Abstract
Using orally administered sucrose as a probe of gastrointestinal permeability, this study focused on determining whether Barrett’s metaplasia exhibits a paracellular transepithelial leak to small nonelectrolytes. Subjects in five separate classes (nonendoscoped, asymptomatic controls; endoscoped, asymptomatic controls; gastroesophageal reflux disease without mucosal complications; grossly visible esophagitis; and Barrett’s esophagus) consumed a sucrose solution at bedtime and collected all overnight urine. Urine volume was measured and sucrose concentration was determined by high-performance liquid chromatography. Patients with Barrett’s were observed to exhibit a transepithelial leak to sucrose whose mean value was threefold greater than that seen in healthy control subjects or patients with reflux but without any mucosal defect. A parallel study of claudin tight junction proteins in endoscopy biopsy samples showed that whereas Barrett’s metaplasia contains dramatically more claudin-2 and claudin-3 than is found in normal esophageal mucosa, it is markedly lower in claudins 1 and 5, indicating very different tight junction barriers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
It has been known for more than 30 years that TJs are altered in transformed and tumor epithelia [1]. Attenuation of TJs is observed in urinary bladder carcinoma and murine mammary adenocarcinoma [2, 3]. TJ strand abnormalities have been seen in human thyroid carcinomas [4]. Poorly differentiated colon adenocarcinomas were observed to have the diminished structural organization seen in fetal colon TJs [5]. M-methyl-N′-nitro-N-nitrosoguanidine (MNNG)-induced gastric cancers and precancerous gastric lesions in rats exhibit disappearance of apical TJs [6]. Decreased numbers of TJ strands have been observed in transitional carcinoma of urinary bladder [7]. TJ disorganization and diminishment was observed in freeze fracture electron microscopy of hepatocellular carcinomas [8]. TJs of human colon adenocarcinomas and certain adenomatous polyps are known to be leaky to electron dense probes [9]. Precancerous colons of mice treated with chemical carcinogens exhibit decreased transepithelial barrier function as measured by transepithelial electrical impedance [10]. A similar pattern of decreased impedance has been observed in inflamed esophageal tissue, and even lower impedance is seen in dysplastic esophageal tissue [11]. In Crohn’s disease, one has human colon tissue at increased risk of cancer onset likewise associating with increased TJ permeability to paracellular probes [12, 13].
Processes and agents known to augment the promotional stage of cancer (e.g., tumor promoting phorbol esters) induce TJ leakiness [14]. Transforming growth factor-alpha is observed to override the induction of TJ formation by glucocorticoids in mammary tumor cells [15]. Oncogene mutations are likewise known to induce TJ leakiness [16–18]. SV-40 virus exposure induces TJ leakiness and down-regulation of the TJ protein, occludin [19]. HGF/scatter factor has been observed to have similar effects [20].
Down-regulation of TJ proteins is common in many tumors. Whereas the epithelial to mesenchymal transition that typifies epithelial neoplasia occurs rather late in the process, decreases of TJ proteins, such as ZO-1, occur earlier [21]. Occludin expression decreases progressively with the increasing carcinoma grade in human endometrial cancer. A similar phenomenon occurring with TJ proteins in colon cancer (e.g., loss of claudin-1 [22]) may have a bearing on the dispersal of carcinoembryonic antigen in colon tumor cells from its normally polarized distribution [23, 24]. Occludin distribution and expression is altered in increasing Gleason grades of prostate cancer [25]. Poorly differentiated adenocarcinomas of the GI tract exhibit reduced occludin expression [26]. Occludin expression is sharply decreased or otherwise altered in hydatiform moles of the placenta [27]. Microvessels of human brain tumors, the leaks in whose TJs are responsible for cerebral edema in certain types of brain cancer, are excellent examples of down-regulation of occludin in cancer [28, 29].
The claudin family of TJ proteins behaves similarly, but in certain tumors, certain claudins can undergo dramatic up-regulation as well. An emerging picture of TJ structure at the molecular level is one of homotypic and heterotypic interactions among occludin and claudin molecules [30]. Simply the mere alteration in proportions of specific TJ proteins, up or down, will disrupt the balance involved in the “weave” of TJ proteins and TJ fibrils. The result may be aberrant pores (in size, charge, and/or number) in the paracellular pathway. Claudin-1 is lost in many tumor microvessels of human glioblastoma multiforme [31] and also seems to be frequently lost or down-regulated in breast tumors, leading to speculation as to its potential role as a tumor suppressor gene [32], a consideration earlier considered for the TJ protein, ZO-1 [33]. However claudin-1 expression has been observed to be up-regulated in certain colorectal cancers compared with adjacent normal mucosa [34]. Claudin-1 is expressed in benign perineuriomas but is absent in (the more serious) fibromyxoid sarcomas, dermatofibrosarcoma protuberans, and desmoplastic fibroblastoma [35]. Claudin-7 is reduced in invasive ductal carcinoma of the breast [36]. Claudins-3 and -4 are overexpressed in some ovarian cancers and claudin-16 is uniquely expressed in certain ovarian tumors—not found in normal ovarian tissue [37]. In summary, TJs are typically deranged, down-regulated, or simply lost during neoplasia, the process starting early in the progression, with the typical result being a functional compromise of normal TJ barrier effectiveness.
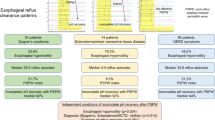
Esophageal adenocarcinoma is one of the fastest rising cancers in the United States and has a 5-year mortality rate exceeding 90% [38, 39]. It is believed to arise out of a disease progression beginning with chronic gastroesophageal reflux disease (GERD), progressing to esophagitis, then to Barrett’s metaplasia, then to Barrett’s with dysplasia, and finally to adenocarcinoma [40]. In Barrett’s esophagus (BE), the normal stratified squamous epithelium of the esophagus is displaced by a columnar epithelium with an apparent high dysplastic potential. This typically occurs in the distal esophagus, arising from the gastroesophageal junction [41]. Whereas the risk of esophageal adenocarcinoma in the general population is only 1 in 10,000, it rises to 1 in 200 in patients with BE, a 50-fold increase [42, 43]. Our hypothesis in this study was that Barrett’s epithelium, a paradigm “precancerous epithelium,” would constitute a region of high paracellular leak across the otherwise highly efficient epithelial barrier of normal esophagus.
Materials and methods
Study population
With the recruitment phase ending in February 2004, 34 outpatients scheduled for upper endoscopy and 13 nonendoscoped control subjects were enrolled in our study. Patients with a previous known history of GERD, dysphagia, or dyspepsia with or without BE were enrolled by gastroenterologists in a teaching community hospital in suburban Philadelphia, PA. They were seen in referral and given appropriate therapy. Patients selected for this study were given medically necessary upper endoscopic examinations. All enrolled subjects gave informed consent and the study was approved by the Main Line Health (Lankenau Hospital) institutional review board committee.
After appropriate endoscopy with biopsies, patients were placed in one of five diagnostic classes: 1) BE, 2) esophagitis, 3) GERD without mucosal abnormality, 4) endoscoped controls (hiatal hernia, Schatzki’s ring, or negative examination), or 5) nonendoscoped healthy control subjects. Patients with observed gastritis or any duodenal abnormalities were excluded from the study. Diagnosis of BE was confirmed by a staff pathologist on the basis of identification of goblet cell metaplasia in hematoxylin and eosin-stained sections, followed by confirmation with Alcian blue staining. These classes are shown in Tables 1 and 2 along with variables pertinent to outcome.
During endoscopic examination, diagnostic esophageal biopsies were taken in four quadrants at 1-cm intervals with standard biopsy forceps and placed immediately in formalin for later paraffin embedding and hematoxylin and eosin and Alcian blue staining. Additional biopsy samples for study purposes were placed in cell lysis buffers with inhibitors of proteolysis as described below.
Criteria for enrollment exclusion were: diabetes mellitus, previous gastric or esophageal surgery, known active gastric or duodenal mucosal ulceration, younger than aged 18 years, abdominal pain, gastrointestinal bleeding, weight loss, or intractable nausea and vomiting.
Sucrose permeability testing
With no net charge and a molecular weight of only 360, sucrose is a workable probe of TJ permeability [44]. Sucrose has already been shown to be a useful probe for gastric ulcer disease that grossly compromises the gastric barrier (by denuding portions of gastric mucosa) [45] and has been used for detecting gastric cancer [46]. Sucrose has only negligible affinity for transcellular transport pathways. Its only significant route across the gastroesophageal barrier is paracellular diffusion. Sucrose in the GI lumen—even the 100 g dose used in this study—actually ceases to exist in the small intestinal lumen because of microvillar hydrolases, thus it can only enter the bloodstream by transepithelial diffusion at sites proximal to this organ. Any sucrose that does diffuse into the bloodstream is quantitatively filtered into urine because sucrose is neither reabsorbed nor metabolized by the kidney. Sucrose appearing in the urine is then analyzed by HPLC as described [45].
At least 14 days after endoscopy, patients and healthy control subjects consumed in their homes a chilled solution of 100 g of sucrose in 200 ml of water containing 5 g of a citric acid-based flavoring agent at bedtime. After 14 days, it is unlikely that sites of previous biopsies could leak or even be identified [47]. An 8-hr overnight urine sample was collected in a container with 5 ml of 10% thymol in isopropanol (as an antimicrobial). Total urine volume was measured and recorded. The concentration of sucrose in the urine sample was then measured by high-performance liquid chromatography (HPLC) after previous desalting of the urine sample by anion and cation exchange resins [45]. Total amount of sucrose in the urine in milligrams was determined by multiplying the urine volume in milliliters by the sucrose concentration in milligrams per milliliter.
Salivary amylase
As a potential endogenous probe of upper gastrointestinal leak, the activity of the saliva enzyme, salivary amylase (SA), was measured in both saliva and blood. The hypothesis was that an uppergastrointestinal leak to sucrose also might permit the paracellular diffusion of SA. At the time of the patient’s endoscopic examination, before administration of anesthesia, a 5-ml blood sample was withdrawn from the patient’s antecubital vein, collected in serum separator tubes, and centrifuged after clotting. The resulting serum supernatant was then analyzed for both pancreatic amylase and total amylase by standard colorimetric methods using monoclonal antibodies to inhibit salivary amylase (ARUP Laboratories, Salt Lake City, UT). The activity of SA in the blood sample was then determined by the difference of total amylase and the pancreatic form. A sputum sample also was obtained at this time by having the patient expectorate before anesthesia administration. Sputum was diluted 1:1000 in 1% bovine serum albumin in phosphate-buffered saline and this solution was likewise assayed for total (salivary) amylase activity (MainLine Clinical Labs, Wynnewood, PA).
Esophageal biopsy sampling and tight junction protein analyses
During the endoscopic examination, in addition to biopsy samples taken for routine histology, three biopsy samples were taken from apparent BE regions and placed immediately in buffers at 4°C. A set of three also was taken from a region of grossly normal esophageal mucosa 2- to 3-cm above the upper limit of the Barrett’s region. Samples were placed immediately in a detergent-free extraction buffer at 4°C with appropriate protease and phosphatase inhibitors [48]. Samples were immediately frozen in a dry ice/methanol bath and stored at −70°C until subsequent protein analysis. Esophageal biopsy samples were not collected into a total lysis buffer as described previously [50] because dilution of the TJ proteins in a total lysate was found to obscure the detection of certain low abundance TJ proteins. Sample extraction involved sonication followed by centrifuging the samples in the above sample buffer at 39,000 rpm for 60 min in a Beckman Ti50 rotor at 4°C. The resulting supernatant comprised proteins freely soluble in the cell and was termed the “cytosolic” fraction. The resulting pellet was resuspended in the above buffer now containing 1% Triton X-100. After subsequent centrifugation as above, the resulting supernatant comprised proteins that were loosely membrane-associated and was termed the “membrane-associated” fraction. The resulting pellet was resuspended in an extraction buffer containing 1% NP-40, 0.1% sodium dodecylsulfate, and 0.1% sodium deoxycholate. After centrifugation, this final supernatant comprised proteins that were strongly lipid-bound or in complexes with other cellular proteins, and was termed the “cytoskeletal” fraction. Samples of each of these three fractions were then analyzed for various members of the claudin family of TJ proteins by PAGE in a Novex Mini Cell Apparatus (Invitrogen, Carlsbad, CA) and Western immunoblot analyses using a Novex XCELL II blotting module. Chemiluminescent-labeled immunoblots were placed against reflection autoradiography film (Kodak, Rochester, NY) and developed in a Kodak M35A X-OMAT processor. For quantitation of Western immunoblots, a Molecular Dynamics Personal Densitometer SI was used.
Statistics
All comparisons were made between nonendoscoped controls and each of the subject groups. All P values are two-sided. Sucrose and age comparisons were performed by using the Wilcoxon rank-sum procedure to avoid problems associated with the raw data distributions and with multiple comparisons using the same control group. Sucrose statistical findings were confirmed using Dunnet t comparisons within a one-way ANOVA performed in logs. Medians are reported because this is the central tendency metric for the Wilcoxon test. Comparisons for gender distributions used Fisher’s exact test.
Materials
Antisera to claudins-1, -2, -3, -4, and -5 were obtained from Zymed, Inc (South San Francisco, CA). Antisera to claudins-1 and -3 were polyclonals; antisera to -2, -4, and -5 were monoclonals. Secondary antibodies conjugated to horseradish peroxidase, in conjunction with Western Lightning® chemiluminescence reagents (Perkin Elmer, Inc.), were used for chemiluminescent detection of protein bands. Reagent chemicals were a product of Fisher Biosciences, Inc. Total amylase activity assays were performed by MainLine Clinical Labs, Inc. Assays for specifically salivary amylase were performed by ARUP Laboratories.
Results
A total of 47 subjects were enrolled. Grouped by disease class their demographic characteristics are shown in Table 1 and clinical parameters pertinent to their conditions in Table 2. Reflecting the community base of the hospital at which recruitment was performed, the great majority of patients in this study were white; only 4% were non-white. Age ranges for each group were similar, as was gender distribution. Tobacco use was higher in the BE group than the simple GERD group or either control group. Where present, esophagitis severity was uniformly low, when present at all, with all Los Angeles scores for the esophagitis class in the A–B range. The overall patient population tended to be on proton pump inhibitor (PPI) therapy at the time of their sucrose permeability test and endoscopy (13/13 patients with BE were taking proton pump inhibitors at the time of the test). The distribution of long vs short segment (short-segment BE defined as <3 cm) BE was approximately even with six long- and seven short-segment patients enrolled. A positive result in Alcian blue staining was the criterion used for all assignments to the BE group.
The BE group (mean value) manifested a near threefold greater leak of sucrose than either control group (which were themselves nearly identical regarding sucrose leak characteristics) (Fig. 1; Table 3). In addition the BE group exhibited a wide range of sucrose leak values for individual subjects. BE patients with the greatest sucrose leak were fivefold to sevenfold greater than the mean sucrose leak values of the two control groups, or the GERD-only group. The first control group was a “healthy control” volunteer group with no history of upper or lower gastrointestinal disease or previous gastrointestinal surgery but did not undergo upper endoscopy. The second control group presented to gastroenterologists for mild dyspepsia or vague gastrointestinal symptoms but did not exhibit typical GERD symptoms or any mucosal abnormality. Perhaps the equally important finding is that the patient group with GERD, showing no observable mucosal pathology during upper endoscopy, exhibited a sucrose leak whose mean was nearly identical to the two control groups, differing only in a slightly increased range of sucrose leak values compared with these two groups. This indicates that GERD per se does not result in an elevated transmural leak to sucrose nor is GERD directly contributing to the elevated sucrose leak that we observe in BE. The esophagitis group showed a sucrose leak whose mean value was intermediate between that of the GERD-only and the BE groups.
The control groups showed no effect of age on sucrose leak (Fig. 2). A similar relationship, showing again no correlation, was seen when the sucrose leaks of the subjects in the BE group were plotted as a function of their age. We concluded that age was not an issue in the results obtained.
Sucrose is—for the purposes of this test—an exogenous probe that is introduced into the lumen of the upper gastrointestinal tract to measure transmural (transepithelial) leak within those organs. While conducting these studies, we attempted to assess whether there may be an endogenous probe that could perform a similar function. We chose for this purpose the enzyme, salivary amylase (SA), which is secreted into the proximal lumen of the upper gastrointestinal tract, by the parotid glands in the oral cavity. SA exists in much higher concentrations in the GI lumen than it does in the antiluminal fluid compartment (vasculature). It fulfills one characteristic that one may wish to see in a probe. It, however, is much larger than sucrose (mw 342), being a 55 kDa protein. Although it is nearly net neutral in charge at normal pH, it is not a nonelectrolyte in the sense of sucrose, because it can carry charged amino acid and carboxyl residues over its surface.
As described in Methods, SA content was measured in sputum samples and serum samples for the majority of subjects enrolled in the study, and data were analyzed for possible differences among the various classes. We did not observe a significant difference between nonscoped control subjects and the BE group, regarding their levels of serum SA, as shown in Fig. 3. Likewise a difference in the ratio of serum SA/sputum SA was not observed for these two different patient classes (data not shown). We conclude that SA is not a useful probe for transmural leak in BE, perhaps because of its size, surface charges, or even its more complicated clinical chemistry, in that unlike sucrose, it is subject to ongoing clearance in the liver or proteolytic digestion in the plasma or other body space.
Comparison of the serum salivary amylase activity between nonendoscoped control subjects and the BE group. Results for individual subjects are shown, the same subjects whose sucrose leak values are reported in Fig. 1. Serum salivary amylase activity was determined as described in Materials and Methods. The two groups were found to not be statistically different
Western immunoblot comparison of TJ proteins in Barrett’s mucosa vs adjacent normal esophageal epithelium. As described in Materials and Methods, biopsies of Barrett’s mucosa and adjacent normal mucosa were fractionated into cell soluble (S), membrane-associated (M), and cytoskeletal-associated (C) samples. Results are shown for one BE patient, and for five separate claudin proteins (claudin-1, -2, -3, -4, -5)
The fact that a threefold greater sucrose leak is observed in BE does not necessarily mean that BE is the source of the transmural leak that we observe in BE patients. As stated earlier, sucrose (with its lack of membrane transport proteins in eukaryotic cells) has only the paracellular pathway to cross the upper gastrointestinal epithelium, i.e., across the epithelial TJ and through the intercellular space, unless there is a large scale break in the epithelial barrier, as in frank ulcerations. The latter possibility is rendered less likely by the seeming inability of SA to cross the epithelial barrier even in the BE population. This rationale prompted the first ever characterization of the TJ proteins of the epithelia of BE vs adjacent normal (stratified squamous) epithelium.
As described in Methods, biopsies were taken during upper endoscopy of the BE region and then 3-cm proximal to the highest margin of the BE region. Even the normal biopsies were always from distal esophagus, because our greatest length of Barrett’s segment was 7 cm (from the Z line). These biopsies were then analyzed by Western immunoblot for different TJ proteins. In an earlier report we showed that although occludin content was surprisingly similar on a per milligram total protein basis between BE and normal esophagus, claudins-1 and -2 were different [49]. Those analyses were hampered somewhat by the low abundance of certain junctional proteins (e.g., claudin-2) and our methods were changed to focus on levels of expression of these junctional proteins in various subcellular fractions rather than a total cell lysate, thereby enriching for these proteins and facilitating their detection.
Figure 4 reports the results for claudins-1 through -5. Each had a mirror image relationship between their content in BE and that in adjacent normal epithelium. Although this does not prove that the BE barrier is leaky, it does indicate that the TJs of the BE barrier are fundamentally and dramatically different from the TJs of normal esophageal epithelium. Although claudins-1 and -5 are abundant in normal esophagus, they are nearly undetectable in BE mucosa (Fig. 4). On the other hand, whereas claudins-2 and -3 may not be present in normal esophageal mucosa, they were readily detectable in BE. Only claudin-4 was easily detected in both, although uniformly greater in abundance in normal esophagus. When these results were quantitated by densitometry for five separate patients with BE (always comparing BE vs normal mucosa for the same individual patient), there was enormous statistical difference between BE and normal mucosa for claudins-1, -2, -3, and -5 (Fig. 5). Only claudin-4 did not exhibit a statistical difference (P < 0.05), although a trend was evident that claudin-4 was lower in BE (P=0.09).
Summary of differences in claudin expression between Barrett’s mucosa and adjacent normal mucosa for BE patients. As described in Materials and Methods, the chemiluminescent film results (Fig. 4) for individual patients were quantitated in a Molecular Dynamics densitometer. Results shown above represent the relative expression of claudins-1 to -5 in Barrett’s mucosa relative to the adjacent normal mucosa. Claudin-1 for example showed an 80% lower expression in BE than in normal mucosa. The extremely higher levels of expression of claudins-2 and -3 in Barrett’s mucosa relative to normal mucosa, reflect their essential undetectability in normal mucosa. Error bars indicate the standard error of the mean for n=6 patients selected at random from our BE cohort. Differences between Barrett’s and normal mucosa were statistically significant for claudins-1, -2, -3, and -5 (P < 0.01, Student t-test, equal variances). *225-fold increase in BE; **36-fold increase in BE
Discussion
In the late 1980s, an elegantly simple, inexpensive test was proposed for screening patients with gastric ulcer disease [45]. The test consisted of patients consuming a concentrated solution of sucrose followed by an overnight urine collection and determination of the amount of sucrose in the urine sample. Because mammalian cells lack a disaccharide transport protein, sucrose cannot be transported by carrier mediation across an epithelial cell barrier. Its only means of transiting an epithelial barrier, such as the upper gastrointestinal tract, would be to diffuse across a breach in the barrier itself, such as a laceration or ulceration. Once gaining access to the bloodstream, sucrose molecules (with normal renal function) would be quantitatively delivered into urine, because the kidney would be unable to reabsorb it because of the same lack of disaccharide transport proteins. Furthermore, the presence of hydrolayses on the microvillar surface of the duodenum means that any sucrose that did leak into the bloodstream must leak before the site of hydrolysis, indicating the site of the leak to be predominantly gastric or esophageal. The present study is an adaptation of this test to the detection of a leak not caused by an injury of the overall epithelium but by altered TJ permeability in a precancerous zone of epithelium. Radiolabeled carbohydrates, such as 14C-mannitol or 14C-polyethyleneglycol (similar to sucrose in their being physiologically inert by being neither metabolized by nor taken up into gastrointestinal cells), have been extensively used to screen for transepithelial leakiness on the molecular level, i.e., permeability across the strands of the TJ complex [50]. Previous observations from our group have shown that exposure to tumor promoting chemicals or mutation of oncogenes, as well as actual preneoplastic epithelium, all associate with a compromise of epithelial barrier function at that site, specifically alteration of the epithelial TJ (zonula occludens) complex itself with resultant increase in TJ and transepithelial permeability [9, 14, 48]. The underlying hypothesis in this study was that patients with BE will manifest increased solute permeability across the metaplastic epithelium of the distal esophagus.
This study has three core findings: 1) Barrett’s esophagus patients have a significant and dramatic transepithelial leak to at least small nonelectrolytes in their upper gastrointestinal tract; 2) reflux per se is not sufficient to generate this leak, because GERD patients who do not have mucosal abnormality do not manifest such leak; and 3) on a molecular level, the paracellular barrier in Barrett’s mucosa is structurally different than the paracellular barrier in normal esophageal mucosa.
Our finding of increased transepithelial leakiness in patients with BE is supported by a recent study that observed decreased electrical impedance across biopsies of Barrett’s tissue compared with normal stratified squamous esophagus [51]. This decreased impedance was moreover accentuated when dysplasia was evident in the tissue, a finding with important clinical significance. Although this finding relates strictly to ion (Na+ and Cl−) permeability through the paracellular pathways, it says that at least some type of increased transmural permeability is present in BE.
Our study is not focused on GERD-related permeability changes occurring in the esophageal mucosa as it transits from a stratified squamous epithelium to the columnar, intestinal-like epithelium in BE. It focuses instead on the end state only: the BE condition. The Orlando group has produced a series of studies on GERD-related changes in the stratified squamous esophageal epithelium, finding most notably that luminal acid and/or pepsin simultaneously produces increased rabbit esophageal transepithelial permeability to a range of dextran probes (up to 20 kDa) and decreased transepithelial electrical resistance while simultaneously causing the appearance of dilated intercellular spaces between cells of the stratum corneum [52, 53]. Similar findings were observed in human esophagus from patients with nonerosive reflux disease and the phenomenon of dilated intercellular spaces reversed with omeprazole treatment [54, 55]. With its patient-based approach, our present study cannot make the cellular or subcellular conclusions reached in these studies. However, based on available literature, except for possibly esophagitis, we can state that BE does not correlate with any pathology in the upper gastrointestinal tract other than the Barrett’s metaplasia itself. Our findings support this in that no other explanation for sucrose leak in patients with BE was identified. We have examined antrum biopsies of patients with BE and compared these to (nondiseased) antrum biopsies of patients who do not have BE. We have not seen any differences in histology, nor do we observe any differences in claudin protein composition that might underlie an altered antral epithelial TJ barrier in BE patients (data not shown).
It is noteworthy that in the course of this study, one patient was reclassified from the esophagitis category to the BE category. This patient was originally diagnosed as having esophagitis when upper endoscopy was first performed. At the request of the gastroenterologist, biopsy tissue samples were sent to a second histology laboratory where Alcian blue staining (not performed at the first laboratory) and/or sections being taken from a different end of the biopsy specimen, resulted in detection of intestinal metaplasia and the diagnosis being changed to BE. This patient’s sucrose leak score at the time of their initial diagnosis was high for the esophagitis group, 269 mg, but was midrange for the BE group. In a sense, that sucrose leak score became predictive for this one patient’s later reclassification to the BE group.
The potential clinical utility of the findings reported here may rest with the ability of sucrose to effectively screen for patients with BE among the general GERD population. If coupled with PPI therapy, the test may have economic and medical value for BE screening. A sucrose test taken before PPI therapy is begun, followed by a second test after 8 weeks of PPI therapy, should show reduction of esophagitis-related leak but no reduction of Barrett’s-associated leak. In the United States, the GERD population is enormous, with at least 20 million PPI prescriptions written per year [56]. Such a large population cannot logistically be screened by the existing number of endoscopists, even if it were financially acceptable to do so. A patient with GERD without any mucosal damage or even mild esophagitis does not have a significantly increased cancer risk and can certainly have their symptoms treated by simply PPI therapy. However, the patient with GERD with BE has a 50-fold increased risk of cancer. A screening test that is safe, simple, and inexpensive may be valuable in that regard. The 90% 5-year mortality rate of esophageal adenocarcinoma suggests that one should not simply treat BE patients’ GERD symptoms with PPI therapy without regard to a screening endoscopy program. However, only 10% of first time presenting GERD patients may in fact have BE, and it would be economically valuable to know overall who does not need endoscopic examination. A sucrose leak test would never take the place of endoscopy but it may have utility as a screening tool to determine those in greatest need of endoscopy or in populations without access to endoscopy. The issue of sensitivity and specificity of this leak phenomenon as a possible future screening tool clearly needs to be addressed in a future study with increased numbers of subjects. An expanded study should indicate whether Barrett’s with known dysplasia evidences greater leak than Barrett’s without detected dysplasia, a finding that may be useful for risk assessment within BE.
A recent report concerning immunohistochemical detection of claudin proteins in BE [57] observed similar and dissimilar findings to the Western immunoblot data presented in this study. As shown by our data, claudin-3 was much more evident in BE, being nondetectable in normal esophageal mucosa. However, where we observed sharply less claudin-1 and claudin-4 in BE than in normal esophagus, their group observed moderate increases of claudin-1 product. Also unlike our results, they did observe staining product for claudin-2 in normal esophagus, and this was lower in BE. However, the claudin-2 staining product in normal esophagus was sometimes found intracytoplasmically and was generally granular. Our two groups both obtained claudin antibodies from Zymed, Inc., so differences in antibodies used is not the reason. The major, obvious difference is detection of claudins by Western immunoblot of cell homogenates vs. immunofluorescence of formalin-fixed samples. In this difference there is opportunity for differences in epitope recognition. Furthermore immunofluorescence shows recognition sites, but unlike Western immunoblot, cannot (by use of molecular weight standards) assign reaction product to a particular protein of interest.
The Western immunoblot data presented in Fig. 4 clearly shows that the TJs in Barrett’s metaplasia are extremely different structurally from the TJs of normal esophageal mucosa. This suggests that permeability of the paracellular pathway in Barrett’s metaplasia would be different from the paracellular permeability in normal esophagus, resulting in different barrier capacities for these two different epithelial tissues.
References
Martinez-Palomo A (1970) Ultrastructural modifications of intercellular junctions between tumor cells. In Vitro 6:15–20
Alroy J (1979) Ultrastructure of canine urinary bladder carcinoma. Vet Pathol 16:693–701
Robenek H, Schopper C, Fasske E, Fetting R, Themann H (1981) Structure and function of the junctional complement of spontaneous and transplanted muring mammary carcinomas. J Submicrosc Cytol 13:347–363
Kerjaschki D, Krisch K, Sleyter U, Umrath W, Jakesz R, Depisch D, Kokoschka R, Horandner H (1979) The structure of tight junctions in human thyroid tumors. A systematic freeze-fracture study. Am J Pathol 96:207–225
Polak-Charcon S, Shoham J, Ben-Shaul Y (1980) Tight junctions of epithelial cells of human fetal hindgut, normal colon and colon adenocarcinoma. J Natl Cancer Inst 65:53–57
Aoyagi K, Kohfiji K, Yano S, Murakami N, Hori H, Terasaki Y, Takeda J, Tanaka M, Shirouzu K (2000) Morphological change in the MNNG-treated rat gastric mucosa. Kurume Med J 47:31–36
Saito T (1984) Ultrastructural changes on the junctional complexes in the human urinary bladder carcinoma by thin sectioning and freeze fracture. J Clin Electron Micros 17:201–209
Swift JG, Mukherjee TM, Rowland R (1983) Intercellular junctions in hepatocellular carcinoma. J Submicrosc Cytol 15:799–810
Soler AP, Miller RD, Laughlin KV, Carp NZ, Klurfeld DM, Mullin JM (1999) Increased tight junctional permeability is associated with the development of colon cancer. Carcinogenesis 20:1425–1431
Davies R, Joseph R, Asbun H, Sedwitz M (1989) Detection of the cancer-prone colon, using transepithelial impedance analysis. Arch Surg 124:480–484
Gonzalez-Mariscal L, Betanzos A, Nava P, Jaramillo B (2003) Tight junction proteins. Prog Biophys Mol Biol 81:1–44
Hollander D (1988) Crohn’s disease: a permeability disorder of the tight junction. Gut 29:1621–1624
Soderholm JD, Olaison G, Peterson KH, Franzen LE, Lindmark T, Wiren M, Tagesson C, Sjodahl R (2002) Augmented increase in tight junction permeability by luminal stimuli in the non-inflamed ileum of Crohn’s disease. Gut 50:307–313
Mullin JM, Peralta Soler A, Laughlin KV, Kampherstein JA, Russo LM, Saladik DT, George K, Shurina RD, O’Brien TG (1996) Chronic exposure of LLC-PK1 epithelia to the phorbol ester TPA produces polyp-like foci with leaky tight junctions and altered protein kinase C-alpha expression and localization. Exp Cell Res 227:12–22
Guan Y, Woo P, Rubenstein N, Firestone G (2002) Transforming growth factor-alpha abrogates the glucocorticoid stimulation of tight junction formation and reverses the steroid-induced down-regulation of fascin in rat mammary epithelial tumor cells by a Ras-dependent pathway. Exp Cell Res 273:1–11
Chen Y, Lu Q, Schneeberger E, Goodenough DA (2000) Restoration of tight junction structure and barrier function by down-regulation of the mitogen-activated protein kinase pathway in ras-transformed Madin-Darby canine kidney cells. Mol Biol Cell 11:849–862
Li D, Mrsny RJ (2000) Oncogenic Raf-1 disrupts epithelial tight junctions via downregulation of occludin. J Cell Biol 148:791–800
Mullin JM, Leatherman JM, Valenzano MC, Huerta ER, Verrechio J, Smith DM, Snetselaar K, Liu M, Francis MK, Sell C (2005) Ras mutation impairs epithelial barrier function to a wide range of nonelectrolytes. Mol Biol Cell 16:5538–5550
Nunbhakdi-Craig V, Craig L, Machleidt T, Sontag E (2003) Simian virus 40 small tumor antigen induces deregulation of the actin cytoskeleton and tight junctions in kidney epithelial cells. J Virol 77:2807–2818
Jiang W, Martin T, Matsumoto K, Nakamura T, Mansel R (1999) Hepatocyte growth factor/scatter factor decreases the expression of occluding and transendothelial resistance (TER) and increases paracellular permeability in human vascular endothelial cells. J Cell Physiol 181:319–329
Blanco D, Vicent S, Elizegi E, Pino I, Fraga M, Esteller M, Saffiotti U, Lecanda F, Montuenga L (2004) Altered expression of adhesion molecules and epithelial-mesenchymal transition in silica-induced rat lung carcinogenesis. Lab Invest 84:999–1012
Resnick MB, Konkin T, Routhier J, Sabo E, Pricolo VE (2004) Claudin-1 is a strong prognostic indicator in stage II colonic cancer: a tissue microarray study. Mod Pathol 18(4):511–518
Tobioka H, Isomura H, Kokai Y, Sawada N (2002) Polarized distribution of carcinoembryonic antigen is associated with a tight junction molecule in human colorectal adenocarcinoma. J Pathol 198:207–212
Tobioka H, Isomura H, Kokai Y, Tokunaga Y, Yamaguchi J, Sawada N (2004) Occludin expression decreases with the progression of human endometrial carcinoma. Hum Pathol 35:159–164
Busch C, Hanssen T, Wagener C, O’Brink B (2002) Down-regulation of CEACAM1 in human prostate cancer: correlation with loss of cell polarity, increased proliferation rate, and Gleason grade 3 to 4 transition. Hum Pathol 33:290–298
Kimura Y, Shiozaki H, Hirao M, Maeno Y, Doki Y, Inoue M, Monden T, Ando-Akatsuka Y, Furuse M, Tsukita S, Monden M (1997) Expression of occludin, tight-junction-associated protein, in human digestive tract. Am J Pathol 151:45–54
Marzioni D, Banita M, Felici A, Paradinas FJ, Newlands E, De Nictolis M, Muhlhauser J, Castellucci M (2001) Expression of ZO-1 and occludin in normal human placenta and in hydatidiform moles. Mol Hum Reprod 7:279–285
Davies R (2002) Blood-brain barrier breakdown in septic encephalopathy and brain tumours. J Anat 200:639–646
Papadopoulos MC, Saadoun S, Woodrow CJ, Davies DC, Costa-Martins P, Moss RF, Krishna S, Bell BA (2001) Occludin expression in microvessels of neoplastic and non-neoplastic human brain. Neuropathol Appl Neurobiol. 27:384–395
Furuse M, Sasaki H, Tsukita S (1999) Manner of interaction of heterogeneous claudin species within and between tight junction strands. J Cell Biol 147:891–903
Liebner S, Kniesel U, Kalbacher H, Wolburg H (2000) Correlation of tight junction morphology with the expression of tight junction proteins in blood-brain barrier endothelial cells. Eur J Cell Biol 79:707–717
Kramer F, White K, Kubbies M, Swisshelm K, Weber BH (2000) Genomic organization of claudin-1 and its assessment in hereditary and sporadic breast cancer. Hum Genet 107:249–256
Willott E, Balda MS, Fanning AS, Jameson B, Van Itallie CM, Anderson JM (1993) The tight junction protein ZO-1 is homologous to the Drosophila discs-large tumor suppressor protein of septate junctions. Proc Natl Acad Sci USA 90:7834–7838
Miwa N, Furuse M, Tsukita S, Niikawa N, Nakamura Y, Furukawa Y (2000) Involvement of claudin-1 in the beta-catenin/Tcf signaling pathway and its frequent upregulation in human colorectal cancers. Oncol Res 12:469–476
Folpe A, Billings S, McKenney J, Walsh S, Nusrat A, Weiss S (2002) Expression of claudin-1, a recently described tight junction-associated protein, distinguishes soft tissue perineurioma from potential mimics. Am J Surg Pathol 12:1620–1626
Kominsky SL, Argani P, Korz D, Evron E, Raman V, Garrett E, Rein A, Sauter G, Kallioniemi OP, Sukumar S (2003) Loss of the tight junction protein claudin-7 correlates with histological grade in both ductal carcinoma in situ and invasive ductal carcinoma of the breast. Oncogene 22:2021–2033
Rangel LB, Agarwal R, D’Souza T, Pizer ES, Alo PL, Lancaster WD, Gregoire L, Schwartz DR, Cho KR, Morin PJ (2003) Tight junction proteins claudin-3 and claudin-4 are frequently overexpressed in ovarian cancer but not in ovarian cystadenomas. Clin Cancer Res 9:2567–2575
Brown LM, Devesa SS (2002) Epidemiologic trends in esophageal and gastric cancer in the United States. Surg Oncol Clin North Am 11:235–256
Eloubeidi MA, Homan RK, Martz MD, Theobold KE, Provenzale D (1999) A cost analysis of outpatient care for patients with Barrett’s esophagus in a managed care setting. Am J Gastroenterol 94:2033–2036
Fitzgerald RC (2005) Genetics and prevention of esophageal cancer. Rec Res Cancer Res 166:35–46
Sampliner RE (2005) Epidemiology, pathophysiology, and treatment of Barrett’s esophagus: reducing mortality from esophageal adenocarcinoma. Med Clin North Am 1989:293–312
Drewitz DJ, Sampliner RE, Garewal HS (1997) The incidence of adenocarcinoma in Barrett’s esophagus: a prospective study of 170 patients followed 4.8 years. Am J Gastroenterol 92:212–215
Shaheen NJ, Crosby MA, Bozymski EM (2000) Is there publication bias in the reporting of cancer risk of Barrett’s esophagus? Gastroenterology 119:333–338
Mullin JM, Kampherstein JA, Laughlin KV, Saladik DT, Peralta Soler A (1997) Transepithelial paracellular leakiness induced by chronic phorbol ester exposure correlates with polyp-like foci and redistribution of protein kinase C-alpha. Carcinogenesis 18:2339–2345
Meddings JB, Sutherland LR, Byles NI, Wallace JL (1993) Sucrose, a novel permeability marker for gastroduodenal disease. Gastroenterology 104:1619–1626
Kawabata H, Meddings JB, Uchida Y, Matsuda K, Sasahara K, Nishioka M (1998) Sucrose permeability as a means of detecting diseases of the upper digestive tract. J Gastroenterol Hepatol 13:1002–1006
Subramanyam K, Patterson M, Gourley WK (1985) Healing of endoscopic biopsy sites in the human rectum. J Clin Gastroenterol 7:266–268
Mullin JM, Agostino N, Rendon-Huerta E, Thornton JJ (2005) Keynote review: epithelial and endothelial barriers in human disease. Drug Discov Today 10:395–408
Rendon-Huerta E, Valenzano MC, Mullin JM, Trembeth SE, Kothari RH, Hameed K, Mercogliano G, Thornton JJ (2003) Comparison of three integral tight junction barrier proteins in Barrett’s epithelium versus normal esophageal epithelium. Am J Gastroenterol 98:1901
Munck BG, Rasmussen SN (1977) Paracellular permeability of extracellular space markers across rat jejunum in vitro. Indication of a transepithelial fluid circuit. J Physiol 271: 473–488
Gonzalez-Correa C, Brown B, Smallwood R, Stephenson T, Stoddard C, Bardhan K (2003) Low frequency electrical bioimpedance for the detection of inflammation and dysplasia in Barrett’s oesophagus. Physiol Meas 24:291–296
Tobey NA, Carson JL, Alkiek RA, Orlando RC (1996) Dilated intercellular spaces: a morphological feature of acid reflux-damaged human esophageal epithelium. Gastroenterology 111:1200–1205
Tobey NA, Hosseini SS, Argote CM, Dobrucali AM, Awayda MS, Orlando RC (2004) Dilated intercellular spaces and shunt permeability in nonerosive acid-damaged esophageal epithelium. Am J Gastroenterol 99:13–22
Calabrese C, Bortolotti M, Fabbri A, Areni A, Cenachi G, Scialpi C, Miglioli M, Di Febo G (2005) Reversibility of GERD Ultrastructural alterations and relief of symptoms after omeprazole treatment. Am J Gastroenterol 100:537–542
Villanacci V, Grigolato PG, Cestari R, Missale G, Cengia G, Klersy C, Rindi G (2001) Dilated intercellular spaces as markers of reflux disease: histology, semiquantitative score and morphometry upon light microscopy. Digestion 64:1–8
Locke GR, Talley NJ, Fett SL, Zinmeister AR, Melton LJ (1997) Prevalence and clinical spectrum of gastroesophageal reflux: a population-based study in Olmsted County, Minnesota. Gastroenterology 112:1448–1456
Gyorffy H, Holczbauer A, Nagy P, Szabo Z, Kupcsulik P, Paska C, Papp J, Schaff Z, Kiss A (2005) Claudin expression in Barrett’s esophagus and adenocarcinoma. Virchows Arch 447:961–968
Acknowledgments
The authors thank Spencer M. Free, Ph.D., for statistical methodologies and insights of study design. The cooperation of the scheduling secretaries and the nurses, patient care technicians, and anesthesiologists of the Main Line Gastroenterology Associates endoscopy facility is much appreciated. The authors thank Jennifer Swauger, Kathleen Ciavarelli, and Loretta Rossino for assistance in preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Support by the Cancer Research and Prevention Foundation, the Sharpe-Strumia Foundation, and the Mary L. Smith Foundation.
Rights and permissions
About this article
Cite this article
Mullin, J.M., Valenzano, M.C., Trembeth, S. et al. Transepithelial Leak in Barrett’s Esophagus. Dig Dis Sci 51, 2326–2336 (2006). https://doi.org/10.1007/s10620-006-9478-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-006-9478-5