Abstract
Aim
Numerous reports in the 1990s pointed to a learning curve for laparoscopic cholecystectomy (LC), critical in achieving excellent outcomes. As LC is now standard therapy for acute cholecystitis (AC), we aimed to determine if surgeon volume is still vital to patient outcomes.
Methods
The Nationwide Inpatient Sample was used to query 80,149 emergent/urgent cholecystectomies performed for AC from 1999 to 2005 in 12 states with available surgeon/hospital identifiers. Volume groups were determined based on thirds of number of cholecystectomies performed per year for AC; two groups were created [low volume (LV): ≤15/year; high volume (HV): >15/year]. Primary endpoints were the rate of open conversion, bile duct injury (BDI), in-hospital mortality, and prolonged length of stay (LOS). Propensity scores were used to create a matched cohort analysis. Logistic regression models were created to further assess the effect of surgeon volume on primary endpoints.
Results
The number of cases performed by HV surgeons increased from 24% to 44% from 1999 to 2005. HV surgeons were more likely to perform LC, had fewer conversions, lower incidence of prolonged LOS, lower BDI, and lower in-hospital mortality. After matching the volume cohorts to create a case-controlled analysis, multivariate analysis confirmed that surgeon volume was an independent predictor of open conversion and prolonged LOS but not BDI and in-hospital mortality.
Conclusions
Increasing surgical volume remains associated with improved outcomes after surgery during emergent/urgent admission for AC with fewer open conversions and prolonged LOS. Our results suggest that referral to HV surgeons has improved outcomes after LC for AC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Surgeon and hospital volume appears to result in improved outcomes after major surgery such as cardiac, vascular, and oncology operations. Other processes of care and specific hospital factors such as intensive care unit support, teaching facilities, and socioeconomic status are also important factors in surgical quality [1–3]. National standards are being created based on outcomes for major surgery such as cardiac surgery, gastric bypass, and transplantation. These checkpoints have created a shift in focus for volume to meet these standards for specialized surgeries under scrutiny.
It remains unclear if a volume standard exists or is needed for common diagnostic problems and surgeries such as acute cholecystitis (AC) and cholecystectomy. Cholecystectomy remains one of the most commonly performed abdominal surgeries in the USA [4–7]. With the advent of laparoscopy, a changing paradigm in surgical training, and the development of acute care surgical services, the quality and outcomes of surgical treatment of AC remains unknown. Numerous reports in the 1990s pointed to a learning curve for laparoscopic cholecystectomy (LC), critical in achieving excellent outcomes [8]. The learning curve manifests early in the surgical experience while the volume–outcome relationship is developed over time with maintaining skills with repeated performance of a single operation. As LC is now standard therapy for acute cholecystitis (AC), we aimed to determine if surgeon volume is still vital to patient outcomes.
Methods and Patients
We used the NIS for the years 1999–2005 to extract data for all patients with AC as previously described [9]. The NIS is the largest national all-payer hospital inpatient care database in the USA. It is supported by the Healthcare Cost and Utilization Project and contains all-payer discharge information for 100% of patient discharges from participating hospitals. Data exist for approximately 7,000,000 hospital discharges per year from a stratified sample of 20% of nonfederal US community hospitals from participating states including academic and specialty hospitals. It contains hospital-level information obtained from a direct link to the American Hospital Association’s annual survey of hospitals, which includes hospital type (teaching/nonteaching) and geographic region (Northeast, West, South or Midwest, as defined by the US Census Bureau). Each record in the NIS represents a single hospital discharge and includes a unique identifier.
The study was reviewed by the University of Massachusetts Institutional Review Board (IRB) as appropriate for exception from IRB oversight as no personal identifiers were used among the registry data.
Study Population
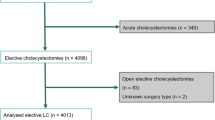
Diagnoses and procedures were identified by the Clinical Modification of the International Classification of Diseases, 9th revision (ICD-9-CM) diagnostic and procedural codes. All patients with a diagnosis of AC were identified (574.0, 574.3, 574.6, 574.8, 575.0, 575.12) as previously described [9, 10]. From this group, patients with an emergent/urgent admission with a primary procedure code of cholecystectomy were then queried [LC 51.23, open cholecystectomy (OC) 51.22]. All patients with primary gastrointestinal (GI) cancer (150–159.9) and metastatic GI cancer (197.4–197.8) were excluded, as were patients for whom the primary procedure was not performed within 7 days of admission. Conversions (any open cholecystectomy with an associated diagnosis code of V64.4, V64.41) were included in the LC group to reflect surgical intent. Intraoperative cholangiogram was also identified (87.53). From 1999 to 2005, 251,956 cholecystectomies (LC and OC) were performed for AC. Surgeries were performed in 2,961 hospitals in 40 different states in the NIS database.
Only patients with the unique surgeon and hospital identifier code were included in the study. This resulted in 80,149 cases (28% of total cohort) from 922 hospitals in 12 different states (median 8; range 5–10) over the 7-year period. States included in at least one year in the study were Florida, Iowa, Maryland, Maine, New Hampshire, New Jersey, Nevada, New York, Pennsylvania, South Dakota, Texas, and Virginia. To evaluate volume, we categorized groups based on surgeon volume of cholecystectomy, in approximately three equal groups for comparison prior to any volume–outcome analysis [11–13]. For presentation of the descriptive and univariate data, volume groups were kept in thirds. To perform dichotomous analyses of the matched cohorts, two groups were then made: high volume (HV) represented the upper-third in surgeon volume (>15 cholecystectomies/year; n = 26.572 admissions) while low volume (LV) accounted for the remaining lower two-thirds of surgeon volume (1–15 cholecystectomies/year; n = 53,577 admissions) in the cohort.
Provider Identifiers
Surgeon identifiers were used to determine the number of LC and OC per individual surgeon. Since record sampling in the NIS does not correlate across years, a continuous single-surgeon identifier was not possible. Each record or identifier is considered a unit assigned to a specific surgeon or hospital; therefore, the same surgeon may operate in different years recorded as a different individual surgeon. For example, due to the sampling in the NIS, it is possible for a surgeon’s hospital to be included in one year and then not included the following year. All volume calculations were done on a year-to-year basis. The range of surgeon volume was 1–122 cases per year. Extrapolation of the dataset using institutional weighting was not performed.
Variables
Demographic and operative characteristics of patients were captured within NIS. Age was maintained as a continuous variable. Race was divided into White, Black, Hispanic or other, which included but was not limited to Asians, Pacific Islanders, and Native Americans. Surgeon volume was also divided into thirds as described above and then split into low and high volume due to the dichotomous analysis. The income bracket was a categorical variable derived from the median household income of the patient’s zip code of residence. From 1999 to 2002, these quartiles were defined based on 1999 demographics such that the maximum of the first quartile was 150% of the poverty level, and the boundary between the second and third quartiles was the national median income. From 2003 to 2005, the quartiles were adjusted annually to divide patients equally, again with the boundary between the second and third quartiles being the national median income. Payer type was divided into four groups: Medicare, Medicaid, private insurance or other. Hospital volume was included in the regression analysis as a continuous variable due to potential confounding between hospital and surgeon volume.
Bile duct injury was identified by a procedure code of bile duct repair (517: repair of bile duct; 513.6: choledochoenterostomy; 513.9: other bile duct anastomosis). This was only accounted for if the procedure for bile duct injury was performed during the same hospital admission as the cholecystectomy.
Prolonged length of stay (LOS) was calculated as any stay that was greater than the 90th percentile of the whole cohort. A categorical value was then assigned if a hospital stay was above the 90th percentile or not. This would capture the complicated cases and long hospitalizations and reflect atypical discharge patterns.
For purposes of risk adjustment, coexisting comorbidity was compiled to create an Elixhauser comorbidity index [14]. This index identifies 29 disease entities that are considered true comorbid diseases associated with adverse outcomes in hospitalized patients. Patients were given a score of 0, 1, 2 or ≥3 based on number of comorbidities.
Case-Controlled Analysis
Propensity scores were used to further investigate whether differences in outcomes by selected surgeon volume groups were dependent on disparities in patient population and disease characteristics as previously described [9, 15]. An advantage to the use of propensity scores is that the model is not constrained with overfitting, multiple testing, and the conventional P < 0.05 criteria for variable inclusion. Candidate factors for the propensity model were important demographic and disease factors including age, gender, race, income bracket, insurance type, year of operation, Elixhauser comorbidity score, and a binary hospital volume variable divided into high or low volume. The propensity groups markedly reduced demographic differences between patients [16, 17]. We used a greedy 5 ≥ 1 matching algorithm for matching [18]. This algorithm first matches on five digits of the propensity score and then subsequently on four, etc. A matched cohort was created in which all demographic/disease characteristic differences between HV and LV surgeons (n = 23,163 in each group) were reduced, allowing us to evaluate the effect of volume in a case-control fashion. Matching with this method allowed 93% of HV surgeons group to be included in the adjust cohort. Within each group, the association between each demographic or disease characteristic was determined by the χ 2 test.
Outcomes
The primary endpoints examined in this study were open conversion from laparoscopic to OC, included in-hospital mortality, bile duct injury (BDI), and prolonged LOS.
Statistical Analysis
Data was analyzed using SAS software release 8.02 (SAS Institute, Cary, NC). Continuous variables were evaluated for normality using Shapiro-Wilk test and tested for statistical significance with a one-way analysis of variance (ANOVA). For more than two continuous variables, ANOVA was used to test for statistical significance. Categorical variables were tested with χ 2 analysis. Temporal trends were assessed using the Cochrane-Armitage trend test. Statistical significance was defined by P < 0.05.
Univariate predictor variables with P < 0.10 were included in the multivariate analysis. A logistic regression was used to determine the effect of different variables on risk of conversion from laparoscopic to OC while controlling for patient and hospital characteristics. A Hosmer–Lemeshow goodness-of-fit test was performed to confirm the final model. A confirmatory analysis using propensity scores to minimize bias from nonrandom assignment of treatments was also performed [19]. All results in the regression model were represented by an odds ratio and 95% confidence interval (CI). All regression models were performed separately with and without variables with missing fields. The data was unchanged in both models. Data presented includes admissions with all data included.
Results
There has been a significant increase in the number of cholecystectomies performed by HV surgeons (Fig. 1) (P < 0.001). Overall, the number of cases performed by surgeon increased each year from 5.6 cases per surgeon per year in 1999 to 7.6 cases per surgeon per year in 2005. Most cases were performed laparoscopically (n = 72,486; 90.4%). The overall conversion rate was 10.5%.
Table 1 displays the demographics of the study cohort. Median age was approximately 54 years and more than 62% of patients were female. About two-thirds of the patients were White, with Hispanic being the second most common race (18%). Patients who underwent surgery for AC were most commonly without comorbidities (38.7%) and had private insurance (42.9%), and 30% were in the highest income bracket (30.3%). The median length of stay for all patients was 4 days (LC: 3 days; OC: 7 days). For patients who underwent LC, only 9.3% had a prolonged LOS while 40.0% who underwent OC had prolonged LOS. Overall in-hospital mortality was 0.76%. Specifically for LC, in-hospital mortality was 0.44% while mortality was 3.78% for OC.
A learning curve in LC may be extrapolated from the data. At the start of the study, LV surgeons started about 16% of cases as OC (Fig. 2). Over time the number of cases started as OC in the lowest-volume group decreased significantly (P < 0.001). Figure 3 shows the open conversion rate stratified by surgeon volume. Throughout the course of the study, HV surgeons had the lowest conversion rate, never exceeding 8.5%. Surgeons in the lowest third improved their conversion rate over the 8-year period from a conversion rate of 14.2% in 1998 to 11.2% in 2005, confirming an improvement in the learning curve. An intraoperative cholangiogram was performed in 37.6% of cases performed by HV surgeons compared with in 30.4% of cases performed by LV surgeons (P < 0.001). High-volume providers operated more frequently at nonteaching hospitals (71% of cases performed versus 64%, P < 0.0001).
Despite a lack of significant differences in demographics, there were important findings in the outcomes after surgery by different volume surgeons (Table 2). From time of admission, HV surgeons operated on patients significantly earlier (1.63 days) than surgeons in the LV group (1.68 days) (P < 0.0001). As a whole, HV surgeons performed more LC (93.1% versus 89.1%; P < 0.0001) and also had fewer open conversions (8.0% versus 11.8%; P < 0.001). The incidence of prolonged length of stay (11.1% versus 12.8%; P < 0.0001), bile duct injury (0.15% versus 0.22%; P = 0.03), and unadjusted mortality (0.67% versus 0.80%; P = 0.05) was also lower with HV surgeons.
Matched cohorts were then created with the use of propensity scores. Demographic differences were reduced between the HV and LV groups in an attempt to eliminate selection bias and referral patterns. Advanced age, gender, race, and hospital volume differences were all eliminated for comparison (P > 0.05). Disparities in payer type and income bracket remained despite the matching method (P < 0.05). Univariate analysis of endpoints revealed that percentage of cases performed laparoscopically (93.0% versus 90.4%; P < 0.0001), conversion rate (7.9% versus 11.1%; P < 0.0001), and prolonged LOS (11.2% versus 12.1%; P = 0.002) remained as significant differences between LV and HV surgeons (Table 3). Bile duct injury (0.13% versus 0.18%) and in-hospital mortality (0.69% versus 0.66%) were no longer significantly different between groups after adjustments in demographics factors.
Multivariate regression analysis of open conversion was performed to determine independent risk factors of conversion (Table 4). Female gender [odds ratio (OR) 0.53; 95% confidence interval (CI) 0.49–0.56], later period in the study (2002–2003: OR 0.92; 95% CI 0.85–0.99 and 2004–2005: OR 0.89; 95% CI 0.82–0.97), and HV surgeons (OR 0.68; 95% CI 0.64–0.73) were all protective factors in reducing the risk of open conversion in surgical therapy for AC. Factors which increased the risk of open conversion included advanced age (OR 1.02; 95% CI 1.02–1.02), Black race (OR 1.35; 95% CI 1.20–1.52), and advanced Elixhauser comorbidity (OR 1.04; 95% CI 1.02–1.07).
A multivariate model was also performed for prolonged LOS (Table 5). Similar to open conversion, HV surgeons independently had a decreased incidence of patients with prolonged LOS (OR 0.91; 95% CI 0.86–0.97).
Discussion
Acute cholecystitis is a significant health-care burden and its treatment, cholecystectomy, is one of the most common general surgical procedures performed today [20]. In this study of the NIS database, we have shown that surgical volume improves patient survival after admission and surgery for AC. With an increased focus on quality improvement and outcomes, there has been a progressive trend towards care with high-volume surgeons in many disciplines [12, 21, 22]. This has been previously described in complex abdominal procedures such as pancreatectomy and colectomy but not for more general procedures such as LC [23–26]. In order to remove selection bias and potential confounding with referral patterns, we performed a matched cohort analysis. Our results confirm that more regionalization and referral to high-volume surgeons has improved outcomes after surgery for AC. High-volume surgeons had a lower open conversion rate, lower likelihood of prolonged length of stay, and a higher likelihood of performing LC overall.
The adoption of LC was initially met with reservation due to development of a learning curve among the surgical community [7, 8]. We have previously shown that, in this decade, LC has become the predominant surgical modality for AC with excellent success [9, 10]. Surgical volume of cholecystectomy is sustained and the laparoscopic method may be easier and safer now than the open approach [9]. There has been no previous study examining surgeon volume with outcomes after LC using US population-based data. Using data from Scottish public-sector hospitals, McMahon et al. showed that increasing laparoscopic experience and annual case load were associated with higher proportion of LCs and shorter hospital stays [27]. We acknowledge that certain confounding must exist between surgeon and center volume to account for some of the observed effects. We therefore included hospital volume in the logistic regression analysis to account for this; surgeon volume remained an important factor for open conversion. Open conversion may not always reflect lack of technical expertise when performing LC; obviously there are instances when surgeon experience and judgment make conversion prudent.
One of our primary endpoints in this study was open conversion after laparoscopic attempt at cholecystectomy. In-hospital mortality and bile duct injury were considered as secondary endpoints primarily due to their low occurrence rate after LC or OC. We felt that these secondary endpoints would not be accurate indicators of surgical quality because low numbers may not reveal significant differences in quality. Due to the rise in cholecystectomy overall for a variety of procedures including biliary colic, we felt it was important for the cohort to be focused using patients that needed surgical intervention and required expertise such as AC. As we have previously described, using the surgeon identifier in the NIS enables us to study over 80,000 cases across 12 states in the 8-year period [15]. With the use of the surgeon identifier, we have shown a shift in volume over time for the highest tertile surgeons. More importantly, outcomes remain excellent for HV surgeons, suggesting that their expertise in LC was gained earlier and remained over the course of the study. This would include open conversion, percentage of cases started laparoscopically, in-hospital mortality, and bile duct injury. It is important to remove inherent differences in cohort with the use of propensity scores to accurately assess whether differences do exist in important endpoints such as conversion and mortality. It was only in the adjusted analysis that we found that bile duct injury and in-hospital mortality were not different among volume groups.
The development of acute-care surgery has led to focused care of patients with urgent general surgery disorders such as appendectomy and cholecystitis [28]. It remains unclear whether this change in approach from the historical “surgeon on call” algorithm has improved the quality and efficiency of surgical care [29]. Timely surgery and a uniform approach to these common disorders was the underlying goal of this systems-based practice and it would appear that acute-care surgeons would become the high-volume surgeons for gastrointestinal disorders such as appendicitis, cholecystitis, and intestinal perforation. Our results would support the use of this approach at both low- and high-volume centers. It appears that, although center volume may be an important factor in management of AC, surgeon volume clearly improves outcome as measured in this study.
The strengths of our study lie in its use of the surgeon and hospital identifier to identify volume cutoffs. We chose to create equal volume cutoffs prior to any analysis to determine the volume cohorts. This is an accepted and rationale method compared with arbitrarily selecting volume cutoffs that may suit statistical thresholds [30, 31]. The NIS is the largest all-payer discharge database in the USA. Unlike other laparoscopic procedures, a unique ICD-9 code exists for LC, OC as well as open conversion, allowing accurate assessment of laparoscopy and surgical intent.
The weaknesses of this study parallel those with the use of retrospective analysis of administrative data. There is a potential for underreporting of events, complications, and diagnoses. Our data contains no information about surgeon specialty, training, experience or other factors that may impact patient outcome. Our study used population-based data with only limited information on patient and treatment factors, thereby limiting our evaluation of medical factors such as presence of cancer, cirrhosis, antibiotic use, mechanical ventilation, and prior surgery. Despite being the largest all-payer database of hospital discharge records in the USA, there is no guarantee that our cohort is representative of local demographics and surgeon practices which may vary by state and community. In this study, only states that had accurate surgeon and hospital identifiers were included in the study. These states reflected a large sample or diverse states across the country and therefore we felt that it was a representative sample of the USA. This may not have been a representational cohort of a large population; demographic and practice patterns of surgeons performing cholecystectomy may vary from state to state. In order to account for this, we calculated the open conversion, mortality rates, and bile duct injury for the whole NIS cohort for cholecystectomy and did not find any difference in mortality among states with surgeon/hospital identifiers and those without. We also did not identify any significant regional differences in our cohort, and states had uniform distribution of demographic and patient characteristics. We were unable to account for patients who received a cholecystostomy tube as well.
The volume–outcome relationship continues to be a charged topic in the surgical arena. The role and importance of surgeon and center remain intertwined and difficult to delineate in most complex medical disorders and surgical procedures [15]. The results of this study confirm that surgeon volume improves outcome as measured by open conversion rate after surgery for AC. Other endpoints improved as well compared with lower-volume surgeons. We attempted to account for as many confounding factors as possible to truly define the difference in surgical expertise with volume. While the role and importance the hospital plays in this benefit remains unclear, referral and formation of acute surgery teams appears to be well suited to meet the demands for high-quality outcomes after patients undergo cholecystectomy for AC.
References
Angus DC, Shorr AF, White A, Dremsizov TT, Schmitz RJ, Kelley MA. Critical care delivery in the United States: distribution of services and compliance with Leapfrog recommendations. Crit Care Med. 2006;34:1016–1024.
Pronovost PJ, Angus DC, Dorman T, Robinson KA, Dremsizov TT, Young TL. Physician staffing patterns and clinical outcomes in critically ill patients: a systematic review. JAMA. 2002;288:2151–2162.
Hannan EL, Popp AJ, Feustel P, et al. Association of surgical specialty and processes of care with patient outcomes for carotid endarterectomy. Stroke. 2001;32:2890–2897.
Legorreta AP, Silber JH, Costantino GN, Kobylinski RW, Zatz SL. Increased cholecystectomy rate after the introduction of laparoscopic cholecystectomy. JAMA. 1993;270:1429–1432.
Rattner DW, Ferguson C, Warshaw AL. Factors associated with successful laparoscopic cholecystectomy for acute cholecystitis. Ann Surg. 1993;217:233–236.
Livingston EH, Rege RV. A nationwide study of conversion from laparoscopic to open cholecystectomy. Am J Surg. 2004;188:205–211.
Zacks SL, Sandler RS, Rutledge R, Brown RS Jr. A population-based cohort study comparing laparoscopic cholecystectomy and open cholecystectomy. Am J Gastroenterol. 2002;97:334–340.
The Southern Surgeons Club. A prospective analysis of 1518 laparoscopic cholecystectomies. N Engl J Med. 1991;324:1073–1078.
Csikesz NG, Tseng JF, Shah SA. Trends in surgical management for acute cholecystitis. Surgery. 2008;144:283–289.
Csikesz N, Ricciardi R, Tseng JF, Shah SA. Current status of surgical management of acute cholecystitis in the United States. World J Surg. 2008;32:2230–2236.
Mcphee JT, Hill JS, Whalen GF, et al. Perioperative mortality for pancreatectomy: a national perspective. Ann Surg. 2007;246:246–253.
Birkmeyer JD, Stukel TA, Siewers AE, Goodney PP, Wennberg DE, Lucas FL. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:2117–2127.
Finlayson EV, Goodney PP, Birkmeyer JD. Hospital volume and operative mortality in cancer surgery: a national study. Arch Surg. 2003;138:721–725.
Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27.
Eppsteiner RW, Csikesz NG, Simons JP, Tseng JF, Shah SA. High volume and outcome after liver resection: surgeon or center? J Gastrointest Surg. 2008.
Cochran WG. The effectiveness of adjustment by subclassification in removing bias in observational studies. Biometrics. 1968;24:295–313.
Cook EF, Goldman L. Asymmetric stratification. An outline for an efficient method for controlling confounding in cohort studies. Am J Epidemiol. 1988;127:626–639.
Austin PC, Grootendorst P, Normand SL, Anderson GM. Conditioning on the propensity score can result in biased estimation of common measures of treatment effect: a Monte Carlo study. Stat Med. 2007;26:754–768.
Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med. 1997;127:757–763.
Everhart JE, Khare M, Hill M, Maurer KR. Prevalence and ethnic differences in gallbladder disease in the United States. Gastroenterology. 1999;117:632–639.
Cowan JA Jr, Dimick JB, Thompson BG, Stanley JC, Upchurch GR Jr. Surgeon volume as an indicator of outcomes after carotid endarterectomy: an effect independent of specialty practice and hospital volume. J Am Coll Surg. 2002;195:814–821.
Harmon JW, Tang DG, Gordon TA, et al. Hospital volume can serve as a surrogate for surgeon volume for achieving excellent outcomes in colorectal resection. Ann Surg. 1999;230:404–411.
Birkmeyer JD, Sun Y, Goldfaden A, Birkmeyer NJ, Stukel TA. Volume and process of care in high-risk cancer surgery. Cancer. 2006;106:2476–2481.
Birkmeyer JD, Siewers AE, Marth NJ, Goodman DC. Regionalization of high-risk surgery and implications for patient travel times. JAMA. 2003;290:2703–2708.
Gordon TA, Bowman HM, Tielsch JM, Bass EB, Burleyson GP, Cameron JL. Statewide regionalization of pancreaticoduodenectomy and its effect on in-hospital mortality. Ann Surg. 1998;228:71–78.
Riall TS, Eschbach KA, Townsend CM Jr, Nealon WH, Freeman JL, Goodwin JS. Trends and disparities in regionalization of pancreatic resection. J Gastrointest Surg. 2007;11:1242–1251.
Mcmahon AJ, Fischbacher CM, Frame SH, Macleod MC. Impact of laparoscopic cholecystectomy: a population-based study. Lancet. 2000;356:1632–1637.
Ekeh AP, Monson B, Wozniak CJ, Armstrong M, Mccarthy MC. Management of acute appendicitis by an acute care surgery service: is operative intervention timely? J Am Coll Surg. 2008;207:43–48.
Green JM. When is faster better? Operative timing in acute care surgery. Curr Opin Crit Care. 2008;14:423–427.
Shah SA, Bromberg R, Coates A, Rempel E, Simunovic M, Gallinger S. Survival after liver resection for metastatic colorectal carcinoma in a large population. J Am Coll Surg. 2007;205:676–683.
Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128–1137.
Acknowledgments
This article was supported by the American Society of Transplant Surgeons Faculty Development Award and Worcester Foundation for Biomedical Research (SAS).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Csikesz, N.G., Singla, A., Murphy, M.M. et al. Surgeon Volume Metrics in Laparoscopic Cholecystectomy. Dig Dis Sci 55, 2398–2405 (2010). https://doi.org/10.1007/s10620-009-1035-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-009-1035-6