Abstract
We performed a phase I pilot study to determine if autologous vaccine HSPPC-96 (gp96, Oncophage®) could be purified from completely resected pancreas adenocarcinomas, to determine patient tolerance of vaccine and to explore immune responses and clinical outcomes of these patients. Subjects were vaccinated with 5 μg of autologous HSPPC-96 weekly for 4 doses. Serial ELISPOT assays of T cells for antitumor reactivity were performed. Subjects received neither adjuvant chemotherapy nor radiation. Ten patients received a full course of vaccinations. No dose-limiting toxicities were encountered. Immediate freezing in liquid nitrogen of the tumor specimen resulted in improved vaccine yield. Median overall survival is 2.2 years (Kaplan–Meier estimate). Autologous anti-HSPPC-96 ELISPOT reactivity increased significantly in 1 of 5 patients examined and a second had an increase of unclear significance. Three of 10 treated patients are alive without disease at 2.6, 2.7, and 5.0 years follow-up. There was no observed correlation between immune response and prognosis. This study demonstrates the feasibility of preparing HSPPC-96 from pancreatic adenocarcinomas. Examination of this novel approach using multiple dose levels is 1 approach to further investigate the immunogenicity and clinical utility of HSPPC-96 vaccination in this setting.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreas adenocarcinoma affects approximately 31,860 people in the United States annually, with 32,180 estimated deaths from disease in 2005 [1]. Fewer than 5% of patients are able to undergo complete surgical resection of their tumor, owing to the high incidence of locally advanced and metastatic disease at the time of presentation [2, 3]. The number of patients undergoing full laparotomy is decreasing as a result of more careful perioperative staging by improved imaging and laparoscopy [4, 5]. The 5-year mortality for patients with pancreatic adenocarcinoma remains in excess of 95% [1, 6]. Despite better overall survival in the setting of resectable disease, the majority of such patients succumb to disease, with median survival of 11–20 months [7, 8] and 5-year survival of 10.2% [8].
Adjuvant radiation, chemotherapy, or both have been employed to attempt to improve patient survival [7, 9–12]. Although there is evidence of modest benefit in some studies, clinical benefit has not been consistently observed [12]. Accordingly, new modalities for eliminating microscopic residual disease are necessary, and studies involving anti-angiogenic and other new therapeutic agents are underway.
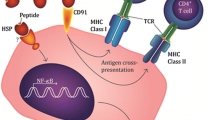
We chose to pursue an adjuvant strategy for pancreatic adenocarcinoma using a novel cancer vaccine based on autologous heat shock protein family member HSPPC-96 (Oncophage®) [13] in patients with completely resected primary tumors who received neither adjuvant radiation nor chemotherapy. The HSPPC-96 vaccine was initially identified in an effort to identify the cellular component responsible for tumor-specific T-cell-mediated immunity in rodents. In animal model systems, HSPPC-96 isolated from individual tumors elicits tumor immunity against the tumor from which the complex is derived, but not unrelated tumors.
The consistent identity between tumor rejection antigens purified from several tumors led to the demonstration that HSPPC-96 and other heat shock proteins serve as carrier molecules for tumor-specific peptides [14]. The heat shock protein itself is not the immunogen, but it acts as a chaperone or carrier of antigenic peptides, carrying a repertoire of cellular peptides for that particular tumor [13]. Pathways were subsequently identified for heat shock proteins that allow for their introduction into antigen-processing pathways that engender major histocompatibility class I-dependent tumor immunity [15, 16].
The HSPPC-96 vaccine is attractive as a treatment modality because it represents a carrier for a library of tumor antigens of the specific cancer, even if there is antigenic heterogeneity within the tumor. HSPPC-96 vaccines are undergoing clinical trials in a variety of cancers [17], including melanoma [18], colon cancer [19], renal cell carcinoma [20], and hematologic malignancies [21]. In this study, our objectives were to test if HSPPC-96 could be purified from this tissue rich in protease activity, to determine patient tolerance of vaccination, and to explore immune responses and clinical outcomes of these patients.
Methods
Patient selection
Patients with pancreatic adenocarcinoma undergoing complete resection at Memorial Sloan-Kettering Cancer Institute (New York, NY) were recruited and consented as part of an institutional review board-reviewed protocol. Five patients were vaccinated in this study between May 1997 and May 1999. The protocol was suspended in May 1999 owing to poor vaccine yield from tissue degradation. After a protocol amendment changing tissue handling, another 5 patients were vaccinated between January and May 2001.
Study design
Ten patients were sought for vaccination in this pilot study. Patients enrolled in this study met the following criteria: (1) resection of their pancreatic cancer at Memorial Sloan-Kettering Cancer Institute; (2) American Joint Committee on Cancer (AJCC; version 5) stage I, II, or III pancreatic adenocarcinoma; (3) Age >18 and able to give informed consent; (4) Karnofsky performance status ≥70%; (5) normal organ function, as characterized by creatinine ≤2 mg/dL, WBC ≥3,000/mm3, lymphocytes ≥700/mm3, and platelets ≥100,000/mm3; and (6) patients could not have received previous chemotherapy, radiation, or immunotherapy. Patients did not have significant heart disease, intercurrent illness, active bleeding, or active infection within 2 weeks of registration. They did not use immunosuppressive agents or have known immunodeficiency, and were not pregnant or lactating.
The schema for vaccination, skin testing, and follow-up is shown in Fig. 1. Patients gave written informed consent before their operation to completely resect their pancreas cancer. Approximately 1 g of tissue, not required for pathologic determination of the presence and extent of the pancreatic cancer, was dissected with an attending pathologist and shipped in cold sterile saline to a central production facility (Woburn, MA, USA) for vaccine preparation. Protease inhibitors were not used because of the concern of introducing substances into the vaccine preparation that are not of a quality sufficient for use in humans. Sections of the tumor mass were examined to confirm they contained tumor cells. Patients not having 1 g of available tumor were considered to have insufficient material for vaccine preparation. Vaccine was produced as previously described [22], vialed, and returned to Memorial Hospital for administration.
Patients signed consent again if it was possible to both completely resect the tumor and successfully prepare HSPPC-96 vaccine. Vaccine samples were prepared by Antigenics, Inc., in 0.5–1.0 mL of phosphate-buffered saline (PBS), and shipped and stored at –70°C or lower. Patients received 5-μg vaccinations of autologous tumor-derived HSPPC-96 intradermally, 1 week apart, for a total of 4 doses. Initially, it was not possible to generate larger amounts of vaccine from tumor samples. Therefore, all 10 patients received the same 5-μg dose in this phase I study. As a test for anergy, patients were sensitized with dinitrochlorobenzene (DNCB) and skin-tested with DNCB 4 and 8 weeks after sensitization.
Patients were seen for follow-up 4 weeks and 3 months following the completion of vaccination, and were followed for at least 2 years with restaging computed tomography scans every 3 months. Toxicity was assessed at each visit.
Because of tissue degradation, only 5 of the first 11 patients with sufficient tumor to make HSPPC-96 vaccine had successful production of vaccine. This led to an amendment in the protocol to change tumor handling. Before the amendment, tumor tissue was shipped on wet ice or in PBS on wet ice to the central processing facility. After the amendment, the portion of tumor used for vaccine production was dissected from the remainder of the resection specimen and snap frozen in liquid nitrogen. The specimen was then shipped on dry ice to the central production facility. After the protocol amendment, 5 of 5 patients with adequate tumor yield at surgery had successful production of HSPPC-96. Thus, in all, 10 patients started and completed the complete set of scheduled vaccinations.
Toxicity assessment
Patient toxicity was evaluated at each clinic visit using the National Cancer Institute Common Toxicity Criteria, version 2.0 (CTC v 2.0) and scores for mild/moderate/severe events. Adverse events were coded according to the MedDRA coding system (version 6.0).
Immune monitoring studies
Delayed-type hypersensitivity testing
Delayed-type hypersensitivity (DTH) testing was performed to determine if a subject had intact cellular immune responses before and after exposure to HSPPC-96 vaccine derived from autologous patient tumor. This was performed by first sensitizing the skin to a compound that produces protein-chemical conjugates recognized by the immune system, then injecting small amounts of the same antigen at a later time to determine if the subject maintained an immune response against the injected antigen.
In this case, DTH testing was performed using DNCB sensitization and skin testing (see Fig. 1). Patients were assessed for DTH responses at the time of DNCB sensitization (before the first vaccination), and at 4 and 8 weeks following the first vaccination. Patients were sensitized by applying 2 mg in 0.1 mL topically overnight, then later skin tested by subcutaneous administration of 25, 50, and 100 μg DNCB subcutaneously at week 4 and week 8 after the start of vaccinations, determining the extent of the reaction. DTH was measured as the maximum dimension of erythema or induration at the injection site 48 hr after skin testing. The subject was not tested with the highest dose(s) of DNCB if there was a brisk reaction to sensitization or skin testing.
CD8+ lymphocyte isolation
Patients underwent phlebotomy of 70–90 cc whole blood for assessment of peripheral blood lymphocyte ELISPOT responses before the first vaccination, and at weeks 1, 3, 5, and 8 after starting vaccinations. Peripheral blood mononuclear cells (PBMC) were obtained from heparinized whole blood by centrifugation on a Ficoll-Hypaque gradient (Pharmacia, Piscataway, NJ) using 50 mL Accuspin conical tubes (Sigma, St. Louis, MO). Cells were stored in 90% fetal calf serum (FCS)/10% dimethyl sulfoxide in aliquots of 5×106 cells per vial at –80°C or colder until use.
At the time of the testing for the ELISPOT assay, 2×107 PBMC were thawed, washed twice in RPMI without additives, and resuspended in ice-cold PBS/2% FCS for isolation of the total CD8+ subpopulation (which includes both memory and naïve T cells). CD8+ cells were purified using Dynabeads M-450 (Dynal, Lake Success, NY) following the manufacturer's instructions. Prior to the use of isolated lymphocytes, magnetic beads were removed with Detachabeads (Dynal) in accordance with the manufacturer's protocol. The purified population was >99% CD8+ after isolation. These are the mononuclear cells most likely to be responsible for any antitumor immunity, and were assayed for that activity using ELISPOT as noted.
Interferon-γ ELISPOT assay
The ELISPOT assay was used to determine the frequency of T cells in the peripheral blood that recognize HSPPC-96-associated autologous pancreas cancer antigens. It is expected that in a vaccinated patient the number of spots seen increases after successive vaccinations, and that the immune response wanes after discontinuing vaccinations. In this case, nitrocellulose 96-well plates (Millipore) were coated with 10 μg/mL anti-interferon-γ (IFN-γ; clone 1-D1K, Mabtech, Sweden) overnight at 4°C. Unbound antibody was removed by 3 washes with PBS. The membrane was blocked with 150 μL RPMI/10% human serum for 1 hr at 37°C. CD8+ effector cells were plated in triplicate at concentrations of 8–10×104 cells/well depending on the yield of CD8+ cells per experiment. The number of plated CD8+ cells/well (8–10×104) was identical throughout an assay.
CD8+ cells were then incubated with the following addition in a final volume of 100 μL: (1) medium alone, (2) autologous tumor suspension (5×104 cells/well), (3) autologous tumor membrane suspension (representing 5×106 cells/well), (4) medium plus anti-class I monoclonal antibody W6/32 (final concentration 10 μg/mL), (5) autologous tumor cell suspension plus W6/32, or (6) autologous tumor cell membrane suspension plus W6/32. All sets of experiments were performed twice, in triplicate. The W6/32 antibody blocks the ability of major histocompatibility (MHC) class I to interact with the T-cell receptor, and therefore does not allow a T cell to become activated and secrete IFN-γ. As a result, if the number of spots in an IFN-γ ELISPOT assay decreased comparing the no-antibody and W6/32 antibody samples, the response was T-cell-mediated through class I MHC molecules.
The tumor suspensions or tumor membranes in these experiments served as a source of antigen-MHC class I complexes in a physiologic context. The number of cells or cell membrane equivalents varied among patients depending on availability. Cell membranes were purified as described [23]. The anti-MHC class I antibody was added for determination of MHC class I restriction of responses. Plates were incubated for 20 hs at 37°C in a 5% CO2 incubator. Cells were removed by extensive washing with PBS/0.05% Tween 20, and 2 μg/mL biotinylated anti-IFN-γ antibody/well was added (clone 7-B6-1, Mabtech, Sweden), followed by a 2-hr incubation at 37°C. After extensive washing with PBS/0.05% Tween 20, 100 μL of avidin-peroxidase complex (Vector, Burlingame, CA) were added, and the plates incubated an additional hour at room temperature. Color development was performed using AEC (Sigma) as a substrate following manufacturer's instructions. Colorimetric reaction was stopped after 4 min, then plates were left to dry overnight. Spot counts were evaluated using an ELISPOT reader system with KS ELISPOT 4.1 software (Carl Zeiss Vision, Oberkochen, Germany).
Statistical considerations
Data analysis was exploratory in nature for this phase I pilot study. To determine the estimated overall survival of this population, a Kaplan–Meier product-limit estimate was generated using standard techniques [24]. One patient was lost to follow-up at 5.0 years with no evidence of disease; up-to-date follow-up data are available for the remaining 9 patients. We also performed a Kaplan–Meier product-limit estimate for patients in whom resection was feasible but vaccine yield was not sufficient or vaccine was found to be degraded (n=12).
Results
Patient characteristics
Patient characteristics are indicated in Table 1. Two patients had AJCC version 6 stage I disease, 2 had stage IIA disease, and 6 had stage IIB disease. All tumors were histologically conventional ductal adenocarcinomas of the head of the pancreas. Six women and 4 men were included in this study and vaccinated. The median age for patients vaccinated was 66.5 (range, 48–73) years. Median Eastern Cooperative Oncology Group performance status and range were 0 and 0–1, respectively. Patients did not receive radiation or chemotherapy as part of their adjuvant treatment.
The initial difficulty in accruing patients to vaccinate in this study and the subsequent improvement in the vaccine preparation method is demonstrated by the number of patients consented for resection in comparison to the actual number of patients vaccinated during the study. Fifty-seven patients were consented to the study before their operation. Of 44 consented between May 1997 and May 1999 before protocol amendment, 18 were found to be unresectable owing to locally advanced or metastatic disease found at the time of surgery, 4 had insufficient amount of tumor for vaccine production (<1 g), 6 had tumor autolysis or yield too low to generate vaccine, 4 had no identifiable pancreas adenocarcinoma found at time of operation (1 had carcinoma in situ), 2 had a final pathology other than pancreatic adenocarcinoma (pancreatic endocrine neoplasm, adenocarcinoma of the ampulla), 1 had an operation that rendered him ineligible for study (splenectomy in addition to the resection of the primary tumor), 1 was ineligible owing to the use of neoadjuvant chemotherapy and radiation, 2 refused treatment or were treated on other protocols, and 1 died 3 days postoperatively, before vaccine administration. Five patients received a full course of 4 vaccines before the amendment. Thus, it was possible to generate HSPPC-96 from 5 of 11 samples with sufficient specimen to make the vaccine (or 5 of 15 subjects including all patients with adenocarcinoma identifiable by pathology review).
After changing the protocol to flash freezing the tumor specimen before shipping, 13 patients were consented for the study between November 2000 and May 2001. Of these, 4 were unresectable, 1 had a diagnosis other than pancreatic adenocarcinoma (ampullary adenocarcinoma), 2 had an insufficient amount of tumor (<1 g) to make vaccine, 1 died at home on postoperative day 10 and before vaccination could be started, and 5 patients received the full course of vaccination. All 5 patients with an adequate sample (or 5 of 7 patients with successful operations demonstrating adenocarcinoma in the specimen) had successful preparation of their HSPPC-96 vaccine.
Skin testing for immune competence
After sensitizing with DNCB before the first vaccine, skin testing for immune competence was performed at the time of the last vaccine (week 4) and 4 weeks later (week 8) to determine immune competence.
Seven of 10 patients were positive at the injection site prior to the administration of the vaccine. Of these 7, 3 patients were positive at week 4 but not week 8, 1 was positive at week 8 only, and 2 were positive at weeks 4 and 8. One patient who was negative at baseline became positive at week 8. No reaction from skin testing was determined to cause grade 3 or worse CTC v. 2.0 adverse events.
Toxicity
The most commonly reported adverse events, independent of attribution, included fatigue (5/10), abdominal pain (4/10), injection site reaction (4/10), flatulence (3/10), and peripheral edema (3/10). Adverse events considered to be possibly, probably, or definitely related to HSPPC-96 treatment are indicated in Table 2, and include an injection site reaction (4/10), upper abdominal pain, fatigue, headache, nodule, peripheral edema, pruritus, and pyrexia. All related events were mild or moderate in severity, with the exception of abdominal pain upper, which was severe for 1 patient. There were no episodes of pancreatitis during or after vaccination in this study (specifically, no clinical or laboratory evidence of pancreatitis in the patient with abdominal pain), indicating that no clinically significant organ-specific autoimmunity developed as a result of the vaccinations. One patient suffered a broken hip that was considered to be severe and led to withdrawal from the study. This event was not considered to be related to HSPPC-96 treatment. All 10 patients completed all 4 scheduled vaccinations, and all were evaluable for toxicity as well as outcome.
All patients were evaluated for induration and erythema at the HSPPC-96 injection site at 1, 24, and 48 hs after each vaccine. Minimal skin reactions were reported. One patient reported an induration 24 hr following the first vaccine (size = 6 mm) and 1 patient reported erythema at 48 hs following vaccines #2 and #4 (size = 40 and 20 mm, respectively). This same patient also reported induration 48 hs following vaccine #4 (size = 20 mm).
Immunologic responses
Complete ELISPOT data for 5 patients were obtained during the course of the phase I study. The remaining 5 sets of lymphocyte samples were lost owing to a freezer malfunction, having been stored in a location different from the first 5 samples. The frequency of autologous anti-HSPPC-96 ELISPOTs increased substantially in 1 patient. A second patient had a modest increase in spot number of unclear significance. ELISPOT reactivity for these 2 patients is indicated in Table 3.
For both patients with increase in ELISPOT reactivity with HSPPC-96 vaccination the response was class I restricted, as indicated by a decrease in the number of spots after the addition of anti-class I MHC antibody W6/32 to the CD8+ lymphocyte/tumor cell or membrane cocultures. The ability of W6/32 to block CD8+ T-cell IFN-γ ELISPOT number for patient 5 is shown in Fig. 2. Patient 5, who died of disease, also had the only response to autologous tumor by ELISPOT prior to vaccination. This was also MHC class I-restricted based on W6/32 blockade of the response.
Clinical outcomes
We evaluated overall survival in this cohort of ten patients, although this was not a primary endpoint of this study. Median overall survival was 2.2 years (95% confidence lower limit 1.1 years; upper boundary not calculable) by Kaplan–Meier product-limit estimate (Fig. 3). Median progression-free survival (Fig. 3) was 0.9 years (95% confidence intervals 0 and 2.5 years). Median follow-up was 2.2 years (range, 0.7–5.0). Three patients remain alive without disease at 2.6, 2.7, and 5.0 years. One patient had recurrence of disease at the time of the first restaging scan, but remained alive for 1.7 years on chemotherapy before expiring; other patients had rapid progression without evidence of sensitivity to chemotherapy after recurrence.
We also evaluated overall survival in patients who were able to undergo complete resection but in whom the vaccine was found to be degraded after preparation, or for whom the yield of vaccine was insufficient to allow for completion of the set of 4 vaccinations. Median survival was 2.7 years by Kaplan–Meier product-limit estimate (95% confidence interval 1.9–5.2 years) Median follow-up was 3.1 years (range, 1.2–6.5). Three patients were alive at 2.9, 4.5, and 6.5 years; the patient alive at 2.9 years is alive with disease and receiving chemotherapy with capecitabine; the other patients have no evidence of disease. The confidence limits for overall survival for the vaccinated group and the group with insufficient or degraded vaccine overlap.
Increase in ELISPOT reactivity in patient 5 after HSPPC-96 vaccinations. The number of spots per 8–10×104 cells is indicated at each time point: (1) before vaccination; (2) after 2 vaccinations; (3) after 3 vaccinations; (4) 1 week following the 4th vaccination; (5) 4 weeks following the 4th vaccination. The ability of the W6/32 anti–class I major MHC antibody to block this response is demonstrated. Solid bars number of spots without W6/32 antibody, hatched bars number of spots in presence of W6/32 antibody. The decreased number of spots indicates the response is MHC class I dependent. Values shown represent the mean of 6 wells (2 experiments, triplicates for each data point in each experiment)
Discussion
In this study, we demonstrated that with improvements in tumor handling, it is possible to generate intact HSPPC-96 vaccine in the protease-rich milieu of the pancreas. However, patients developing immune responses by DTH or by ELISPOT analysis were not the same patients who experienced long-term survival in this study. Those patients who had resection of their tumors but did not receive vaccination had a similar outcome to those who received the vaccinations. Technical problems with autologous vaccine production in this study included obtaining adequate supply of tumor tissue for vaccine production and proteolysis of the vaccine by endogenous proteases.
The reason for the discrepancy between clinical outcome and quality of the immune response is unclear. An immune response after HSPPC-96 vaccination was associated with a better clinical outcome in patients with colon cancer metastatic to liver in whom the vaccine was generated from their metastatic disease [19], a finding also observed in patients vaccinated with HSPPC-96 for melanoma [18]. One explanation is that the dose of the vaccine or number of vaccinations was inadequate; patients on other studies of HSPPC-96 vaccine have received up to 100 μg of vaccine as single doses [18, 19]. In addition, the use of peripheral blood to determine the immune response to the HSPPC-96 vaccine is likely an insensitive assay for immunity that should occur in situ. A phase III study of HSPPC-96 in patients with resected renal adenocarcinomas recently completing accrual should definitively determine the relationship between immunologic response to the vaccine and clinical outcome for at least 1 solid tumor type.
A number of other studies have begun to examine the relevance of tumor-specific vaccines in pancreatic cancer [25–28]. Perhaps the most intriguing to date has been the generation of autologous whole-cell GM-CSF producing pancreas adenocarcinoma lines that are then irradiated and administered to the patient as part of a program of adjuvant therapy [25]. The results of the phase I study of this whole-cell vaccine technology were that GM-CSF could be found in the serum of patients after vaccination, that immune cell infiltrates could be found in the vaccination sites after immunization, and that 3 patients of the first 14 treated in the phase I setting were still alive without disease >25 months after diagnosis. Two of the 3 surviving patients were treated with the highest dose of GM-CSF-producing irradiated tumor cells, hinting at a dose–response relationship for this therapy.
Early stage investigations with other peptide and protein vaccines involving mutated ras peptides [26], carcinoembryonic antigen [28], and cell-surface mucins [27] as antigens are ongoing, as are studies involving viruses encoding cancer cell-surface molecules and immune system costimulatory molecules [29]. Vaccine studies are hindered by an inconsistent association of immune responses with clinical outcome, as noted in this study. However, in at least 2 studies with HSPPC-96, there appears to be an association between tumor-specific immunity and clinical outcome [18, 19].
Although it was not the primary goal of this study, we found the median overall survival in this cohort of 10 patients to be 2.2 years by Kaplan–Meier estimate. Three of 10 patients experienced long-term survival, better than the typically quoted long-term survival rate of ∼10% [5]. Given the small number of highly selected subjects in this nonrandomized study, randomized phase III data are necessary to determine if there is genuine benefit of autologous HSPPC-96 vaccination in the adjuvant setting.
Our pilot study only examined patients able to have complete resection of primary disease. For the remainder of patients with unresectable disease, such vaccination therapy is untested. In future studies, it will also be important to have more thorough immunologic monitoring and to consider giving several dose levels or a longer course of vaccinations. Finally, given increasing data that chemotherapy may be helpful for patients with resected primary localized pancreatic adenocarcinoma, it will also be challenging to incorporate vaccine therapy into adjuvant therapeutic plans involving other modalities.
References
Jemal A, Tiwari RC, Taylor-Murray EW, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ (2005) Cancer statistics, 2005. CA Cancer J Clin 55:10–30
Geer RJ, Brennan MF (1993) Prognostic indicators for survival after resection of pancreatic adenocarcinoma. Am J Surg 165:68–72
Begg CB, Cramer LD, Hoskins WJ, Brennan MF (1998) Impact of hospital volume on operative mortality for major cancer surgery. JAMA 280:1747–1751
Callery MP, Strasberg SM, Doherty GM, Soper J, Norton JA (1997) Staging laparoscopy with laparoscopic ultrasonography: optimizing resectability in hepatobiliary and pancreatic malignancy. J Am Coll Surg 185:33–39
Brooks AD, Mallis MJ, Brennan MF, Conlon KC (2002) The value of laparoscopy in the management of ampullary, duodenal, and distal bile duct tumors. J Gastrointest Surg 6:139–145
Gold EB, Goldin SB (1998) Epidemiology of and risk factors for pancreatic cancer. Surg Oncol Clin North Am 7:67–91
Evans DB, Abbruzzese JL, Willett CG (2001) Cancer of the pancreas. In: DeVita VT, Hellman S, Rosenberg SA (eds) Cancer: Principles and practice of oncology (6th edn). Lippincott Williams & Wilkins, Philadelphia, pp 1126–1161
Conlon KC, Klimstra DS, Brennan MF (1996) Long-term survival after curative resection for pancreatic ductal adenocarcinoma. Clinicopathologic analysis of 5-year survivors. Ann Surg 223:273–279
Kalser MH, Ellenberg SS (1985) Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg 120:899–903
Klinkenbijl JH, Jeekel J, Sahmoud T, van Pel R, Couvreur ML, Veenhof CH, Arnaud JP, Gonzalez DG, de Wit LT, Hennipman A, Wils J (1999) Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg 230:776–782
Neoptolemos JP, Dunn JA, Stocken DD, Almond J, Link K, Beger H, Bassi C, Falconi M, Pederzoli P, Dervenis C, Fernandez-Cruz L, Lacaine F, Pap A, Spooner D, Kerr DJ, Friess H, Buchler MW (2001) Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet 358:1576–1585
Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, Beger H, Fernandez-Cruz L, Dervenis C, Lacaine F, Falconi M, Pederzoli P, Pap A, Spooner D, Kerr DJ, Buchler MW (2004) A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 350:1200–1210
Srivastava PK (2000) Immunotherapy of human cancer: lessons from mice. Nat Immunol 1:363–366
Srivastava PK, Maki RG (1991) Stress-induced proteins in immune response to cancer. Curr Top Microbiol Immunol 167:109–123
Basu S, Binder RJ, Ramalingam T, Srivastava PK (2001) CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity 14:303–313
Binder RJ, Srivastava PK (2004) Essential role of CD91 in re-presentation of gp96-chaperoned peptides. Proc Natl Acad Sci U S A 101:6128–6133
Janetzki S, Palla D, Rosenhauer V, Lochs H, Lewis JJ, Srivastava PK (2000) Immunization of cancer patients with autologous cancer-derived heat shock protein gp96 preparations: a pilot study. Int J Cancer 88:232–238
Belli F, Testori A, Rivoltini L, Maio M, Andreola G, Sertoli MR, Gallino G, Piris A, Cattelan A, Lazzari I, Carrabba M, Scita G, Santantonio C, Pilla L, Tragni G, Lombardo C, Arienti F, Marchiano A, Queirolo P, Bertolini F, Cova A, Lamaj E, Ascani L, Camerini R, Corsi M, Cascinelli N, Lewis JJ, Srivastava P, Parmiani G (2002) Vaccination of metastatic melanoma patients with autologous tumor-derived heat shock protein gp96-peptide complexes: clinical and immunologic findings. J Clin Oncol 20:4169–4180
Mazzaferro V, Coppa J, Carrabba MG, Rivoltini L, Schiavo M, Regalia E, Mariani L, Camerini T, Marchiano A, Andreola S, Camerini R, Corsi M, Lewis JJ, Srivastava PK, Parmiani G (2003) Vaccination with autologous tumor-derived heat-shock protein gp96 after liver resection for metastatic colorectal cancer. Clin Cancer Res 9:3235–3245
Caudill MM, Li Z (2001) HSPPC-96: a personalised cancer vaccine. Expert Opin Biol Ther 1:539–547
Younes A, Fayad LE, Pro B, McLaughlin P, Hagemeister FB, Mansfield P, Clayman G, Medeiros LJ, Lewis J, Srivastava P (2003) Safety and efficacy of heat shock protein-peptide 96 complex (HSPPC-96) in low-grade lymphoma. Proc Am Soc Clin Oncol 22:570 [Abstr 2294]
Srivastava PK, Jaikaria NS (2001) Methods of purification of heat shock protein-peptide complexes for use as vaccines against cancers and infectious diseases. Methods Mol Biol 156:175–186
Heike M, Blachere NE, Wolfel T, Meyer zum Buschenfelde KH, Storkel S, Srivastava PK (1994) Membranes activate tumor- and virus-specific precursor cytotoxic T lymphocytes in vivo and stimulate tumor-specific T lymphocytes in vitro: implications for vaccination. J Immunother Emphasis Tumor Immunol 15:165–174
Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457–481
Jaffee EM, Hruban RH, Biedrzycki B, Laheru D, Schepers K, Sauter PR, Goemann M, Coleman J, Grochow L, Donehower RC, Lillemoe KD, O'Reilly S, Abrams RA, Pardoll DM, Cameron JL, Yeo CJ (2001) Novel allogeneic granulocyte-macrophage colony-stimulating factor-secreting tumor vaccine for pancreatic cancer: a phase I trial of safety and immune activation. J Clin Oncol 19:145–156
Gjertsen MK, Buanes T, Rosseland AR, Bakka A, Gladhaug I, Soreide O, Eriksen JA, Moller M, Baksaas I, Lothe RA, Saeterdal I, Gaudernack G (2001) Intradermal ras peptide vaccination with granulocyte-macrophage colony-stimulating factor as adjuvant: Clinical and immunological responses in patients with pancreatic adenocarcinoma. Int J Cancer 92:441–450
Goydos JS, Elder E, Whiteside TL, Finn OJ, Lotze MT (1996) A phase I trial of a synthetic mucin peptide vaccine. Induction of specific immune reactivity in patients with adenocarcinoma. J Surg Res 63:298–304
Marshall J (2003) Carcinoembryonic antigen-based vaccines. Semin Oncol 30(3 Suppl 8):30–36
Schuetz T, Marshall J, Kaufman HL, Safran H, Panicali D (2004) Two phase I studies of prime-boost vaccinations with vaccinia-fowlpox vaccines expressing CEA, MUC-1, and TRICOM costimulatory molecules (B7.1/ICAM-1/LFA-3) in patients with advanced pancreatic cancer. J Clin Oncol 22(Suppl 14S):2564
Acknowledgments
The authors are grateful to all patients agreeing to enroll on this study. We wish to thank Jennifer Yamada and Cate Hirst for their assistance with data management over the several years of this study, and to H. Kelley for her assistance with the biostatistical analysis. Portions of this study were presented in abstract form at the American Society for Clinical Oncology meeting in 1999 (abstract 1687), and European Cancer Conference 12 in 2003 (ECCO, abstract 48). P.K.S. is supported by an NIH grant (CA 44786) and by a research agreement with Antigenics, Inc., in which he has significant financial interest. P.L. is supported in part by NIH grant CA 33049.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maki, R.G., Livingston, P.O., Lewis, J.J. et al. A Phase I Pilot Study of Autologous Heat Shock Protein Vaccine HSPPC-96 in Patients With Resected Pancreatic Adenocarcinoma. Dig Dis Sci 52, 1964–1972 (2007). https://doi.org/10.1007/s10620-006-9205-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-006-9205-2