Abstract
Introduction
Recent data suggest a wide range of conversion (4.9–20%) from laparoscopic (LC) to open cholecystectomy (OC) despite increasing surgeon familiarity and superior equipment. Previously identified risk factors for conversion include increased age, male gender, diabetes, and emergent surgeries. Recent studies also suggest that formal minimally invasive surgical training (MIST) reduces conversion rates. We sought to determine conversion rates in our population, a rural academic medical center, and identify any significant risks for conversion.
Methods
We conducted a single-center retrospective review of 2810 cholecystectomies performed over a seven-year period (2009–2016).
Results
Our study included 837 (29.8%) males and 1973 (70.2%) females with a mean age of 49.2 years. Forty-two percent of cases were done by surgeons with MIST. A total of 139 (4.95%) cases were converted to OC. Univariate predictors of conversion to OC included male gender, age ≥65, urgent and emergent admissions, and MIST of the surgeon. In multivariate modeling, which included significant univariate predictors of conversion, independent predictors of conversion to OC included urgent or emergent admission, male gender, and age ≥65. MIST status was no longer a significant predictor.
Conclusion
Our conversion rate from LC to OC falls within the lower range of recently published rates. This is likely multifactorial, and reflects increasing familiarity of the laparoscopic technique, improved quality of laparoscopic equipment, and/or prior knowledge of preoperative risk factors for conversion. Our results, consistent with previous literature, show a reduced conversion rate among surgeons with MIST. This finding, albeit not significant on multivariate analysis, may offer insight into a potential alterable preoperative risk factor for conversion and warrants further research. Further knowledge about the impact MIST has on conversion may provide a feasible preoperative approach to reducing conversion to OC, thereby reducing costs and overall patient morbidity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Cholecystectomy continues to be one of the most commonly performed surgical procedures in the United States, with estimates exceeding 600,000 cases per year [1]. The laparoscopic approach has been the gold standard of treatment for gallbladder disease for over two decades. The first recorded laparoscopic cholecystectomy (LC) was performed by Prof Dr. Erich Mühe of Germany in 1985 [2]. The procedure was originally met with much criticism, but by the 1990s popularity and subsequent credibility of the procedure grew globally, propelling its prevalence in the field of surgery today [3]. It has been reported that the prevalence of open cholecystectomy (OC) has decreased by roughly 67 and 92% in the 1990s and 2000s, respectively [4]. Furthermore, significantly reduced morbidity has contributed to the increased prevalence of laparoscopic cholecystectomy, with postoperative morbidity rates approximately half of open cholecystectomies as shown by recent meta-analysis [5].
Despite increasing surgeon familiarity with the laparoscopic technique, as well as improved quality of the surgical equipment, recent data shows a wide and relatively stable range of intraoperative conversion rates to OC; albeit, much lower than the traditional rates shown during and shortly thereafter the advent of the laparoscopic technique. Reported rates have ranged from 4.9 to 20.0% depending on the emergence of the case and preoperative risk factors [5,6,7,8,9,10].
Preoperative risk factors for conversion have been identified by recent studies and include increased age, male gender, pre-existing diabetes mellitus, and acute cholecystitis [5, 6, 8, 11, 12]. In addition, emergent surgical cases have been shown to result in higher conversion rates as compared to elective procedures [9]. Recent studies have also shown that prior formal minimally invasive surgical training (MIST) may reduce the risk of intraoperative conversion. The high prevalence of laparoscopic treatment of acute cholecystitis, in conjunction with growing evidence of decreased morbidity and length of hospital stay as compared to the open approach, warrants further knowledge about the potential risks for conversion and appropriate preoperative counseling [5]. This study sought to determine conversion rates from LC to OC in patients admitted to the University of Vermont Medical Center (UVMMC), a rural academic hybrid medical center, as well as identify any significant risk factors for conversion. Furthermore, given the minimal retrospective data investigating the effects of MIST on conversion, we sought to provide more data and insight for this potential preoperative risk factor.
Methods and materials
Patient population
We performed a single-center retrospective review of cholecystectomies performed from 2009 to 2016. Data were extracted from the clinical data warehouse with the assistance of the Jeffords Institute for Quality and Clinical Effectiveness at UVMMC. Cases were identified by CPT procedure codes for cholecystectomy (47562–47564, 47600, 47605, 47610, 47612, 47620) performed between 1/1/2009 and 12/31/2016. Cases identified as using intraoperative cholangiogram were also derived and categorized via CPT procedure codes (47563, 47564, 47605, 47610, 47612, 47620). The laparoscopic cases converted to open were identified via review of formal operative reports and each case was categorized as laparoscopic or laparoscopic converted to open cholecystectomy. Covariates extracted for each case included previously identified preoperative risk factors for conversion such as age (65 and older), gender, BMI (<30 versus ≥30), diabetes mellitus, and admit type (elective, urgent, or emergent). Surgeons were categorized as having participated in formal MIST (completion of an advanced laparoscopic fellowship) and surgeons without formal MIST.
Statistical analysis
We first examined the univariate associations of our predictor variables with our main outcome variable, conversion to open cholecystectomy using the Pearson χ 2 test; a p value of <0.05 was considered statistically significant. Odds ratio and 95% confidence intervals for conversion were calculated. In order to determine the independent association of the predictors we performed multivariate logistic regression, which included statistically significant variables on univariate analysis. Mean operative time was calculated via the two sample test. Statistical analyses were performed using SPSS version 23 (Armonk, NY: IBM Corp.).
Results
Patient characteristics
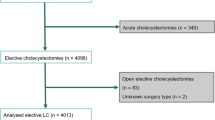
A total of 2810 laparoscopic cholecystectomies were reviewed over a 7-year period (Table 1). After excluding incomplete data, 2419 patients were included in the data analysis for preoperative BMI. The patient population included 837 (29.8%) males and 1973 (71.2%) females, with a mean age of 49.2 years. A total of 139 (4.95%) cases were converted from laparoscopic to open (Table 2). Furthermore, median length of hospital stay among converted cases was 5 days as opposed to 1 day for the laparoscopic group (p < 0.001). The mean operation time across the entire cohort equaled 85.0 min, with mean times of 81.2 (53.8) and 159.0 (70.2) min for LC and C, respectively (p < 0.001). Surgeons with MIST yielded a mean operative time of 86.5 (61.5) min as compared to 84.0 (54.0) min for surgeons without MIST (p = 0.51). Mean operative times during laparoscopic cases were 83.4 (58.4) and 80.0 (50.1) min for surgeons with MIST and surgeons without MIST, respectively (p = 0.07). Mean operative times during cases converted to open were 189.4 (75.4) and 149.1 (65.9) min for surgeons with MIST and surgeons without MIST, respectively (p = 0.003). A total of 980 (34.9%) of the 2810 cholecystectomies had IOC, including 17 (48.6%) and 49 (47.1%) of the converted cases for surgeons with MIST and surgeons without MIST, respectively (p = 0.022).
Risks for conversion
Eighty (9.6%) of the male patients converted to open, as compared to 59 (3.0%) females (OR 3.43, 95%CI [2.42, 4.85], p < 0.001) (Tables 2, 3). Sixty-four (11.9%) of the 537 patients ≥65 years old were converted to open, as compared to 75 (3.3%) of the 2273 patients less than 65 years old (OR 3.97, 95%CI [2.80, 5.62], p < 0.001). Of the admit types (elective, urgent, emergent), 20 (1.0%), 52 (24.5%), and 67 (11.4%) of the cases were converted to open, respectively (p < 0.001). The odds ratio using elective cases as the reference group are as follows: urgent cases (OR 32.33, 95%CI [18.87, 55.56]), emergent cases (OR 12.66, 95%CI [7.63, 21.28]). A total of 196 (7.0%) of the 2810 patients had pre-existing diabetes mellitus, of these, 15 (7.7%) were converted to open, as compared to 124 (4.7%) of the 2614 patients without pre-existing diabetes (OR 1.66, 95%CI [0.95, 2.90], p = 0.07). Of the 1150 patients that had a preoperative BMI of ≥30.0, 50 (4.3%) were converted to open, as compared to 62 (4.9%) of the 1269 patients with BMI of less than 30.0 (p = 0.53). A total of 1172 (41.7%) of the 2810 cases were performed by surgeons with MIST; of these, 35 (3.0%) were converted to open, as compared to 104 (6.3%) of the 1638 cases performed by surgeons without MIST (OR 2.20, 95%CI [1.49, 3.26], p < 0.001). There was a significant difference among the MIST and no-MIST patients, with the no-MIST cohort containing more males, patients ≥65 years old, and emergent/urgent cases (Table 4). Upon analysis of individual risk factors among the MIST and No-MIST cohorts, MIST showed conversion rates of 11.8, 6.5, and 6.2% for urgent/emergent admit types, male gender, and age ≥65 years old, respectively (Table 5). In comparison, the No-MIST cohort resulted in conversion rates of 16.3% (OR 1.45, 95%CI [0.94, 2.26], p = 0.09), 11.4% (OR 1.85, 95%CI [1.09, 3.13], p = 0.02), and 14.7% (OR 2.60, 95% CI [1.32, 5.10], p = 0.004) for urgent/emergent admit types, male gender, and age ≥65 years old, respectively. Admit type (p < 0.001), including emergent (p < 0.001) and urgent (p < 0.001) subtypes, age ≥65 years old (p < 0.001), and male gender (p < 0.001) were found to be significant on multivariate logistic regression, but minimally invasive training was not (p = 0.087) (Table 6). On exploratory multivariate analysis, including each of the significant univariate risks individually with no-MIST, all three factors slightly attenuated the OR for no-MIST.
Discussion
Laparoscopy is widely regarded as the procedure of choice for gallbladder disease, including both emergent and elective cases. Despite its vast prevalence, there remains an inherent risk for conversion to open cholecystectomy (OC) when performing the laparoscopic technique. Several studies have looked at this risk in order to garner a better understanding of the rate, as well as any predictable preoperative risk factors for conversion. While recent findings show an improvement in conversion rates as compared to historical data, the rates among recent studies have shown minimal decline with a wide variability of conversion [5,6,7,8,9,10]. As mentioned prior, preoperative risks for conversion include increasing age, male gender, diabetes, and emergent cases, among others. The question remains, are there any predictable risk factors that can accurately influence clinical decision making and subsequently reduce the risk of conversion? Additionally, it is uncertain at this time whether the decline in conversion rates as compared to historical data is due to prior knowledge of risk factors, a function of time, and increasing familiarity of the laparoscopic technique, or both. Eliciting further knowledge of the risks for conversion and subsequently reducing the rate may have vast implications, including decreased spending secondary to shorter hospital stays, and reduced overall patient morbidity [5].
Our study showed similar trends in regards to previously identified preoperative risk factor for conversion. Notably, male gender, age ≥65, and emergence of the case (urgent, emergent) were found to be statistically significant on univariate analysis and multiple variate logistic regression. These findings reflect and further solidify a growing collaboration of preoperative risk factors that can ultimately alter patient’s clinical outcomes. Our overall conversion rate of 4.95% was comparable to the lower end of the range rates shown in recently published literature (4.9–20.0%) [5,6,7,8,9,10]. This downward trend is likely multifactorial, but may be influenced via prior knowledge of risk factors for conversion, increasing familiarity with the laparoscopic technique, and improved equipment.
Our overall conversion rate (3.0%) among surgeons with MIST as compared to surgeons without MIST (6.3%) was found to be statistically significant on univariate analysis; it was not, however, found to be significant on multivariate logistic regression. There was no significant difference in mean operative times among MIST and no-Mist surgeons upon analyzation of the entire cohort. However, MIST surgeons yielded a significantly higher mean operative time of 189.4 (75.4) min during cases converted to open as compared to 149.1 (65.9) min for surgeons without MIST (p = 0.003). This finding may reflect a tendency among MIST surgeons to insist in completing the procedure laparoscopically, due to experience, preference, underlying perceptions of the MIS technique, among other factors. Further data, optimally in conjunction with qualitative surgeon input, is needed to garner better insight into the variance in operative times reflected by these data. As shown by Table 4, the variation among the MIST and No-MIST cohorts was significant across each of the observed statistically significant (on univariate analysis) risk factors for conversion. This observed skew towards a larger percentage of males, patients greater than age of 65, and emergent/urgent cases likely contributed to the higher rate of conversion among the No-MIST cohort and subsequent significance on univariate analysis. However, upon analysis of individual risk factors among the MIST and No-MIST cohorts, the MIST group showed significantly lower conversion rates for male gender and age ≥65, and a marginally significant difference in conversion for urgent/emergent admit types (Table 5). Despite the lack of significance on multivariate analysis, the large variation of conversion rate among the MIST and No-MIST groups warrants further research and insight into whether MIST does indeed reduce the risk for conversion. Recent studies have interpreted this phenomenon and have found similar findings. In 2015, a retrospective study analyzing 592 cholecystectomies showed an overall conversion rate of 5.7%. Surgeons with MIST performed 40% of the cases, similar to our cohort. The rate among surgeons with MIST was 1.7% as compared to 8.5% among surgeons without MIST, despite the fact that surgeons with MIST performed 70% of the acute cholecystitis cases [6].
Similarly to prior knowledge of preoperative risk factors for conversion, MIST may have implications on overall conversion rate, as well as both economic and clinical outcomes. Furthermore, MIST may offer a predictable and alterable variable which can guide preoperative management and allocation of cases based upon risk, should the resources and appropriate faculty exist, and subsequently minimize conversion to open procedures. The question remains, can cases with high risk for conversion, including those with the preoperative factors discussed in this study, be allocated to surgeons with MIST in a timely and safe manner? And if so, will this alterable preoperative allocation of cases result in decreased conversions? Or were these trends merely a function of case acuity and patient characteristics?
Limitations of the study include the patient population associated with a single academic center, which may limit generalizability. Despite gathering preoperative diagnostic data via our cohort, primary diagnosis codes were often variable and difficult to classify in a methodical manner, thus prompting their absence from this study’s analysis. We recognize the prognostic importance of the several well documented preoperative diagnoses, such as acute cholecystitis, that increase the risk for conversion [5, 7, 11, 12]. Additionally, the inherent limitations due to our lack of reliable preoperative diagnoses subsequently decreased our ability to assess disease timing and severity at the time of the operation. However, we believe it can be implied (though preoperative diagnostic data cannot confirm) that higher case acuity (emergent/urgent) can help elucidate acute processes such as acute cholecystitis from elective cases and chronic processes such as biliary colic, dyskinesia, or chronic cholecystitis. Furthermore, we were unable to obtain reliable preoperative diagnoses in regards to concern for malignancy, which often times results in prompt conversion to open after initial laparoscopic visualization. With that being said, we do not feel as though this skewed our data significantly, due to the fact that the prevalence of malignancy (after histopathological interpretation) reported in prior literature has been found to be as low as 0.25 and 0.70% for gallbladder carcinoma and dysplasia, respectively [13].
Additionally, the retrospective nature of this study added several inherent limitations. It was very difficult to objectively classify intraoperative reasons for conversion, such as dense adhesions, inflammation and subsequent poorly visualized anatomy, and common bile duct injury or concern for thereof which have been published as significant risks for conversion [10]. Upon review of formal operative reports of converted cases, individual surgeon descriptions of reasons for conversion and/or complications were often variable and lacked granularity, thus prompting our exclusion from this study. Furthermore, prior number of intraabdominal operations, a risk for formation of adhesions and subsequent conversions, was not included in our data due to the scope and design of the study. The difference in IOC rate during converted cases for MIST (48.6%) and no-MIST (47.1%), albeit small, was found to be significant. Due to lack of data on other pertinent forms of intraoperative imaging, such as ultrasound, in conjunction with lack of qualitative surgeon input in regards to use of IOC and relation to conversion, we decided to exclude intraoperative imaging from multivariate analysis. Similarly, as discussed above, the retrospective nature of the study made it difficult to ascertain the driving factor causing variance in conversion among the MIST and No-MIST groups. After controlling for significant preoperative risk factors on multivariate analysis, MIST was no longer found to be significant for conversion; however, univariate analysis of individual risk factors among the MIST and No-MIST cohorts resulted in lower conversion rates among the MIST cohort, with male gender and age ≥65 showing significant variance.
We recognize that prior studies have incorporated several measures of surgeon experience, such as timing of residency training and surgeon caseload at the time of the operation as potential variables in regards to conversion [8]. However, due to the vast sample size and duration of our study, the variability in the length of training/caseload at the particular time of the procedure could not be accurately and completely coded with our resources at hand and retrospective study design. Furthermore, surgeon perception and value of the MIS technique may affect their willingness and timing of conversion. As noted above, the significantly higher mean operative time among MIST surgeons during cases converted to open in this study may be a representation of this phenomenon, and this qualitative parameter could be incorporated into future studies through conjunctive surgeon surveys. Subsequently, a prospective, multi-center analysis, incorporating the limiting factors listed above in concert with the parameters evaluated throughout this study, would provide more insight into the nature of formal advanced laparoscopic fellowship training and risk for conversion.
Conclusions
Our conversion rate from LC to OC falls within the lower range of recently published rates. This is likely multifactorial, and reflects increasing familiarity of the laparoscopic technique, improved quality of laparoscopic equipment, and/or prior knowledge of preoperative risk factors for conversion. Procedure urgency, male gender, and advanced age were found to be statistically significant on multivariate logistic regression. Our results, consistent with previous literature, show a reduced rate of conversion among surgeons with prior minimally invasive surgical training [6, 8]. However, formal MIST was no longer found to be significant on multivariate analysis. It remains to be shown whether the reduction in conversions among surgeons with MIST is due to training, or a patient population with an inherently decreased acuity and collaboration of preoperative risk factors as discussed in this study. Future prospective and multi-center studies are needed to provide more definitive insight into this relationship. Despite this, the reduced rates observed in this study (request to delete ‘trend’ via reviewers) among surgeons with MIST offers a potential alterable preoperative risk factor for conversion, and therefore warrants further research. Further knowledge about the impact MIST has on conversion rates may provide a feasible preoperative approach to reducing conversion to OC, thereby reducing costs and overall patient morbidity and mortality [5].
References
Goldman L (2011) Goldman’s cecil medicine (ed 24). Elsevier Saunders, Philadelphia
Reynolds W Jr (2001) The first laparoscopic cholecystectomy. JSLS 5(1):89–94
Blum CA, Adams DB (2011) Who did the first laparoscopic cholecystectomy? J Minim Access Surg 7(3):165–168. doi:10.4103/0972-9941.83506
Sirinek KR, Willis R, Schwesinger WH (2016) Who will be able to perform open biliary surgery in 2025? J Am Coll Surg 223(1):110–115. doi:10.1016/j.jamcollsurg.2016.02.019
Coccolini F, Catena F, Pisano M, Gheza F, Fagiuoli S, Saverio Di, Gioacchino L, Giulia M, Ceresoli M, Corbella D, Sartellia M, Sugrue M, Ansaloni L (2015) Open versus laparoscopic cholecystectomy in acute cholecystitis. Systematic review and meta-analysis. Int J Surg 18:196–204. doi:10.1016/j.ijsu.2015.04.083
Abelson JS, Afaneh C, Rich BS, Dakin G, Zarnegar R, Fahey TJ 3rd, Pomp A (2015) Advanced laparoscopic fellowship training decreases conversion rates during laparoscopic cholecystectomy for acute biliary diseases: a retrospective cohort study. Int J Surg 13:221–226. doi:10.1016/j.ijsu.2014.12.016
Licciardello A, Arena M, Nicosia A, Di Stefano B, Cali G, Arena G, Minutolo V (2014) Preoperative risk factors for conversion from laparoscopic to open cholecystectomy. Eur Rev Med Pharmacol Sci 18(2 Suppl):60–68
Sakpal SV, Bindra SS, Chamberlain RS (2010) Laparoscopic cholecystectomy conversion rates two decades later. JSLS 14(4):476–483. doi:10.4293/108680810X12924466008240
To KB, Cherry-Bukowiec JR, Englesbe MJ, Terjimanian MN, Shijie C, Campbell DA Jr, Napolitano LM (2013) Emergent versus elective cholecystectomy: conversion rates and outcomes. Surg Infect (Larchmt) 14(6):512–519. doi:10.1089/sur.2012.160
Yajima H, Kanai H, Son K, Yoshida K, Yanaga K (2014) Reasons and risk factors for intraoperative conversion from laparoscopic to open cholecystectomy. Surg Today 44(1):80–83
Philip Rothman J, Burcharth J, Pommergaard HC, Viereck S, Rosenberg J (2016) preoperative risk factors for conversion of laparoscopic cholecystectomy to open surgery—a systematic review and meta-analysis of observational studies. Dig Surg 33(5):414–423. doi:10.1159/000445505
Yang TF, Guo L, Wang Q (2014) Evaluation of preoperative risk factor for converting laparoscopic to open cholecystectomy: a meta-analysis. Hepatogastroenterology 61(132):958–965
Wrenn SM, Callas PW, Abu-Jaish W (2016) Histopathological examination of specimen following cholecystectomy: are we accepting resect and discard? Surg Endosc. doi:10.1007/s00464-016-5002-y
Acknowledgements
Aggregate patient data were provided by the Jeffords Institute for Quality at the University of Vermont Medical Center. We greatly appreciate the assistance from Jeffords Institute, particularly Dr. Allison Holm (Senior Research Specialist) and Mia Nowlan (Measurement Analyst). The authors would also like to thank Charles Maclean MD for his insight and suggested revisions throughout the drafting of this manuscript.
Author information
Authors and Affiliations
Contributions
Author contributions
SC is the primary author of manuscript text and major contributor to study design, and preformed data collection, review of literature, and statistical analysis. PC provided critical statistical analysis and support and assisted with manuscript revisions. SW assisted with the procurement of data and study design, as well as provided critical revisions to the manuscript. WA-J (senior author) is the principal investigator for the study, designed the idea for the study, and assisted with manuscript drafting and revision.
Corresponding authors
Ethics declarations
Disclosures
Mr. Steven J. Coffin, and Drs. Sean M. Wrenn, Peter W. Callas, and Wasef Abu-Jaish have no conflicts of interest or financial ties to disclose.
Rights and permissions
About this article
Cite this article
Coffin, S.J., Wrenn, S.M., Callas, P.W. et al. Three decades later: investigating the rate of and risks for conversion from laparoscopic to open cholecystectomy. Surg Endosc 32, 923–929 (2018). https://doi.org/10.1007/s00464-017-5767-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-017-5767-7