Abstract
Background
The relationship of gastric cancer to the presence of interleukin-8 (IL-8) 251 T/A has been reported with conflicting results.
Aim
To further explore the association of IL-8 251 allele polymorphism with gastric cancer susceptibility.
Methods
We performed an extensive search of relevant studies and carried out a meta-analysis, including ten studies with 2,195 gastric cancer cases and 3,505 controls, to obtain a more precise estimate.
Results
The combined results based on all studies showed that the IL-8 251 allele AA genotype was a risk factor for gastric cancer [AA versus TT: odds ratio (OR) = 1.363, 95% confidence interval (CI): 1.199–1.527]. In subgroup analysis, a clear effect of AA in IL-8 251 allele was shown in Asians (AA versus TT: OR = 1.593, 95% CI: 1.013–2.173) but not in Caucasians or Mexicans. When stratified by Lauren classification, we found that the IL-8 251 allele TA and AA polymorphism was significantly associated with the diffuse type of gastric cancer (TA versus TT: OR = 1.448, 95% CI: 1.177–1.720; AA versus TT: OR = 1.586, 95% CI: 1.128–2.044). The IL-8 251 AA genotype was found to be a risk factor for cardiac gastric cancer (AA versus TT: OR = 1.840, 95% CI: 1.112–2.568) but not for noncardiac gastric cancer.
Conclusions
This meta-analysis suggested that IL-8 251 allele A>T polymorphism might be a risk factor for gastric cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer (GC) is one of the most common cancers worldwide and the second leading cause of cancer death [1, 2]. There is substantial geographic variation in the incidence of gastric cancer internationally, with higher rates in Asia and some parts of South America [1]. GC is one of the most prevalent malignant tumors in China, where 38% of worldwide cases occur every year [2]. Although prominent improvements in early diagnosis of and synthesized therapy for GC have been reported, many patients are not treated before the disease advances because of the lack of specific symptoms early in the disease course [3]. Being the second leading cause of tumor death, GC has become a public health priority [4]. A major strategy for tackling this health care problem is to identify individuals at risk and hence contribute to prevention and early detection of this disease [3, 4]. Knowledge of molecular pathology mechanism of GC might lead to a new and hopefully more effective means of controlling this lethal disease.
Persistent inflammation could cause cellular damage and proliferation, which might be associated with tumor progression [5]. It is clear that sustained cell proliferation in tissue with abundant inflammatory cells, cell factors, activated stroma, angiogenesis, and DNA-damaging agents would provide necessary conditions for tumor formation and progression; for example, gastric mucosa would progress to carcinoma from chronic gastritis [6]. As a member of the α-chemokine family and a chemoattractant of neutrophils and lymphocytes, IL-8 plays an important role in progression of inflammation [7]. It has been reported that expression of IL-8 in GC specimens was significantly higher than in corresponding normal gastric mucosa [8]. IL-8 is a potent angiogenic factor in tumor invasion and metastasis [9]. Therefore, it is reasonable to deduce that IL-8 plays a certain role in the formation and progression of gastric tumor. At present, the molecular mechanism of GC is still relatively unclear; single-nucleotide polymorphism (SNP) could be used as a tool to detect the genetic variation of the disease gene and protect individuals with susceptibility to the disease. Whether SNP of IL-8 is associated with increased risk of GC and how it affects prognosis remain unclear. Recently, a study suggested that SNPs of IL-8 251 allele located at the promoter sequence of IL-8 gene (4q13–q21) might be associated with tumorigenesis of gastric cancer [10]. There are also some other studies focused on the relationship of GC with three genotypes of IL-8 251 allele: IL-8 251 AA, IL-8 251 AT, and IL-8 251 TT [11–20]. However, these studies reported conflicting results.
Therefore, in the present study, we aim to provide a quantitative estimation of various studies and explain their diversity by means of meta-analysis.
Meta-analysis is a powerful tool for summarizing the results from different studies by producing a single estimate of the major effect with enhanced precision. One of the major advantages of meta-analysis is to increase sample size, which may reduce the probability that random error will produce false-positive or false-negative associations [21].
Materials and Methods
Literature Search Strategy
Published studies that focused on IL-8 polymorphism and GC were reviewed. A literature search for papers published from the years 1970 to 2009 on human subjects was retrieved using PubMed and the Cochrane Library. The search was restricted to English-language papers. The following key search terms were used: “gastric” or “stomach,” “cancer” or “carcinoma,” “interleukin-8,” and “polymorphism.” The reference lists of reviews and retrieved articles were reviewed for other relevant publications at the same time. Abstracts or unpublished reports were not considered.
Inclusion and Exclusion Criteria
The inclusion criteria of these studies were as follows: (1) The type of study was case–control or cohort study; (2) The number of GC cases or controls studied was provided; (3) Frequencies of individuals by IL-8 genotype (IL-8 251 AA, IL-8 251 AT, IL-8 251 TT) were provided for cases or controls; (4) Characteristics of individuals including race, population source, age, etc. were provided; and (5) Cases were histologically diagnosed. The exclusion criteria were as follows: (1) The study was on animals; (2) The study was a case report, review, descriptive or qualitative research; (3) The study was not about polymorphism; and (4) fewer than ten participants were involved in the study.
Data Extraction
All data were carefully extracted from all eligible publications independently by two of the authors based on the inclusion criteria. Studies were appraised for quality using the Critical Appraisal Skills programme including sample size, participant characteristics at baseline and on completion, attrition rates and reporting, and the application of controls or multiple measures. Disagreement was resolved by discussion. The following information was extracted from the studies: chief researcher, nation, study design and period, statistical methods, population, number of GC cases and controls studied, and study results.
Statistical Analysis
Statistical manipulations were conducted using Stata statistical software, release 9.0 (Stata Corporation, College Station, TX; 2005).
Firstly, a heterogeneity test was performed to assess the null hypothesis that all studies were evaluating the same effect by testing Cochran’s Q statistic. P < 0.05 was taken to indicate heterogeneity across studies. A fixed-effects model was used if the studies exhibited homogeneity. When the test found significant study heterogeneity, the random-effects model was used for meta-analysis and it was assumed that the studies were a random sample of a hypothetical population of studies. Pooled estimate of OR was obtained by the Dersimonian-Laird method in the random-effects model. Pooled OR in the meta-analysis was performed by weighting each individual OR with the inverse of its variance.
Publication bias was tested by Egger’s test, a linear regression approach. The standard normal deviate (SND), defined as the odds ratio divided by its standard error, was regressed against the estimate’s precision, the latter being defined as the inverse of the standard error, using a regression equation of: SND = a + b × precision [22]. If the line passed through the origin at standard normal deviate zero (a = 0) without significance (P > 0.1), this would suggest that publication bias did not exist; otherwise it existed.
Results
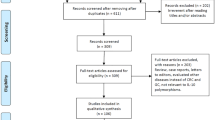
Characteristics of Eligible Studies (Fig. 1)
About 432 papers relevant to the search terms were identified. By screening, 400 of these articles were excluded because they were not about polymorphism. Full texts of the remaining 32 articles were reviewed and 20 of them were excluded (18 were not about polymorphism or GC, 1 contained too few cases, and 2 were reviews), leaving 10 studies for full publication review. So, ten papers [11–20] with 2,195 GC cases and 3,505 controls, including three population-based and seven hospital-based studies, were included in the meta-analysis. These studies were carried out in Asia [11–15, 17], Europe, and Mexico [16, 18–20]. Characteristics of the studies included in this meta-analysis are presented in Table 1.
Quantitative Data Synthesis
The combined OR of AA/AT versus TT was not significant (OR = 1.229, 95% CI: 0.943–1.514). The combined result of AA versus TT genotype of IL-8 251 allele to GC was found to be significant (OR = 1.363, 95% CI: 1.199–1.527), with adjustment factor D of 0.11; TA versus TT genotype of IL-8 251 allele to GC was not significant, with adjustment factor D of 0.42 (Table 2). Weights of the ten studies in meta-analyses of TA versus TT or AA versus TT had no significant difference.
When stratified by race, the combined result of AA versus TT genotype of IL-8 251 allele to GC in Asians was significant (OR = 1.593, 95% CI: 1.013–2.173). No significance was found in AA versus TT or TA versus TT genotype of IL-8 251 allele to GC of Caucasians and Mexicans (Table 2). Stratifying by tumor location, we only found that AA versus TT genotype of IL-8 251 allele to cardiac GC was significant (OR = 1.840, 95% CI: 1.112–2.568; Table 2). Significant differences were found for TA versus TT and AA versus TT genotype of IL-8 251 allele to diffuse GC (OR = 1.448, 95% CI: 1.177–1.720; OR = 1.586, 95% CI: 1.128–2.044, respectively; Table 2).
Some combinations were not examined, as indicated in Table 2, because the results of those studies of the same subtype were coordinate with each other. Only two combinations were fixed-effects models with homogeneity between studies of the same subtype: TA versus TT genotype of IL-8 251 allele to noncardiac GC or GC of Caucasians and Mexicans. The other combinations were random-effects models with heterogeneity.
Egger’s test was performed to test if publication bias existed according to the combined results with significance. For the overall ten studies and for the four studies of Asians of AA versus TT genotype of IL-8 251 allele to GC, the intercepts a of the regression lines were −0.138 and −0.854, respectively (P all >0.1); for the four studies of cardiac GC of AA versus TT genotype of IL-8 251 allele, the intercept a of the regression line was −0.695 (P > 0.1); for the four studies of diffuse GC of TA versus TT and AA versus TT genotype of IL-8 251 allele, the intercepts a of the regression lines were −2.699 and 0.313, respectively (P all >0.1).
Discussion
It is well known that there is individual susceptibility to GC even when exposed to the same environmental factors. Therefore, genetic factors must also play an important part in pathogenesis of GC. Recently, a growing number of studies have suggested that SNPs of IL-8 251 allele, which is located at the promoter sequence of the IL-8 gene (4q13–q21), might be associated with GC tumorigenesis. However, the results are contradictory. To better understand the association of this polymorphism with GC susceptibility, pooled analysis with a large sample, subgroup analysis, and heterogeneity exploration is necessary.
In our meta-analysis, we found that presence of an A allele versus the TT homozygote was not a risk factor for GC, similar to the study of Lu et al. [23]. However, the combined result of AA versus TT genotype of IL-8 251 allele to GC was found to be significant (OR = 1.363, 95% CI: 1.199–1.527), which suggested that those subjects with IL-8 251 AA genotype had a risk 1.363 times that of subjects with IL-8 251 TT genotype. This would be related to the fact that presence of IL-8 251 AA at the transcription start site exerts great influence on IL-8 production, and increased transcriptional activity of the IL-8 promoter was confirmed in an in vitro assay [7].
Subgroup meta-analyses were conducted on race, cancer location, and Lauren classification at the same time. In fact, six studies were conducted among Asians, whereas the other studies were conducted among Caucasians and Mexicans. When stratified by race, we found that the pooled effect of studies of Asian origin reported significant association between the IL-8 251 AA polymorphism and risk for development of GC. However, the pooled effect of studies on Europeans showed conflicting results, the reasons for which might be the following: (1) There were differences of genetic background among Asians and other races. Junko Fujiharaf reported that Japanese population exhibits interprefecture differences in IL-8 251 A>T polymorphism as well as a certain genetic heterogeneity in the worldwide distribution of IL-8 251 A>T polymorphism and that Asians have higher frequencies of IL-8 251 T allele [24]. (2) The genes interacted with human behavior: Asian diets are high in salt and nitrates and low in milk, which are risk factors for development of GC [1, 4]. (3) Association studies of the same disease are often inconsistent in their findings, and the first study to report an association often indicates a stronger effect than subsequent studies. This could lead to a distorted impression of the genetic etiology underlying a given disease [25]. Thus, it is suggested that future research should be designed with an ethnically different population. When stratified by location, we found that IL-8 251 AA was a risk factor for cardiac GC. Meta-analysis was not done for noncardiac GC because none of the results in those studies were significant. Gastric cardiac adenocarcinoma (GCC) differed from gastric noncardia cancer (GNCC) in that it rose more proximally in the stomach and was associated with younger age at presentation [1, 26]. This meta-analysis hinted that the upper part of stomach might be different from the lower part of stomach in terms of the tumorigenesis process and that IL-8 might be involved in this progress. When stratified by Lauren classification, we found that the IL-8 251 allele A>T polymorphism was significantly associated with the diffuse type of GC. The gastric mucosa progressed into carcinoma via gastric atrophy, intestinal metaplasia, and adenomatous dysplasia, leading to intestinal carcinoma or via hyperplastic into diffuse-type carcinoma, starting from chronic inflammation [27]. IL-8 was a strong activator of neutrophils which infiltrate the mucosa leading to atrophy, and the atrophy of gastric mucosa was associated with GC [27]. Ohyauchi [14] proposed that IL-8 251 allele A transcription was activated more strongly. Thus, IL-8 251 allele A was associated with the intestinal type. However, our results indicated that the IL-8 251 AA genotype was associated with higher risk of diffuse type.
When we explore the association of IL-8 SNPs and GC, the following might be considered: (1) IL-8 was just one of the cytokines taking part in progression of inflammation. Other cytokines might also be involved in tumorigenesis of GC as well. Moreover, they could either magnify or conceal the real effect of IL-8. (2) We just studied SNPs of IL-8 251 allele, but there are other SNPs sites of IL-8 such as the IL-8 +396 allele, the IL-8 +781, and several alleles on the IL-8 receptor [19]. Polymorphism of these alleles might be associated with activity, ligand binding, and subsequent signaling of IL-8. To further explore the role of IL-8 in the tumorigenesis of GC, studies on IL-8 polymorphism other than 251 allele variants are needed. (3) Human behavior or environment factors are associated with human diseases. For example, human behavior might be affected by human genes. Individuals with IL-8 251 TT genotype were more frequent among current smokers (52.5%), former smokers (51.0%), and ever smokers (51.9%) than among never smokers [28]. However, cigarette smoking is an established risk factor for GC [1, 4]. According to the announcement of Hidemi Ito, individuals with IL-8 251 AA allele might consume fewer cigarettes. Thus, smoking might have masked the effects of gene polymorphism.
It should be noted that there are some limitations to this study. Firstly, we were not able to obtain information about presence or absence of history of infection with Helicobacter pylori in most studies. Secondly, the availability of gene-environment interaction data on gastric cancer is limited [7]. There were no synchronous questionnaires designed in these studies and we were not able to obtain details about the subjects in terms of alcohol or cigarette consumption, diet, etc., which would eventually lead to identification of population subgroups at greater risk of gastric cancer because of concomitant environmental coexposure [7]. Lastly, not all researchers used Lauren classification of GC or reported tumor stage, thus we were not able to obtain all of the information we needed.
In spite of the limitations listed above, our meta-analysis suggests that IL-8 251 AA polymorphism is associated with susceptibility to GC. However, a study with a large sample of different ethnic groups should be considered in future associated studies to confirm our results. In addition, further evaluation of the effect of gene–gene and gene–environment interactions on IL-8 251 AA polymorphism and GC susceptibility is necessary.
References
Terry MB, Gaudet MM, Gammon MD. The epidemiology of gastric cancer. Semin Radiat Oncol. 2002;12:111–127.
Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153–156.
Smith RA, Cokkinides V, Eyre HJ. American cancer society guidelines for the early detection of cancer, 2006. CA Cancer J Clin. 2006;56:11–25.
Hohenberger P, Gretschel S. Gastric cancer. Lancet. 2003;362:305–315.
Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545.
Macarthur M, Hold GL, EI-Omar EM. Inflammation and cancer II. Role of chronic inflammation and cytokine gene polymorphism in the pathogenesis of gastrointestinal malignancy. Am J Physiol Gastrointest Liver Physiol. 2004;286:G515–G520.
Gianfagna F, De Feo E, van Duijn CM, Ricciardi G, Boccia S. A systematic review of meta-analyses on gene polymorphisms and gastric cancer risk. Curr Genomics. 2008;9(6):361–374.
Kitadai Y, Haruma K, Sumii K, et al. Expression of interleukin-8 correlates with vascularity in human gastric cancers. Am J Pathol. 1998;152:93–100.
Koch AE, Polverini PJ, Kunkel SL, et al. Interleukin-8 as a macrophage derived mediator of angiogenesis. Science. 1992;258:1798–1801.
Kamali-Sarvestani E, Bazargani A, Masoudian M, Lankarani K, Taghavi AR, Saberifiroozi M. Association of H pylori cagA and vacA genotypes and IL-8 gene polymorphism with clinical outcome of infection in Iranian patients with gastrointestinal diseases. World J Gastroenterol. 2006;12(32):5205–5210.
Savage SA, Abnet CC, Mark SD, et al. Variants of the IL8 and IL8RB genes and risk for gastric cardia adenocarcinoma and esophageal squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2004;13:2251–2257.
Lee WP, Tai DI, Lan KH, et al. The 251 T allele of the interleukin-8 promoter is associated with increased risk of gastric cancer featuring diffuse-type histopathology in Chinese population. Clin Cancer Res. 2005;11:6431–6441.
Lu WL, Pan KF, Zhang L, Lin DX, Miao XP, You WC. Genetic polymorphism of interleukin (IL)-1B, IL-1RN, IL-8, IL-10 and tumor necrosis factor alpha and risk of gastric cancer in a Chinese population. Carcinogenesis. 2005;26:631–636.
Ohyauchi M, Imatani A, Yonechi M, et al. The polymorphism interleukin 8–251 A/T influences the susceptibility of Helicobacter pylori related gastric diseases in the Japanese population. Gut. 2005;54:330–335.
Taguchi A, Ohmiya N, Shirai K, et al. Interleukin-8 promoter polymorphism increases the risk of atrophic gastritis and gastric cancer in Japan. Cancer Epidemiol Biomarkers Prev. 2005;14:2487–2493.
Kamangar F, Abnet CC, Hutchinson AA, et al. Polymorphism in inflammation-related genes and risk of gastric cancer (Finland). Cancer Causes Control. 2006;17:117–125.
Shirai K, Ohmiya N, Taguchi A, et al. Interleukin-8 gene polymorphism associated with susceptibility to non-cardiac gastric cancer with micro satellite instability. J Gastroenterol Hepatol. 2006;21(7):1129–1135.
Savage SA, Hou L, Lissowska J, Chow WH, Zatonski W, Chanock SJ. Yeager M Interleukin-8 polymorphism are not associated with gastric cancer risk in a polish population. Cancer Epidemiol Biomarkers Prev. 2006;15:589–591.
Garza-Gonzalez E, Bosques-Padilla FJ, Mendoza-Ibarra SI, et al. Thr399Ile and interleukin-8 -251 polymorphism in the risk for the development of distal gastric cancer. BMC Cancer. 2007;7:70.
Canedo P, Castanheira-Vale AJ, Lunet N, Pereira F, et al. The interleukin-8–251*T/*A polymorphism is not associated with risk for gastric cancer development in a Portuguese population. Eur J Cancer Prev. 2008;17(1):28–32.
Hardy CJ, Palmer BP, Muir KR, Sutton AJ, Powell RJ. Smoking history, alcohol consumption, and systemic lupus erythematosus: a case–control study. Ann Rheum Dis. 1998;57:451–455.
Harris T, Creed F, Brugha TS. Stressful life events and Graves’ disease. Br J Psychiatry. 1992;161:535–541.
Lu Y, Wang ZD, Shen J, Xu YC. Meta-analysis on the relationship between IL8–251 gene polymorphism and gastric cancer. Zhonghua Yu Fang Yi Xue Za Zhi. 2007;41(Suppl.):39–42.
Fujihara J, Shiwaku K, Yasuda T, et al. Variation of interleukin 8 −251 A>T polymorphism worldwide populations and intra-ethnic differences in Japanese populations. Clin Chim Acta. 2007;377:79–82.
Ioannidis JP, Ntzani EE, Trikalinos TA, Contopoulos-Ioannidis DG. Replication validity of genetic association studies. Nat Genet. 2001;29:306–309.
Wang LD, Zheng S, Zheng ZY, et al. Primary adenocarcinomas of lower esophagus, esophagogastric junction and gastric cardia: in special reference to China. World J Gastroenterol. 2003;9:1156–1164.
Correa P. Human gastric carcinogenesis: a multistep and multifactorial process—first American cancer society award lecture on cancer epidemiology and prevention. Cancer Res. 1992;52:6735–6740.
Ito H, Matsuo K, Hamajima N, et al. Significant association of interleukin 8-251T/A polymorphism with smoking behavior in a Japanese population. J Hum Genet. 2005;50:567–573.
Acknowledgments
This research was supported by the Humane and Social Sciences Research Fund, Education Department of Anhui Province (reference: 2009sk192zd), Cademic Leader Foundation of Anhui Medical University, and Doctor’s Scientific Research Foundation of Anhui Medical University. At the same time, thanks are due to Dr. De bin Wang for help in the revision of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, J., Pan, HF., Hu, YT. et al. Polymorphism of IL-8 in 251 Allele and Gastric Cancer Susceptibility: A Meta-Analysis. Dig Dis Sci 55, 1818–1823 (2010). https://doi.org/10.1007/s10620-009-0978-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-009-0978-y