Abstract
Interleukin-6 (IL-6) is a multifunctional cytokine, which plays a vital role in inflammation as well as tumorigenesis. Several studies have demonstrated that the association of IL6 -174 G/C (rs1800795) and -572 G/C (rs1800796) promoter polymorphisms influences transcription and has been found to trigger the risk of gastric tumor advancement with inconsistent and controversial result. The present study aims at collecting eligible articles through extensive search in PubMed, MEDLINE, and Embase databases. Additionally, the analysis also included 15 case–control investigations. MetaGenyo web tool was used to perform the meta-analysis. No substantial association was observed between IL6 polymorphisms and GC. In conclusion, our study signifies that polymorphisms of IL6, -174 G/C, and -572 G/C are not linked with GC risk.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
10.1 Introduction
Inflammation is an essential innate immune response induced by microbial infection and tissue damage. Numerous studies have provided a wide range of clinical evidence that chronic inflammation is linked with elevated risk of Gastric cancer (GC) (Greten and Grivennikov 2019; Multhoff et al. 2011; Bockerstett and DiPaolo 2017). Cytokines are a wide range of small proteins secreted by immune cells, including nucleated cells and work as an intracellular messenger in the immune system (Lowry 1993). Cytokine is a key mediator of diagnosis and treatment during inflammation in many diseases (Verma et al. 2016). The potential association between multifunctional cytokines and GC has been examined by several investigators. Among those, Interleukin 6 (IL-6) is known to function as both a pro-inflammatory cytokine as well as an anti-inflammatory myokine regulator (Tanaka et al. 2014). IL-6 belongs to a family of pleiotropic cytokine and modulates cell proliferation and differentiation. Previous data has demonstrated that IL-6 level was increased in mucosa, which leads to the inflammatory microenvironment in Helicobacter pylori (H. pylori) related gastritis (Yamaoka et al. 1996). Further, overexpression of IL6 is strongly associated with an increased risk of GC development and progression (Madej-Michniewicz et al. 2015). Therefore, based on the earlier reports, IL-6 is closely linked to occurrence and development of cancer.
The gene coding for IL6 is located on chromosome 7p21 and comprises 184 amino acids, which fold as a 4 alpha-helix bundle structure (Choy and Rose-John 2017). Understanding the genetic diversity with population genetic structure of IL6 will aid in predicting tumor risk as well as in reducing mortality. To date, several single nucleotide polymorphisms (SNPs) have been identified in the promoter region of IL6 (Terry et al. 2000). Among them, IL6 -174 G/C (rs1800795) and -572 G/C (rs1800796) are the most widely studied polymorphisms in several cancers including GC. However, previous investigations have yielded varying results regarding the relationship between IL6 promoter polymorphisms and gastric cancer (Chakraborty et al. 2017; Markkula et al. 2014). It could be because of the insignificant sample size, variations in genotyping methods, and ethnicity of the populations. In order to assess the precise role of IL6 promoter polymorphism on GC susceptibility, we have conducted this meta-analysis of all existing case–control studies.
10.2 Methods
10.2.1 Study Selection Strategy
To evaluate the relation between IL6 promoter polymorphisms and the risk of GC, all potentially pertinent articles were searched and identified according to the PRISMA guidelines (Liberati et al. 2009). Pubmed, Web of Science, and EMBASE Database were searched using the following keywords: Interleukin-6 and gastric cancer, IL6, IL6 -174 G/C, rs1800795, -572 G/C, and rs1800796. The last search was executed on 26 April 2020.
10.2.2 Literature Inclusion and Exclusion Criteria
Two investigators selected eligible studies independently. Studies that met the following criteria were included in this meta-analysis: (1) case–control study on GC and IL6 promoter polymorphisms; (2) genotypes available for calculating odds ratio (OR) and 95% confidence interval (CI). The exclusion criteria for this meta-analysis were as follows: (1) studies with no specific control group; (2) non-availability of genotype data. The quality evaluation of all eligible studies and data extraction of information was made with consensus and the discrepancy between investigators was resolved by cross-checking the data. From each paper, name of the first author, publication year, country and ethnicity of the participants, genotypes in cases and control subjects were collected and documented in Table 10.1.
10.2.3 Statistical Analysis
The strength of association between IL6 polymorphism (-174 G/C and -572 G/C) and GC was assessed for all studies. The crude ORs and their corresponding 95% confidence interval (CI) limits were calculated. The presence of heterogeneity was evaluated with the Cochran’s Q test and inconsistency I2 statistics. Based on the extent of heterogeneity, fixed effects model (FEM) or random effects model (REM) were adopted for pooled analysis. The association between IL6 polymorphisms and GC was analyzed in dominant, recessive, and allelic genetic models. To assess the robustness of the study, sensitivity analysis was performed by overlooking each study one time and estimating the Odd Ratio (OR) for the remaining studies. Publication bias was measured by the use of a funnel plot and Egger’s test. MetaGenyo web tool was used to perform the meta-analysis (Martorell-Marugan et al. 2017).
10.3 Results
10.3.1 Characteristics of Published Studies
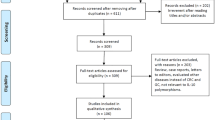
Our systematic literature search identified 436 articles. Based on the inclusion and exclusion criteria, unrelated or duplicate studies were excluded by reading titles and abstracts. Ninety-six relevant articles were selected for further assessment and 71 studies were consequently excluded after reading the full text to avoid discrepancy. Finally, 15 case–control studies fulfilled our study criteria (Fig. 10.1). Out of which, IL6 -174 G/C genotypes were extracted from thirteen papers (Dos Santos et al. 2019; Attar et al. 2017; Ramis et al. 2017; Sampaio et al. 2015; Pohjanen et al. 2013; Yong et al. 2010; Crusius et al. 2008; Deans et al. 2007; Gatti et al. 2007; Kamangar et al. 2006; Xing et al. 2006; El-Omar et al. 2003; Hwang et al. 2003). The IL6 -572 G/C genotypes were extracted from six papers (Dos Santos et al. 2019; Xing et al. 2006; Hwang et al. 2003; Martínez-Campos et al. 2019; Kang et al. 2009). Hwang et al. paper has analyzed samples from two ethnicities, hence it is considered as two studies (Hwang et al. 2003). The genotype distributions and main characteristics of studies are presented in Table 10.1. For IL6 -174 G/C, the heterogeneity test indicated significant heterogeneity between studies (CG+CC vs. GG: Pheterogeneity <0.001, I2 = 72%), but no heterogeneity was observed between studies of IL6 -572 G/C (CG+CC vs. GG: Pheterogeneity = 0.232, I2 = 27%) (Table 10.2).
10.4 Quantitative Data Synthesis
To explore the correlation between IL6 promoter polymorphisms and the risk of GC, 15 studies of IL6 -174 G/C polymorphism (1311 cases/ 2855 control), and six studies of IL6 -572 G/C polymorphism (631 cases /666 controls) were used. Meta-analysis of IL6 -174 G/C polymorphism and GC is documented in Fig. 10.2a, which did not reveal significant association between IL6 -174 G/C polymorphism and gastric cancer in the allelic model (C vs. G; OR = 0.96, 95% CI: 0.74–1.24, P = 0.738), recessive model (CC vs. GC+GG; OR = 0.95, 95% CI: 0.77–1.16, P = 0.584), and dominant models (CG+CC vs. GG; OR = 1.01, 95% CI: 0.69–1.48, P = 0.960). The pooled effect estimates presented in Fig. 10.2b shows that IL6 -572 G/C is not associated with GC in allelic model (C vs. G; OR = 0.99, 95% CI: 0.82–1.18, P = 0.872), recessive model (CC vs. GC+GG; OR = 1.11, 95% CI: 0.74–1.66, P = 0.627), and dominant models (CG+CC vs. GG; OR = 0.94, 95% CI: 0.74–1.19, P = 0.611).
10.4.1 Sensitivity Analysis and Publication Biases
Sensitivity analysis was carried out with pooled effect estimates by omitting each study one time to evaluate the stability of the outcomes. The outcomes of sensitivity analysis presented in Fig. 10.3 suggest that no single study could influence the pooled ORs of IL6 -174 G/C and IL6 -572 G/C polymorphisms. Visual inspection of Begg’s funnel plots did not show asymmetry for both IL6 -174 G/C and IL6 -572 G/C polymorphisms (Fig. 10.4a, b) indicating that there is no publication bias. The same was confirmed by Egger’s test p values (P > 0.05).
10.5 Discussion
Despite recent progress in clinical practice, GC remains the third most common cancer-related death worldwide. According to current data, in 2017, more than 1.22 million new cases of GC occurred and nearly 8,65,000 patients have died due to GC (Russi et al. 2019; Etemadi et al. 2020). To date, the exact causes of GC still remain unknown. Nevertheless, it has been proven that cytokines play a role in inflammation, and can also induce cell transformation in the development of cancer and chemoresistance (Conlon et al. 2019; Verma et al. 2020). Interleukins are low-molecular-weight cytokine expressed by leukocytes and are involved in normal functioning of the immune system. Further, disruptions of interleukins level may lead to immune deficiencies and tumorigenesis (Larsen et al. 2018). Subsequently, it has been reported that some mutations in interleukin genes lead to increased risk of GC development (Wang et al. 2014).
To date, several case–control studies have explored the association between IL6 -174 G/C and IL6 -572 G/C polymorphism on the susceptibility to GC. However, small sample sizes, different genotyping methods, and variation in minor allele frequencies across ethnicities leads to the lack of consistency in results. Therefore, we have performed the present meta-analysis to precisely study the association of IL6 polymorphism with GC risk. In this comprehensive meta-analysis we have observed that the IL6 -174 G/C and IL6 -572 G/C polymorphisms are not significantly associated with the risk of GC. The results of this meta-analysis are consistent with the results of previous meta-analysis in which no association between GC risk and IL6 -174 G/C (Jafari-Nedooshan et al. 2019; Yunxia Liu et al. 2018; Wang et al. 2018, 2012) or IL6 -572 G/C (Wang et al. 2018, 2012; Peng et al. 2018; Du et al. 2015) was documented. However, some meta-analyses have demonstrated increased GC risk for IL6 -174 G/C (Wang et al. 2018; Tian et al. 2015) or IL6 -572 G/C (Liu et al. 2018) in Asian populations.
In conclusion, our study indicates that the IL6 -174 G/C and IL6 -572 G/C polymorphisms are not correlated with GC risk. Soon, a large population based case–control studies would be potentially needed for validation of Interleukin 6 gene association with GC risk.
Abbreviations
- GC:
-
Gastric cancer
- IL-6:
-
Interleukin 6
- H. pylori :
-
Helicobacter pylori
- NLM:
-
National Library of Medicine
- SNPs:
-
Single nucleotide polymorphisms
- CBLD:
-
Chinese Biomedical Literature Database
- HWE:
-
Hardy–Weinberg equilibrium
- P value:
-
Probability value
- OR:
-
Odds ratio
References
Attar M, Mansoori M, Shahbazi M (2017) Interleukin-6 genetic variation and susceptibility to gastric cancer in an Iranian population. APJCP 18(11):3025–3029
Bockerstett KA, DiPaolo RJ (2017) Regulation of gastric carcinogenesis by inflammatory cytokines. Cell Mol Gastroenterol Hepatol 4(1):47–53
Chakraborty B, Vishnoi G, Gowda SH, Goswami B (2017) Interleukin-6 gene-174 G/C promoter polymorphism and its association with clinical profile of patients with multiple myeloma. Asia Pac J Clin Oncol 13(5):e402
Choy E, Rose-John S (2017) Interleukin-6 as a multifunctional regulator: inflammation, immune response, and fibrosis. J Scleroderma Relat Disord 2(2_suppl):S1–S5
Conlon KC, Miljkovic MD, Waldmann TA (2019) Cytokines in the treatment of cancer. J Interf Cytokine Res 39(1):6–21
Crusius JB, Canzian F, Capella G, Pena AS, Pera G, Sala N et al (2008) Cytokine gene polymorphisms and the risk of adenocarcinoma of the stomach in the European prospective investigation into cancer and nutrition (EPIC-EURGAST). Ann Oncol 19(11):1894–1902
Deans C, Rose-Zerilli M, Wigmore S, Ross J, Howell M, Jackson A et al (2007) Host cytokine genotype is related to adverse prognosis and systemic inflammation in gastro-oesophageal cancer. Ann Surg Oncol 14(2):329–339
Dos Santos MP, Sallas ML, Zapparoli D, Orcini WA, Chen E, Smith MAC et al (2019) Lack of association between IL-6 polymorphisms and haplotypes with gastric cancer. J Cell Biochem 120(6):9448–9454
Du Y, Gao L, Zhang K, Wang J (2015) Association of the IL6 polymorphism rs1800796 with cancer risk: a meta-analysis. Genet Mol Res 14(4):13236–13246
El-Omar EM, Rabkin CS, Gammon MD, Vaughan TL, Risch HA, Schoenberg JB et al (2003) Increased risk of noncardia gastric cancer associated with proinflammatory cytokine gene polymorphisms. Gastroenterology 124(5):1193–1201
Etemadi A et al (2020) The global, regional, and national burden of stomach cancer in 195 countries, 1990-2017: a systematic analysis for the Global Burden of Disease study 2017. Lancet Gastroenterol Hepatol 5(1):42–54
Gatti LL, Burbano RR, Zambaldi-Tunes M, de Lábio RW, de Assumpção PP, de Arruda Cardoso-Smith M et al (2007) Interleukin-6 polymorphisms, Helicobacter pylori infection in adult Brazilian patients with chronic gastritis and gastric adenocarcinoma. Arch Med Res 38(5):551–555
Greten FR, Grivennikov SI (2019) Inflammation and cancer: triggers, mechanisms, and consequences. Immunity 51(1):27–41
Hwang IR, Hsu PI, Peterson LE, Gutierrez O, Kim JG, Graham DY et al (2003) Interleukin-6 genetic polymorphisms are not related to Helicobacter pylori-associated gastroduodenal diseases. Helicobacter 8(2):142–148
Jafari-Nedooshan J, Dastgheib SA, Kargar S, Zare M, Raee-Ezzabadi A, Heiranizadeh N et al (2019) Association of IL-6 -174 G>C polymorphism with susceptibility to colorectal cancer and gastric cancer: a systematic review and meta-analysis. Acta Med 62(4):137–146
Kamangar F, Abnet CC, Hutchinson AA, Newschaffer CJ, Helzlsouer K, Shugart YY et al (2006) Polymorphisms in inflammation-related genes and risk of gastric cancer (Finland). Cancer Causes Control 17(1):117–125
Kang JM, Kim N, Lee DH, Park JH, Lee MK, Kim JS et al (2009) The effects of genetic polymorphisms of IL-6, IL-8, and IL-10 on Helicobacter pylori-induced gastroduodenal diseases in Korea. J Clin Gastroenterol 43(5):420–428
Larsen KM, Minaya MK, Vaish V, Peña MMO (2018) The role of IL-33/ST2 pathway in tumorigenesis. Int J Mol Sci 19(9):2676
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 151(4):W65–W94
Liu Y, Xu Y, Wang Y, Yao Y, Yang J (2018) Associations between interleukin gene polymorphisms and the risk of gastric cancer: a meta-analysis. Clin Exp Pharmacol Physiol 45(12):1236–1244
Lowry SF (1993) Cytokine mediators of immunity and inflammation. Arch Surg 128(11):1235–1241
Madej-Michniewicz A, Budkowska M, Sałata D, Dołęgowska B, Starzyńska T, Błogowski W (2015) Evaluation of selected interleukins in patients with different gastric neoplasms: a preliminary report. Sci Rep 5:14382
Markkula A, Simonsson M, Ingvar C, Rose C, Jernstrom H (2014) IL6 genotype, tumour ER-status, and treatment predicted disease-free survival in a prospective breast cancer cohort. BMC Cancer 14:759
Martínez-Campos C, Torres-Poveda K, Camorlinga-Ponce M, Flores-Luna L, Maldonado-Bernal C, Madrid-Marina V et al (2019) Polymorphisms in IL-10 and TGF-β gene promoter are associated with lower risk to gastric cancer in a Mexican population. BMC Cancer 19(1):453
Martorell-Marugan J, Toro-Dominguez D, Alarcon-Riquelme ME, Carmona-Saez P (2017) MetaGenyo: a web tool for meta-analysis of genetic association studies. BMC Bioinf 18(1):563
Multhoff G, Molls M, Radons J (2011) Chronic inflammation in cancer development. Front Immunol 2:98
Peng X, Shi J, Sun W, Ruan X, Guo Y, Zhao L et al (2018) Genetic polymorphisms of IL-6 promoter in cancer susceptibility and prognosis: a meta-analysis. Oncotarget 9(15):12351–12364
Pohjanen VM, Koivurova OP, Makinen JM, Karhukorpi JM, Joensuu T, Koistinen PO et al (2013) Interleukin 6 gene polymorphism -174 is associated with the diffuse type gastric carcinoma. Genes Chromosomes Cancer 52(10):976–982
Ramis IB, Vianna JS, Gonçalves CV, von Groll A, Dellagostin OA, da Silva PEA (2017) Polymorphisms of the IL-6, IL-8 and IL-10 genes and the risk of gastric pathology in patients infected with Helicobacter pylori. J Microbiol Immunol Infect 50(2):153–159
Russi S, Verma HK, Laurino S, Mazzone P, Storto G, Nardelli A et al (2019) Adapting and surviving: intra and extra-cellular remodeling in drug-resistant gastric cancer cells. Int J Mol Sci 20:15
Sampaio AM, Balseiro SC, Silva MR, Alarcão A, d'Aguiar MJ, Ferreira T et al (2015) Association between IL-4 and IL-6 expression variants and gastric cancer among Portuguese population. GE Port J Gastroenterol 22(4):143–152
Tanaka T, Narazaki M, Kishimoto T (2014) IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol 6(10):a016295
Terry CF, Loukaci V, Green FR (2000) Cooperative influence of genetic polymorphisms on interleukin 6 transcriptional regulation. J Biol Chem 275(24):18138–18144
Tian G, Mi J, Wei X, Zhao D, Qiao L, Yang C et al (2015) Circulating interleukin-6 and cancer: a meta-analysis using Mendelian randomization. Sci Rep 5:11394
Verma H, Mishra H, Khodiar PK, Patra PK, Bhaskar LV (2016) NOS3 27-bp and IL4 70-bp VNTR polymorphisms do not contribute to the risk of sickle cell crisis. Turk J Haematol 33(4):365–366
Verma HK, Falco G, Bhaskar LVKS (2020) Molecular signaling pathways involved in gastric cancer chemoresistance. In: Raju GSR, Bhaskar LVKS (eds) Theranostics approaches to gastric and colon cancer. Springer, Singapore, pp 117–134
Wang J, He W, Liu J, Nong L, Wei Y, Yang F (2012) Association of IL-6 polymorphisms with gastric cancer risk: evidences from a meta-analysis. Cytokine 59(1):176–183
Wang XQ, Terry PD, Cheng L, Yan H, Wang JS, Wu WA et al (2014) Interactions between pork consumption, CagA status and IL-1B-31 genotypes in gastric cancer. World J Gastroenterol 20(25):8151–8157
Wang X, Yang F, Xu G, Zhong S (2018) The roles of IL-6, IL-8 and IL-10 gene polymorphisms in gastric cancer: a meta-analysis. Cytokine 111:230–236
Xing P, Xiao D, Zeng Q, Gao W, Wang Y, Wang H (2006) Relationship between cytokine gene polymorphisms on development and clinical characteristics of gastric adenocarcinoma. Chin J General Surg 15:659–663
Yamaoka Y, Kita M, Kodama T, Sawai N, Imanishi J (1996) Helicobacter pylori cagA gene and expression of cytokine messenger RNA in gastric mucosa. Gastroenterology 110(6):1744–1752
Yong Z, Ying X, Feng G, Xin Z, Feng S (2010) Relativity research on the association between the interleukin-6 gene polymorphisms and risk of gastric cancer in Wuwei Area of Gansu Province. Clin J Med Officers 1:23199
Yunxia Liu YX, Wang Y, Yao Y, Yang J (2018) Associations between interleukin gene polymorphisms and the risk of gastric cancer: a meta-analysis. Clin Exp Pharmacol Physiol 45(12):1236–1244
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 The Editor(s) (if applicable) and The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Verma, H.K., Merchant, N., Bhaskar, L.V.K.S. (2020). Association Between IL6 Gene Polymorphisms and Gastric Cancer Risk: A Meta-Analysis of Case-Control Studies. In: Vadde, R., Nagaraju, G.P. (eds) Immunotherapy for Gastrointestinal Malignancies. Diagnostics and Therapeutic Advances in GI Malignancies. Springer, Singapore. https://doi.org/10.1007/978-981-15-6487-1_10
Download citation
DOI: https://doi.org/10.1007/978-981-15-6487-1_10
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-6486-4
Online ISBN: 978-981-15-6487-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)