>30 % reduction), progressive disease (>20 % increase), and stable disease.
The TACE group showed variable degrees of tumor necrosis on both preoperative imaging studies and surgical specimen pathology analyses. Moreover, widely variable interval periods existed between the last TACE and HR in this study, so we simply divided the degrees of tumor necrosis based on pathological examination as >50 and ≤50 %, respectively [11]. We also defined the complete pathological response (complete necrosis) as an area of necrosis ≥95 % of the total tumor volume [21].
Postoperative surveillance and treatments for HCC recurrence
In principle, patients were followed every 1 to 3 months during the first year after HR, and thereafter every 3 months. Most hepatitis B virus (HBV)-associated patients became HBV DNA-negative during the outpatient clinic follow-up through vigorous antiviral treatment. The general principles of treatment for recurrent HCC lesions were applied to the patients enrolled in this study. Detailed postoperative patient follow-up profiles were previously presented [14].
Statistical analysis
The primary endpoints of this study were the overall patient survival and tumor recurrence after macroscopic curative resection. Numeric data are reported as means with standard deviation or as medians with range. Continuous variables were compared using Student’s t test and incidence variables were compared using the Chi-square test. Survival curves were estimated using the Kaplan–Meier method and compared using the log-rank test. Comparisons were made among the three TACE subgroups and one control group, but our primary concern was paid to the comparison between the TACE A subgroup and control group. A p-value <0.05 was considered to indicate a statistically significant difference. Statistical analyses were performed using SPSS (version 20; IBM, New York, NY) and Statistica (version 6.0; StatSoft, Tulsa, OK) software.
Results
Patient demographics
In the 935 patients enrolled in this study, most HCC lesions were detected in an asymptomatic state through regular health screening or routine follow-up for chronic liver diseases [n = 796 (85.1 %)]. HBV infection was detected in 737 patients (78.8 %) and antiviral agents were administered to 627 patients (85.1 %), starting before or after HR. Baseline characteristics of the three TACE subgroups and control group were generally comparable (Table 1). Moreover, the patient profiles of these 4 groups were similar. The mean tumor diameter was 3.8 ± 1.0 cm in the TACE group and 3.6 ± 0.9 cm in the control group (p = 0.093).
Responses to preoperative TACE
Among the 105 patients who underwent preoperative TACE, 72 (68.6 %) had undergone the last TACE session at our institution with definite therapeutic or curative intention (n = 54) or as a routine preparation for preoperative PVE (n = 18), whereas the other 33 patients were referred to our institution after TACE for further treatment, including surgery. The mean number of TACE sessions was 1.8 ± 1.6 (range: 1–7), among which 64 patients received a single session, 15 patients received two sessions, and 26 patients received three or more sessions. The mean interval period between the last TACE session and HR was 23.5 ± 36.8 weeks (range: 2–252), including <3 months in 64 patients, 3–12 months in 27 patients, and >12 months in 14 patients.
As mentioned above, patients were divided into the following three subgroups based on the timing and number of TACE sessions: TACE A subgroup (n = 68), TACE B subgroup (n = 23), and TACE C subgroup (n = 14). Among the 50 patients in TACE A subgroup after exclusion of patients who would undergo PVE, the last TACE session was performed primarily with a therapeutic intent in 29 patients and with a neoadjuvant intent in 21 patients. Among the TACE A subgroup, for the final TACE session, the TACE responses at 1 month based on the mRECIST criteria were a complete response in 16 patients (23.5 %), partial response in 32 patients (47.1 %), and stable or progressive disease in 20 patients (29.4 %). In the TACE A, B, and C subgroups, the number of patients showing tumor necrosis >50 % at a median post-TACE period after the last TACE session was 41 patients (60.3 %) at 1.9 months, 10 patients (43.5 %) at 2.1 months, and 2 patients (14.3 %) at 18.9 months, respectively.
Tumor recurrence and overall survival outcomes
During a mean follow-up period of 60.1 ± 28.1 months (median, 56; range, 4–151) in a total of 935 patients, tumor recurrence occurred in 481 patients (51.4 %) and death from all causes occurred in 186 patients (19.9 %). The 1-, 3-, 5-, and 10-year tumor recurrence rates were 20.9, 45.8, 52.9, and 63.5 %, respectively (Fig. 1a). The 1-, 3-, 5-, and 10-year overall patient survival rates were 97.8, 89.2, 81.9, and 69.0 %, respectively (Fig. 1b).
Tumor recurrence and patient survival curves. Tumor recurrence is presented as a cumulative tumor recurrence curve (a) and patient survival is illustrated by an overall patient survival curve (b) in a total of 935 patients. Comparison of the TACE (n = 105) and control (830) groups is shown as cumulative tumor recurrence curves (c) and overall patient survival curves (d)
In the control group of 830 patients, the 1-, 3-, 5-, and 10-year tumor recurrence rates were 18.3, 41.2, 51.4, and 63.4 %, respectively; in the TACE group of 105 patients, the postoperative 1-, 3-, 5-, and 10-year tumor recurrence rates were 29.9, 57.2, 62.8, and 68.8 %, respectively (Fig. 1c; p = 0.005).
In the control group, the 1-, 3-, 5-, and 10-year overall patient survival rates were 97.7, 90.3, 83.4, and 69.6 %, respectively; in the TACE group, the postoperative 1-, 3-, 5-, and 10-year overall patient survival rates were 97.1, 80.0, 70.4, and 60.1 %, respectively (Fig. 1d; p = 0.003). The survival interval between TACE and HR was not taken into account in this survival analysis.
Outcomes among TACE subgroups
Postoperative outcomes were compared among the three TACE subgroups and the control group. The median postoperative tumor recurrence rates in the TACE A, B, and C subgroups and control group were 35, 13, 14, and 55 months, respectively (Fig. 2a; p < 0.001). The postoperative survival periods at a 75 % survival rate in the TACE A, B, and C subgroups and control group were 51, 38, 51, and 98 months, respectively (Fig. 2b; p = 0.003).
Outcomes in patients with PVE
The TACE A subgroup was divided into PVE (n = 18) and non-PVE (n = 50) subgroups. A comparison of these two groups alone did not show significant differences in postoperative tumor recurrence (p = 0.088) or postoperative patient survival (p = 0.477) rates probably due to small case numbers. However, comparisons with inclusion of the control group showed significant differences in postoperative tumor recurrence (Fig. 3a; p = 0.038) and postoperative patient survival (Fig. 3b; p = 0.018) rates, in which comparisons between the PVE and control groups showed no significant difference in tumor recurrence (p = 0.052) or patient survival (p = 0.839) rates.
Outcomes according to pathological response in the TACE A subgroup
The TACE A subgroup could be divided into >50 % (n = 41) and ≤50 % (n = 27) necrosis. A comparison of these two groups alone did not show significant differences in postoperative tumor recurrence (p = 0.854) or postoperative patient survival (p = 0.711) rates. Comparisons of these groups with the control group showed no significant differences in postoperative tumor recurrence (Fig. 4a, p = 0.305) or patient survival (Fig. 4b, p = 0.053) rates. Additionally, we divided the TACE A subgroup into complete (≥95 %) necrosis (n = 16) and incomplete (<95 %) necrosis (n = 52). A comparison of these two groups alone did not show significant differences in postoperative tumor recurrence (p = 0.854) or patient survival (p = 0.996) rates. Comparisons with the control group also showed no significant differences in postoperative tumor recurrence (Fig. 4c; p = 0.239) or patient survival (Fig. 4d; p = 0.051) rates.
Comparison of the tumor recurrence and overall patient survival curves in the TACE A subgroup according to pathological responses. Panels show the tumor recurrence (a) and overall patient survival (b) with a cutoff of 50 % necrosis, and tumor recurrence (c) and overall patient survival (d) with a cutoff of 95 % necrosis
Intention-to-treat survival outcomes among TACE subgroups
The overall survival periods after the first and last TACE sessions were also analyzed. The overall survival period after the first TACE session (sum of the interval period between first TACE and HR and the postoperative survival period) in the TACE A, B, and C subgroups were 53, 65, and 95 months at a 75 % survival rate, respectively (p = 0.441). Comparisons with the control group did not show significant differences in intention-to-treat overall patient survival rates (Fig. 5a; p = 0.176). The overall survival period after the last TACE session (sum of the interval period between last TACE and HR and the postoperative survival period) in the TACE A, B, and C subgroups were 53, 40, and 95 months at a 75 % survival rate, respectively (p = 0.248). However, comparisons with the control group showed significant differences in the overall patient survival rates (Fig. 5b; p = 0.006).
Discussion
So far to date, four RCTs have assessed the prognostic effects of preoperative TACE [6, 7, 12, 13], and all concluded that pretreatment with TACE did not improve post-resection survival. However, some other studies have presented conflicting results, so the postoperative survival benefit of preoperative TACE for HCC remains the subject of debate. We previously presented that a single session of preoperative TACE for initially resectable HCC worsened the overall survival rate and increased the risk of tumor recurrence in patients who achieved incomplete tumor necrosis [21]. However, the patient profiles were rather heterogeneous in our precedent study, so we considered that a further validation study was needed to provide more robust evidence about the postoperative survival risk-benefit of preoperative TACE. To reduce such heterogeneity in patient profiles, we confined our patients in the present study to those who had undergone HR for only small solitary HCCs.
In the design step of the present study, strict patient selection was important to reduce the heterogeneity of patient profiles. The size of HCC tumors has been traditionally considered to be one of the important prognostic factors, although this concept has been modified after several studies showed that survival outcomes were independent of tumor size in patients who underwent HR of solitary HCCs [14, 22–26]. The original Barcelona Clinic Liver Cancer (BCLC) system had size cutoffs set at 2 and 5 cm [4], but the cutoff at 5 cm was omitted when the guidelines were updated in 2014 [5]. The Hong Kong Liver Cancer staging system has a cutoff at 5 cm [25]. In our recent study on HR of solitary HCCs in 2558 patients, the independent risk factors for both tumor recurrence and overall survival were non-anatomical resection, microvascular invasion, and tumor size >5 cm [14]. Thus, we confined the study patients with a solitary tumor between 2 and 5 cm in size.
The various purposes of TACE for initially resectable HCCs can be divided into three categories. The first category is a neoadjuvant chemotherapy for resectable HCCs with an expectation of improvement in post-resection survival [6, 7, 11]. We have rarely performed preoperative TACE in selected patients with this intention because we already considered that it may not improve and could even worsen the postoperative prognosis [21]. The second category is a preparation for PVE for major hepatectomy to reduce the risk of rapid tumor growth [17]. The third category is an independent treatment with a therapeutic or even curative intent, in which additional treatments, including surgery, would be provided if the TACE response was suboptimal. Because the present study was performed retrospectively, we could not clearly classify our patients according to these purpose categories. Instead, we roughly divided the study patients into three subgroups according to the timing and number of TACE sessions.
The results of this study revealed that both tumor recurrence and patient survival rates worsened, or were at best similar, in all three TACE subgroups, which were comparable to the findings of our precedent study [21]. Except for complete tumor necrosis and very late tumor recurrence after TACE, the patients in the TACE group showed poorer postoperative outcomes compared with the control group. We presume that two underlying causes might account for such negative prognostic effects of preoperative TACE. First, partial tumor necrosis induced by preoperative TACE can increase the risk of tumor recurrence after HR, which may be a consequence of tumor cell dislodgement into the bloodstream [27, 28]. The extent of tumor vascularization is significantly associated with the degree of TACE efficacy, and a high degree of vascularization is therefore considered to be predictive of a good response to TACE [11, 29]. Thus, preoperative TACE may be permissible in HCC patients with a high degree of tumor vascularity [30]. By contrast, if incomplete tumor necrosis occurs, the remaining viable tumor cells are less firmly attached, and are thus more likely to be dislodged into the bloodstream before surgery where they can promote the hematogenous spread of residual tumor cells during HR [21, 28]. The second potential cause may be associated with biased patient selection in this retrospective study because there is a high probability of requiring surgery as a rescue therapy in patients who show suboptimal responses to TACE of a therapeutic intent.
It is generally accepted that no size limit precludes HR, especially for solitary HCCs that are resectable [14, 31]. In practice, such surgery-oriented treatment policies are not well matched with the guidelines of the BCLC and Hong Kong Liver Cancer staging systems because of different socio-medical environments regarding HCC treatment [4, 25]. In contrast, almost all of the patients in this study were initially indicated as HR according to the general HCC treatment guidelines, but ~10 % of our patients underwent TACE instead of HR because of various underlying causes, such as patient reluctance to undergo surgery, a major co-morbidity, and liver cirrhosis. If we have examined the entire patient population undergoing TACE at our institution, a much higher proportion of patients would have received TACE or radiofrequency ablation therapy as the first treatment for resectable HCCs with a therapeutic intent [32]. Most of these patients have received repeated non-surgical locoregional treatments for recurrent intrahepatic HCC lesions, and only a small proportion of these patients finally received HR, likely because of a refractory response to non-surgical treatments. Along with the intention-to-treat concept, we found that the intention-to-treat survival outcomes after the first TACE session during the first 5 years were quite similar in those patients in the TACE B and C subgroups and the control group, with inferior outcomes occurring only in the TACE A subgroup. These findings indicate that HR is readily indicated for the residual or recurrent HCCs if they are resectable after repeated sessions of TACE.
Uniquely, TACE is highly recommended before preoperative PVE for major hepatectomy. The beneficial effects of preoperative TACE before PVE compared with PVE alone have been established [17, 33], but the risk of TACE-associated tumor spread has not yet been thoroughly investigated. Our findings with 18 patients who underwent preoperative sequential TACE and PVE revealed that their post-resection prognosis was comparable to that of the control group. It is well known that HCC tumor cell spread usually occurs via the portal venous system. Initially, we had presumed that PVE induces near complete blockage of the ipsilateral hemiliver portal venous system, thus effectively preventing transportal HCC tumor cell spread, but this concept could not clearly explain the prognostic differences between PVE alone and TACE followed by PVE. The primary intention of precedent TACE is to prevent tumor growth resulting from PVE-associated buffering increase in hepatic arterial flow, but it also increases the risk of tumor cell spread. In our 18 patients treated with sequential TACE and PVE, tumor necrosis >50 % was observed in 14 cases (77.8 %), compared with 27 of the other 50 patients (54 %) in the TACE A subgroup; however, complete tumor necrosis was observed in 3 of 18 patients (16.7 %) and 13 of 50 patients (26 %), respectively. The proportion of complete tumor necrosis after sequential TACE and PVE was lower in the present study than in a previous study with French patients [33], in which complete tumor necrosis was achieved in more than 80 % of cases, but only in 5 % of patients after PVE alone. Considering these findings, we speculate that the cytoreductive effect from precedent TACE might offset the risk of PVE-induced tumor growth, so it may be reasonable to perform TACE before preoperative PVE. On the other hand, we recently reported that such prognostic offset following sequential TACE and PVE was weakened in patients with solitary HCCs >5 cm, by which larger tumors may not be adequately indicated for PVE regardless of precedent TACE [34].
Our current study had several limitations. First, our analyses were retrospectively performed, thus the purpose of preoperative TACE could not be clearly categorized. Second, the sample size of the TACE subgroups was not large enough, so it was not balanced well with that of the control group. A uniquely strong point of this study was that the survival status of all patients was completely followed up.
In conclusion, preoperative TACE may adversely affect the post-resection survival of patients with solitary small HCCs. If the pathological response to TACE is incomplete, it can increase the risk of tumor recurrence. Even in patients with complete pathological responses, the rates of overall survival and recurrence are similar to those of patients without preoperative TACE. Therefore, we suggest that preoperative TACE should be avoided for patients with resectable small HCC unless preoperative PVE for major hepatectomy is planned.
References
Forner A, Llovet JM, Bruix J (2012) Hepatocellular carcinoma. Lancet 379:1245–1255
El-Serag HB (2011) Hepatocellular carcinoma. N Engl J Med 365:1118–1127
Rahbari NN, Mehrabi A, Mollberg NM, Müller SA, Koch M, Büchler MW et al (2011) Hepatocellular carcinoma: current management and perspectives for the future. Ann Surg 253:453–469
Llovet JM, Brú C, Bruix J (1999) Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis 19:329–338
Bruix J, Sherman M (2011) Management of hepatocellular carcinoma: an update. Hepatology 53:1020–1022
Zhou WP, Lai EC, Li AJ, Fu SY, Zhou JP, Pan ZY et al (2009) A prospective, randomized, controlled trial of preoperative transarterial chemoembolization for resectable large hepatocellular carcinoma. Ann Surg 249:195–202
Kaibori M, Tanigawa N, Kariya S, Ikeda H, Nakahashi Y, Hirohara J et al (2012) A prospective randomized controlled trial of preoperative whole-liver chemolipiodolization for hepatocellular carcinoma. Dig Dis Sci 57:1404–1412
Rahman A, Assifi MM, Pedroso FE, Maley WR, Sola JE, Lavu H et al (2012) Is resection equivalent to transplantation for early cirrhotic patients with hepatocellular carcinoma? A meta-analysis. J Gastrointest Surg 16:1897–1909
Toro A, Pulvirenti E, Palermo F, Di Carlo I (2012) Health-related quality of life in patients with hepatocellular carcinoma after hepatic resection, transcatheter arterial chemoembolization, radiofrequency ablation or no treatment. Surg Oncol 21:e23–e30
Lee KT, Lu YW, Wang SN, Chen HY, Chuang SC, Chang WT et al (2009) The effect of preoperative transarterial chemoembolization of resectable hepatocellular carcinoma on clinical and economic outcomes. J Surg Oncol 99:343–350
Nishikawa H, Arimoto A, Wakasa T, Kita R, Kimura T, Osaki Y (2013) Effect of transcatheter arterial chemoembolization prior to surgical resection for Hepatocellular carcinoma. Int J Oncol 42:151–160
Yamasaki S, Hasegawa H, Kinoshita H, Furukawa M, Imaoka S, Takasaki K et al (1996) A prospective randomized trial of the preventive effect of pre-operative transcatheter arterial embolization against recurrence of hepatocellular carcinoma. Jpn J Cancer Res 87:206–211
Wu CC, Ho YZ, Ho WL, Wu TC, Liu TJ (1995) P’eng FK. Preoperative transcatheter arterial chemoembolization for resectable large hepatocellular carcinoma: a reappraisal. Br J Surg 82:122–126
Hwang S, Lee YJ, Kim KH, Ahn CS, Moon DB, Ha TY et al (2015) The impact of tumor size on long-term survival outcomes after resection of solitary hepatocellular carcinoma: single-institution experience with 2558 patients. J Gastrointest Surg 19:1281–1290
Korean Association for the Study of the Liver (2012) KASL Clinical Practice Guidelines: management of chronic hepatitis B. Clin Mol Hepatol 18:109–162
Suk KT, Baik SK, Yoon JH, Cheong JY, Paik YH, Lee CH et al (2012) Korean Association for the Study of the Liver. Revision and update on clinical practice guideline for liver cirrhosis. Korean J Hepatol 18:1–21
Yoo H, Kim JH, Ko GY, Kim KW, Gwon DI, Lee SG et al (2011) Sequential transcatheter arterial chemoembolization and portal vein embolization versus portal vein embolization only before major hepatectomy for patients with hepatocellular carcinoma. Ann Surg Oncol 18:1251–1257
Hwang S, Ha TY, Song GW, Jung DH, Ahn CS, Moon DB et al (2015) Quantified risk assessment for major hepatectomy via the indocyanine green clearance rate and liver volumetry combined with standard liver volume. J Gastrointest Surg 19:1305–1314
Choi JY (2011) Treatment algorithm for intermediate and advanced stage hepatocellular carcinoma: Korea. Oncology 81(Suppl 1):141–147
Lencioni R, Llovet JM (2010) Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 30:52–60
Kim IS, Lim YS, Lee HC, Suh DJ, Lee YJ, Lee SG (2008) Pre-operative transarterial chemoembolization for resectable hepatocellular carcinoma adversely affects post-operative patient outcome. Aliment Pharmacol Ther 27:338–345
Vauthey JN, Lauwers GY, Esnaola NF, Do KA, Belghiti J, Mirza N et al (2002) Simplified staging for hepatocellular carcinoma. J Clin Oncol 20:1527–1536
Ariizumi S, Kotera Y, Takahashi Y, Katagiri S, Yamamoto M (2013) Impact of hepatectomy for huge solitary hepatocellular carcinoma. J Surg Oncol 107:408–413
Shindoh J, Andreou A, Aloia TA, Zimmitti G, Lauwers GY, Laurent A et al (2013) Microvascular invasion does not predict long-term survival in hepatocellular carcinoma up to 2 cm: reappraisal of the staging system for solitary tumors. Ann Surg Oncol 20:1223–1229
Yau T, Tang VY, Yao TJ, Fan ST, Lo CM, Poon RT (2014) Development of Hong Kong Liver Cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology 146:1691–1700
Kudo M, Chung H, Osaki Y (2003) Prognostic staging system for hepatocellular carcinoma (CLIP score): its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score). J Gastroenterol 38:207–215
Adachi E, Matsumata T, Nishizaki T, Hashimoto H, Tsuneyoshi M, Sugimachi K (1993) Effects of pre-operative transcatheter hepatic arterial chemoembolization for hepatocellular carcinoma. The relationship between postoperative course and tumor necrosis. Cancer 72:3593–3598
Ravaioli M, Grazi GL, Ercolani G, Fiorentino M, Cescon M, Golfieri R et al (2004) Partial necrosis on hepatocellular carcinoma nodules facilitates tumor recurrence after liver transplantation. Transplantation 78:1780–1786
Sergio A, Cristofori C, Cardin R, Pivetta G, Ragazzi R, Baldan A et al (2008) Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastroenterol 103:914–921
Zhang Z, Liu Q, He J, Yang J, Yang G, Wu M (2000) The effect of preoperative transcatheter hepatic arterial chemoembolization on disease-free survival after hepatectomy for hepatocellular carcinoma. Cancer 89:2606–2612
Hwang S, Lee YJ, Kim KH, Ahn CS, Moon DB, Ha TY et al (2015) Long-term outcome after resection of huge hepatocellular carcinoma ≥10 cm: single-institution experience with 471 patients. World J Surg 39:2519–2528. doi:10.1007/s00268-015-3129-y
Kim JW, Kim JH, Sung KB, Ko HK, Shin JH, Kim PN et al (2014) Transarterial chemoembolization vs. radiofrequency ablation for the treatment of single hepatocellular carcinoma 2 cm or smaller. Am J Gastroenterol 109:1234–1240
Ogata S, Belghiti J, Farges O, Varma D, Sibert A, Vilgrain V (2006) Sequential arterial and portal vein embolizations before right hepatectomy in patients with cirrhosis and hepatocellular carcinoma. Br J Surg 93:1091–1098
Choi JH, Hwang S, Lee YJ, Kim KH, Ko GY, Gwon DI et al (2015) Prognostic effect of preoperative sequential transcatheter arterial chemoembolization and portal vein embolization for right hepatectomy in patients with solitary hepatocellular carcinoma. Korean J Hepatobiliary Pancreat Surg 19:59–65
Author contributions
Hwang S and Ha TY designed the study; Lee YJ, Kim KH, Ko GY, Gwon DI, Ahn CS, Moon DB, Song GW, Jung DH, Lee HC, Lim YS, Kim KM, Shim JH, Choi JH, and Lee SG contributed to data acquisition and analysis; and Hwang S drafted and revised the text.
Funding
None of the authors were supported financially for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have any conflict of interest.
Rights and permissions
About this article
Cite this article
Ha, TY., Hwang, S., Lee, YJ. et al. Absence of Benefit of Transcatheter Arterial Chemoembolization (TACE) in Patients with Resectable Solitary Hepatocellular Carcinoma. World J Surg 40, 1200–1210 (2016). https://doi.org/10.1007/s00268-015-3373-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-015-3373-1