Abstract
Background
Nonalcoholic fatty liver disease is one of the most prevalent forms of chronic liver disease in the Western world. Taurine is a conditionally essential amino acid in humans that may be a promising therapy for treating this disease.
Aim
To evaluate the effect of taurine on hepatic steatosis induced by thioacetamide in Danio rerio.
Methods
Animals were divided into four groups: control (20 μl of saline solution), taurine (1,000 mg/kg), thioacetamide (300 mg/kg), and the taurine–thioacetamide group (1,000 + 300 mg/kg). Thioacetamide was injected intraperitoneally three times a week for 2 weeks. The mRNA expression, lipoperoxidation, antioxidant enzymatic activity, and histological analyses were evaluated in the liver and the triglyceride content was assessed in the serum.
Results
Thioacetamide injection induced steatosis, as indicated by histological analyses. The lipoperoxidation showed significant lipid damage in the thioacetamide group compared to the taurine–thioacetamide group (p < 0.001). Superoxide dismutase (SOD) activity in the taurine–thioacetamide group (5.95 ± 0.40) was significantly increased compared to the thioacetamide group (4.14 ± 0.18 U SOD/mg of protein) (p < 0.001). The mRNA expression of SIRT1 (0.5-fold) and Adiponectin receptor 2 (0.39-fold) were lower in the thioacetamide group than the control (p < 0.05). TNF-α mRNA expression was 6.4-fold higher in the thioacetamide group than the control (p < 0.05). SIRT1 mRNA expression was 2.6-fold higher in the taurine–thioacetamide group than in the thioacetamide group.
Conclusions
Taurine seems to improve hepatic steatosis by reducing oxidative stress and increasing SIRT1 expression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nonalcoholic fatty liver disease (NAFLD) has been increasingly recognized as the most common form of chronic liver disease in both adults and children [1]. In humans, it is histologically characterized by the accumulation of fat, which is predominantly comprised of mainly triglycerides in more than 5% of hepatocytes [2]. It is one of the most prevalent forms of chronic liver disease, and its prevalence is estimated to be 31% in American adults [3]. In addition, the prevalence increases in risk populations, such as persons with a large waist circumference, obesity, insulin resistance, or diabetes. In Brazil, more than 80% of patients admitted for bariatric surgery have hepatic steatosis [4, 5]. The pathogenesis of hepatic steatosis is still poorly understood. There is no consensus regarding therapies for NAFLD, and many pharmacological agents are being tested for this purpose [6]. Nevertheless, it is known that free fatty acids (FFA) and adipocytokines, such as TNF-α (tumor necrosis factor α) and adiponectin have a central role in its development.
Taurine (2-aminoethanesulfur acid) is a conditionally essential amino acid in humans and important for the development of the nervous system, xenobiotic conjugation, osmoregulation, and other processes [7]. Taurine also presents hypolipidemic and antioxidant effects, which is the main reason why it has been tested as a therapeutic agent against steatosis [8]. Interestingly, Chen et al. [9] observed a reduction of oxidative stress, an increase in expression of adiponectin, a decrease in expression of TNF-α, and an improvement of histological parameters of steatosis in rats with nonalcoholic steatohepatitis treated with taurine compared to controls.
Among the animal models currently available for the study of liver diseases, the zebrafish (Danio rerio) has gained prominence for its easy and cheap culture system, short life cycle and molecular homology with humans [10]. Several authors have used this freshwater fish in experimental studies [11, 12]. Amali et al. [13] recently proposed a model of hepatic steatosis in zebrafish with intraperitoneal injections of thioacetamide (300 mg/kg), which is a potent hepatotoxic agent. Disease models induced by drugs in zebrafish are cheaper than mice, because zebrafish are small organisms that only require limited amounts of drugs to induce disease development [14].
The aim of this study was to evaluate whether taurine treatment has a protective effect on hepatic steatosis induced by thioacetamide in zebrafish.
Materials and Methods
Animals
One hundred twenty adults Danio rerio (3–6 months-old; weight: 334 ± 23.75 mg) of both sexes were purchased from a commercial supplier (Delphis, Brazil) and acclimated to soft water (25–28°C; pH 6.8) over a 14-day period in an aerated 40-l aquarium. After acclimation, the zebrafish were housed in four 10-l aquariums containing 30 fish each. Fish were fed daily with a commercial tropical fish food (Alcon Basic, Brazil) and maintained on a 14:10 h light–dark photoperiod regimen. Zebrafish were fasted for 24 h prior to the beginning of experimentation. All procedures used were approved by the Ethics Committee of the Hospital de Clínicas de Porto Alegre (protocol number 09 393).
Experimental Design
Animals were divided into the following four groups: control (Ctrl—20 μl of saline solution), taurine (TAU—1,000 mg/kg diluted in 20 μl of saline solution), thioacetamide (TAA—300 mg/kg diluted in 20 μl of saline solution) [12], and a thioacetamide–taurine group (TAU + TAA—1,000 + 300 mg/kg diluted in 20 μl of saline solution). The experiment duration was 2 weeks and the drugs were injected intraperitoneally three times a week. Zebrafish were cryo-anesthetized and killed at the end of the experiment.
Histological Analysis
Livers from the zebrafish were fixed in 10% formalin and embedded in paraffin wax, sectioned (6 μm), and stained with hematoxylin and eosin (H&E). The liver tissue was also frozen at −20°C, cryosectioned (6–8 μm thick), and stained with Oil Red O to assess the fatty droplet accumulation [15]. The lipid content of pictures of Oil Red slides was quantified with Adobe® Photoshop® CS3 (Adobe Systems, USA) and expressed as pixels per 1,000. To quantify the fat positivity by Oil Red O staining, we selected the color representing fat positivity as the foreground color, and the color representing the liver as the background. The value of the foreground and of the sum of the first and second plans were recorded and converted into a percentage.
Biochemical Analysis
To evaluate serum triglycerides, 12 animals of each group were cryo-anesthetized and had their blood collected as previously described [16]. Briefly, a transverse incision was made just before the tail, and the blood was immediately collected with an automatic pipette pre-washed with 5 M EDTA. The blood of three pools of four animals each was centrifuged for 10 min at 3,200 rpm (Eppendorf Centrifuge 5415D, Eppendorf, Germany). The serum was collected and triglycerides were analyzed using a colorimetric test (Labtest Diagnóstica, Brazil).
Nine milliliters of phosphate buffer (140 mM KCL, 20 mM sodium phosphate, pH 7.4) per liver tissue gram was added and the tissue was homogenized in an Ultra Turrax (IKA-WERK, Germany) for 40 s at 4°C. It was then centrifuged for 10 min at 4,000 rpm (SORVALL RC-5B Refrigerated Superspeed Centrifuge, Du Pont Company, USA). The supernatant was placed into Eppendorf tubes and the precipitate was discarded. The samples were stored at −80°C for posterior analyses.
The amount of aldehydes generated by lipid peroxidation was measured by the thiobarbituric acid reactive substances assay (TBARS). Briefly, the samples were incubated at 100°C for 30 min after addition of 0.37% thiobarbituric acid in 15% trichloroacetic acid and centrifuged at 3,000 rpm for 10 min at 4°C. Absorbance was determined by a spectrophotometer at 535 nm [17].
The superoxide dismutase (SOD) catalyzes the reaction of two superoxide anions to form hydrogen peroxide, which is less reactive and can be degraded by enzymes such as catalase (CAT). The analysis of SOD is based on the ability of the sample to inhibit the superoxide-mediated adrenaline oxidation [18]. One unit of SOD activity was defined as the amount of enzyme required to inhibit the reaction of oxidation by 50%, and was measured by absorbance at 480 nm and expressed as unit mg protein−1. The analysis of CAT activity is based on the sample ability to consume hydrogen peroxide. One unit of CAT activity was defined as the amount of enzyme required to consume 1 μmol H2O2 in 1 s, and was measured by absorbance at 240 nm and expressed as unit mg protein−1 [19]. Four pools of five livers were used for all biochemical analyses. The protein content was evaluated by the Bradford method using bovine albumin as the standard (Sigma–Aldrich, USA). The absorbance was measured with a spectrophotometer at 595 nm and all biochemical analyses were normalized by protein content [20].
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
The liver samples (n = 8) from each fish were obtained by microsurgery and immediately immersed in TRIZOL reagent (Invitrogen, USA). Total RNA was isolated according to the manufacturer’s instructions and the concentrations were quantified by UV spectrophotometry (Nanodrop, Thermo Fisher Scientific, USA) at 260 nm. RNA purity was verified by a 260/280 nm ratio of 1.8 or greater. First-strand cDNA was synthesized from 800 ng of total RNA using the Superscript™ II RT system (Invitrogen, USA). Gene expression analysis was performed in duplicate on a Stratagene MX3000P real-time PCR machine using TaqMan Gene Expression Assays (Applied Biosystems, USA) for the following genes: Tumor Necrosis Factor Alpha (TNF-α; NM_212859.2), Adiponectin Receptor 2 (ADIPOR2; NM_001025506.2), Sirtuin 1 (SIRT1; ENSDART00000098209) and Elongation Factor 1-alpha (EF1-α; NM_131263.1). PCR was performed with 10 μl of TaqMan Gene Expression PCR Master Mix (Applied Biosystems, Foster City, CA), 5 μM of the probe, 18 μM of each primer, 4 μl of diluted cDNA (100 ng), and 5 μl of RNAse-free water in a 20-μl final reaction mixture. The two-step PCR conditions were as follows: 2 min at 50°C, 10 min at 95°C, and 40 cycles with 15 s at 95°C and 1 min at 60°C.
Gene expression was quantified using the 2−∆∆Ct (threshold cycle) method [21]. Each sample was analyzed in duplicate and the ΔC T value was obtained by subtracting the elongation factor-α C T value from the C T value of the gene of interest. To calculate the difference between groups, the ΔCT mean value obtained for the control group was used to calculate the ΔΔCt of each gene (2−ΔΔCt).
Statistical Analysis
Statistical analyses were performed by using Prism 5 (GraphPad, San Diego, USA). Distributions were first tested for normality using the Shapiro–Wilk test. Multiple comparisons between Gaussian distributions were performed using one-way ANOVA with Tukey–Kramer post hoc tests. For non-normal distributions, the Kruskal–Wallis test was used with Dunn post hoc tests for multiple comparisons. Results with p < 0.05 were considered statistically significant.
Results
Taurine Promotes Change in Hepatic Lipid Content
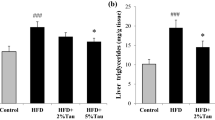
There was an increase of lipid content in the livers of animals receiving a TAA injection (Figs. 1, 2), which was determined by measuring the number of pixels that corresponded to fat positivity by Oil Red staining (n = 3; p < 0.0001). The TAA group had a significantly greater content of lipids in the liver than the control (p < 0.001), TAU (p < 0.001), and TAU + TAA (p < 0.001) groups. There was no difference in serum triglycerides between the groups (n = 3).
Histological appearance of liver sections from the four experimental groups (×1,000). The first column a, c, e, g was staining with hematoxylin and eosin and the second column b, d, f, h was stained with Oil Red O. a, b Control group. c, d Taurine group. e, f Thioacetamide group. g, h Taurine–thioacetamide group
The effect of taurine and thioacetamide on the hepatic accumulation of lipids (a) and serum triglycerides (b). To test differences between groups, ANOVA followed by the Tukey test were used (p < 0.05). Data are presented as mean and SE. a Difference between control (Ctrl) and thioacetamide (TAA) group. b Difference between the TAA group and the co-treatment of taurine and thioacetamide (TAA + TAU) group
Taurine Prevents Hepatic Lipoperoxidation
To evaluate the peroxidative damage in hepatic lipids, we used the TBARS method (Fig. 3a). After 15 days, lipid peroxidation was significantly different between the groups (n = 4; p < 0.0001). The TAA group showed higher levels of lipoperoxidation compared to the control (p < 0.001) and taurine group (p < 0.001). Importantly, the TAU + TAA group was effective in preventing TAA-induced lipid oxidation (p < 0.001).
The effect of taurine and thioacetamide on hepatic lipoperoxidation a, SOD b, and CAT c activity. To test differences between groups, ANOVA and the Tukey test were used (p < 0.05). Data are presented as mean and SE. a Difference between the control (Ctrl) and thioacetamide (TAA) group. b Difference between the TAA group and the co-treatment of taurine and thioacetamide (TAA + TAU) group
Taurine Alters SOD but Not CAT Activity
The antioxidant defenses were evaluated by determining SOD (Fig. 3b) and CAT (Fig. 3c) enzyme activities. Exposure to TAA for 2 weeks significantly reduced the SOD activity (n = 5) when compared to the control (p < 0.001) and TAU group (p < 0.01). Treatment of these fish with taurine (the TAU + TAA group) prevented the TAA-mediated SOD inhibition (p < 0.001). In contrast, CAT showed no statistically significant changes in its activity profile between the groups (n = 4).
The Effect of Taurine on the Expression of Cytokine mRNA
The relative mRNA expression of ADIPOR2 (Fig. 4a), SIRT1 (Fig. 4b), and TNF-α (Fig. 4c) in hepatic tissue was analyzed by qRT-PCR. The Kruskal–Wallis test revealed a statistically significant difference in mRNA expression of ADIPOR2 (p = 0.0014), SIRT1 (p = 0.0038), and TNF-α (p = 0.0079) between the groups. The expression of ADIPOR2 was lower in the TAA group than in the control (p < 0.05) and TAU (p < 0.01) groups; however, no difference was observed with the TAU + TAA group. In contrast, SIRT1 showed a reduction in mRNA expression in the TAA group when compared to the control (p < 0.05), TAU (p < 0.05), and TAU + TAA (p < 0.01) groups. The mRNA expression of TNF-α was increased in the TAA group compared to the control (p < 0.05) and TAU (p < 0.05) groups; but, no difference was observed with the TAU + TAA group (p > 0.05).
The effect of taurine and thioacetamide on the mRNA expression of ADIPOR2 (a), SIRT1 (b), and TNF-α (c). To test differences between groups, the Kruskal–Wallis test followed by the Dunn test were used (p < 0.05). Each box represents the median and interquartile range of values, with the ends of the vertical lines indicating the minimum and maximum data values. a Difference between the control (Ctrl) and thioacetamide (TAA) group. b Difference between the TAA group and the co-treatment of taurine and thioacetamide (TAA + TAU) group
Discussion
NAFLD is a spectrum of disorders that ranges from asymptomatic steatosis to nonalcoholic steatohepatitis (NASH) and cirrhosis. Many factors are involved in the pathogenesis and progression of NAFLD, such as insulin resistance, oxidative stress, and inflammatory cascade [22]. A two-hit theory has been accepted as the hypothesis for the chain of events that has been implicated in the progression of steatosis to NASH [23]. This theory postulates that steatosis, the first hit, increases the liver sensitivity to oxidative stress and to proinflammatory cytokines, which characterizes the second hit [24]. Recently, a third hit—hepatocyte death and lack of repair—was included to explain the NAFLD progression [25].
TAA is a selective and potent hepatotoxin widely used in research as an inducer of hepatic damage [26]. To exert its hepatotoxic effect, TAA needs to undergo two steps of bioactivation mediated by CYP2E1. The metabolites of TAA then form covalent bonds with liver macromolecules [27]. Moreover, the oxidative stress seems to have an important role in TAA-induced liver injury [28]. In humans, greater than 5% accumulation of lipids in hepatocytes is characterized as NAFLD. However, in zebrafish, there is no cut-off percentage for this disease. Because of this, we considered the group with a significant difference in hepatic lipid content compared to the control group as the definition of hepatic steatosis. The results of this study showed that TAA treatment of zebrafish induced hepatic steatosis in zebrafish similar to that previously described [12].
Hepatic triglyceride formation is a protective strategy for the liver to defend itself from lipotoxicity caused by an overload of FFA from adipose tissue [29, 30]. These FFA also undergo β-oxidation or become esterified with glycerol to form triglycerides, which are then packaged and exported as very low density lipoproteins (VLDL). Therefore, hepatic fat accumulation can occur as a result of fat synthesis or increase in delivery, reduction of fat oxidation, or decrease in fat exportation [31]. Nevertheless, hypertriglyceridemia is an independent risk factor associated with NAFLD [32]. Contrary to our expectations, this study did not find a significant difference in serum triglycerides between the groups. However, TAA-treated zebrafish that received taurine showed an improvement in fat lipid accumulation compared to TAA-treated zebrafish alone. This result is in agreement with others studies that observed a reduction in hepatic steatosis after treatment with taurine in models of hepatic injury [9, 33, 34].
Several studies have demonstrated the antioxidant role of taurine [35, 36]. In this study, we found an increase of lipoperoxidation in the group exposed to TAA and a protective effect of taurine on lipoperoxide formation in zebrafish. We have not found any study to date that has analyzed the effect of taurine on oxidative stress in the zebrafish liver. Taurine has been previously evaluated in the zebrafish brain exposed to ethanol and was shown to prevent the increase of lipid peroxidation induced by ethanol [37]. Our finding is consistent with previous studies in rats, which also observed a higher content of malondialdehydes in the TAA-induced hepatic damage group as well as a reduction of hepatic lipoperoxidation when taurine was co-administered with TAA [8, 28]. Considering that taurine is a very stable molecule, it may have a scavenger-type role against reactive oxygen species (ROS). In fact, it has been demonstrated that taurine inhibits lipoperoxidation induced by tert-butyl hydroperoxide in slices of rat liver and can protect thiol groups from oxidation [38].
SOD and CAT act coordinately to control ROS levels. SOD catalyzes the reaction of two superoxide anions, resulting in the formation of hydrogen peroxide, which is less reactive and can be degraded by enzymes such as CAT. Our results indicated a decrease in SOD activity in the group with TAA-induced liver damage compared to the control; however, there were no changes in CAT activity. It is well known that an imbalance in antioxidant defenses promotes lipoperoxidation and oxidative stress, which is an important factor in the progression of NAFLD, and some studies have reported a reduction of SOD activity in the liver of patients with NAFLD [39–41]. On the other hand, the TAU + TAA group presented higher levels of SOD activity compared to the TAA group. This result differs from some previous studies that have shown that SOD activity was not altered after taurine treatment, however this analysis was done in cirrhosis [8, 28]. In contrast, Chang et al. [42] found an enhancement of SOD activity in hamsters with steatosis induced by a high-fat/cholesterol diet that were treated with taurine. The increase in SOD activity and decrease in lipid peroxidation in the TAU + TAA group in our study confirms the hypothesis that taurine exerts a protective effect on the oxidant-antioxidant imbalance, thereby preventing oxidative stress.
Many genes are the focus of studies that attempt to elucidate the molecular mechanisms involved in NAFLD development and progression [43]. In this study, we found an enhancement in TNF-α and decrease in ADIPOR2 and SIRT1 mRNA expression in the TAA group compared to the Ctrl group. In fact, an increase in TNF-α and a reduction in ADIPOR2 levels has been observed in individuals with NAFLD [44, 45]. Adiponectin is an anti-inflammatory adipokine that is antagonized by TNF-α. Low circulating levels of adiponectin seem to contribute to the progression of NAFLD [44]. The adiponectin effects in the liver are predominantly mediated by the receptor ADIPOR2 and the mRNA expression of this receptor negatively correlates with hepatic aminotransferases in NAFLD patients [45]. Higher ADIPOR2 expression in the liver was shown in transgenic mice with moderate overexpression of SIRT1 [46]. In this study, we found an increase in SIRT1 expression in the TAU + TAA group compared to the TAA group alone.
SIRT1 is an NAD+-dependent protein deacetylase that has been recently associated with a protective effect in NAFLD [47]. No published study to date has evaluated the effect of taurine on SIRT1 expression in hepatic steatosis in adult zebrafish. The expression of SIRT1 is highest in the male gonads and liver compared to other tissues, and has the highest expression among other isoforms in zebrafish [48]. Deng et al. [49] found a reduction of SIRT1 expression in rats with NAFLD caused by high-fat diets. In addition, Costa et al. [50] found that SIRT1 expression was decreased in visceral adipose tissue of morbidly obese patients with severe steatosis compared to patients with slight or moderate steatosis. Moreover, the function of SIRT1 in hepatic steatosis seems to be due to the induction of SOD and lower activation of TNF-α and interleukin 6 (IL-6), which confers a protective effect during the second hit of NASH [46].
In conclusion, the results of this study revealed that taurine can improve oxidative stress parameters in a model of hepatic steatosis induced by TAA in adult wild-type zebrafish. Furthermore, we found that taurine prevented the decrease of SIRT1 mRNA expression caused by TAA exposure. Based on our results, taurine may be a promising therapy for treating hepatic steatosis.
References
Roberts EA. Pediatric nonalcoholic fatty liver disease (NAFLD): a “growing” problem? J Hepatol. 2007;46:1133–1142.
Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231.
Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395.
Moretto M, Kupski C, Mottin CC, et al. Hepatic steatosis in patients undergoing bariatric surgery and its relationship to body mass index and co-morbidities. Obes Surg. 2003;13:622–624.
Cotrim HP, Parise ER, Oliveira CP, et al. Nonalcoholic fatty liver disease in Brazil. Clinical and histological profile. Ann Hepatol. 2011;10:33–37.
OH MK, Winn J, Poordad F. Review article: diagnosis and treatment of non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2008;28:503–522.
Huxtable RJ. Physiological actions of taurine. Physiol Rev. 1992;72:101–163.
Balkan J, Dogru-Abbasoglu S, Kanbagli O, et al. Taurine has a protective effect against thioacetamide-induced liver cirrhosis by decreasing oxidative stress. Hum Exp Toxicol. 2001;20:251–254.
Chen SW, Chen YX, Shi J, et al. The restorative effect of taurine on experimental nonalcoholic steatohepatitis. Dig Dis Sci. 2006;51:2225–2234.
Lam SH, Gong Z. Modeling liver cancer using zebrafish: a comparative oncogenomics approach. Cell Cycle. 2006;5:573–577.
Passeri MJ, Cinaroglu A, Gao C, et al. Hepatic steatosis in response to acute alcohol exposure in zebrafish requires sterol regulatory element binding protein activation. Hepatology. 2009;49:443–452.
Rekha RD, Amali AA, Her GM, et al. Thioacetamide accelerates steatohepatitis, cirrhosis and HCC by expressing HCV core protein in transgenic zebrafish Danio rerio. Toxicology. 2008;243:11–22.
Amali AA, Rekha RD, Lin CJ, et al. Thioacetamide induced liver damage in zebrafish embryo as a disease model for steatohepatitis. J Biomed Sci. 2006;13:225–232.
McGrath P, Li CQ. Zebrafish: a predictive model for assessing drug-induced toxicity. Drug Discov Today. 2008;13:394–401.
Kinkel AD, Fernyhough ME, Helterline DL, et al. Oil red-O stains non-adipogenic cells: a precautionary note. Cytotechnology. 2004;46:49–56.
Jagadeeswaran P, Sheehan JP, Craig FE, et al. Identification and characterization of zebrafish thrombocytes. Br J Haematol. 1999;107:731–738.
Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310.
Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–3175.
Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J. 1973;134:707–716.
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2[−delta delta C(T)] method. Methods. 2001;25:402–408.
Lewis J, Mohanty S. Nonalcoholic fatty liver disease: a review and update. Dig Dis Sci. 2010;55:560–578.
Day CP. Pathogenesis of steatohepatitis. Best Pract Res Clin Gastroenterol. 2002;16:663–678.
Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842–845.
Tiniakos DG, Vos MB, Brunt EM. Nonalcoholic fatty liver disease: pathology and pathogenesis. Annu Rev Pathol. 2010;5:145–171.
Chilakapati J, Shankar K, Korrapati MC, et al. Saturation toxicokinetics of thioacetamide: role in initiation of liver injury. Drug Metab Dispos. 2005;33:1877–1885.
Tunez I, Munoz MC, Villavicencio MA, et al. Hepato- and neurotoxicity induced by thioacetamide: protective effects of melatonin and dimethylsulfoxide. Pharmacol Res. 2005;52:223–228.
Dogru-Abbasoglu S, Kanbagli O, Balkan J, et al. The protective effect of taurine against thioacetamide hepatotoxicity of rats. Hum Exp Toxicol. 2001;20:23–27.
Law K, Brunt EM. Nonalcoholic fatty liver disease. Clin Liver Dis. 2010;14:591–604.
Yamaguchi K, Yang L, McCall S, et al. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology. 2007;45:1366–1374.
Dowman JK, Tomlinson JW, Newsome PN. Pathogenesis of non-alcoholic fatty liver disease. Qjm. 2010;103:71–83.
Assy N, Kaita K, Mymin D, et al. Fatty infiltration of liver in hyperlipidemic patients. Dig Dis Sci. 2000;45:1929–1934.
Tasci I, Mas N, Mas MR, et al. Ultrastructural changes in hepatocytes after taurine treatment in CCl4 induced liver injury. World J Gastroenterol. 2008;14:4897–4902.
Chen X, Sebastian BM, Tang H, et al. Taurine supplementation prevents ethanol-induced decrease in serum adiponectin and reduces hepatic steatosis in rats. Hepatology. 2009;49:1554–1562.
Schaffer SW, Azuma J, Mozaffari M. Role of antioxidant activity of taurine in diabetes. Can J Physiol Pharmacol. 2009;87:91–97.
Lakshmi Devi S, Anuradha CV. Mitochondrial damage, cytotoxicity and apoptosis in iron-potentiated alcoholic liver fibrosis: amelioration by taurine. Amino Acids. 2010;38:869–879.
Rosemberg DB, da Rocha RF, Rico EP, et al. Taurine prevents enhancement of acetylcholinesterase activity induced by acute ethanol exposure and decreases the level of markers of oxidative stress in zebrafish brain. Neuroscience. 2010;171:683–692.
Oliveira MW, Minotto JB, de Oliveira MR, et al. Scavenging and antioxidant potential of physiological taurine concentrations against different reactive oxygen/nitrogen species. Pharmacol Rep. 2010;62:185–193.
Oliveira CP, Coelho AM, Barbeiro HV, et al. Liver mitochondrial dysfunction and oxidative stress in the pathogenesis of experimental nonalcoholic fatty liver disease. Braz J Med Biol Res. 2006;39:189–194.
Videla LA, Rodrigo R, Orellana M, et al. Oxidative stress-related parameters in the liver of non-alcoholic fatty liver disease patients. Clin Sci (Lond). 2004;106:261–268.
Yesilova Z, Yaman H, Oktenli C, et al. Systemic markers of lipid peroxidation and antioxidants in patients with nonalcoholic fatty liver disease. Am J Gastroenterol. 2005;100:850–855.
Chang YY, Chou CH, Chiu CH, et al. Preventive effects of taurine on development of hepatic steatosis induced by a high-fat/cholesterol dietary habit. J Agric Food Chem. 2011;59:450–457.
Malaguarnera M, Di Rosa M, Nicoletti F, et al. Molecular mechanisms involved in NAFLD progression. J Mol Med. 2009;87:679–695.
Jou J, Choi SS, Diehl AM. Mechanisms of disease progression in nonalcoholic fatty liver disease. Semin Liver Dis. 2008;28:370–379.
Kaser S, Moschen A, Cayon A, et al. Adiponectin and its receptors in non-alcoholic steatohepatitis. Gut. 2005;54:117–121.
Banks AS, Kon N, Knight C, et al. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab. 2008;8:333–341.
Colak Y, Ozturk O, Senates E, et al. SIRT1 as a potential therapeutic target for treatment of nonalcoholic fatty liver disease. Med Sci Monit. 2011;17:HY5–HY9.
Pereira TCB, Rico EP, Rosemberg DB, et al. Zebrafish as a model organism to evaluate drugs potentially able to modulate sirtuin expression. Zebrafish. 2011;8:9–16.
Deng XQ, Chen LL, Li NX. The expression of SIRT1 in nonalcoholic fatty liver disease induced by high-fat diet in rats. Liver Int. 2007;27:708–715.
dos Costa CS, Hammes TO, Rohden F, et al. SIRT1 transcription is decreased in visceral adipose tissue of morbidly obese patients with severe hepatic steatosis. Obes Surg. 2010;20:633–639.
Acknowledgments
This work was financially supported by Fundo de Incentivo à Pesquisa e Eventos of Hospital de Clínicas de Porto Alegre (FIPE-HCPA).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hammes, T.O., Pedroso, G.L., Hartmann, C.R. et al. The Effect of Taurine on Hepatic Steatosis Induced by Thioacetamide in Zebrafish (Danio rerio). Dig Dis Sci 57, 675–682 (2012). https://doi.org/10.1007/s10620-011-1931-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-011-1931-4