Abstract
At present, the main therapy for chronic renal failure (CRF) is dialysis and renal transplantation, but neither obtains satisfactory results. Human umbilical cord mesenchymal stem cells (huMSCs) are isolated from the fetal umbilical cord which has a high self-renewal and multi-directional differentiation potential. Icariin (ICA), a kidney-tonifying Chinese Medicine can enhance the multipotency of huMSCs. Therefore, this work seeks to employ the use of ICA-treated huMSCs for the treatment of chronic renal failure. Blood urea nitrogen and creatinine (Cr) analyses showed amelioration of functional parameters in ICA-treated huMSCs for the treatment of CRF rats at 3, 7, and 14 days after transplantation. ICA-treated huMSCs can obviously increase the number of cells in injured renal tissues at 3, 7, and 14 days after transplantation by optical molecular imaging system. Hematoxylin-eosin staining demonstrated that ICA-treated huMSCs reduced the levels of fibrosis in CRF rats at 14 days after transplantation. Superoxide dismutase and Malondialdehyde analyses showed that ICA-treated huMSCs reduced the oxidative damage in CRF rats. Moreover, transplantation with ICA-treated huMSCs decreased inflammatory responses, promoted the expression of growth factors, and protected injured renal tissues. Taken together, our findings suggest that ICA-treated huMSCs could improve the kidney function in CRF rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The number of patients suffering from CRF, a pattern of chronic disease, is growing and increasing the need for renal replacement therapy [1]. Despite pharmacologic therapy that could improve survival in early CRF patients, the effect is not obvious in patients with end-stage disease. End-stage CRF patients mainly take dialysis and renal transplantation treatment. Dialysis as continuous renal replacement therapy cannot complete the kidney secretion and metabolism to maintain homeostasis and a series of other important functions, and there are many complications. Renal transplantation can completely replace kidney function, and its mortality rate is lower than dialysis patients, but the lack of donor kidneys, xenotransplantation failures, and need for long-term use of immunosuppressive agents restrict the application of organ transplantation [2]. Therefore, there is an urgent need to develop new therapy strategies for CRF.
Bone marrow-derived mesenchymal stem cells (BM-MSCs) have been used for treating Acute renal failure (ARF) [3]. Studies show that BM-MSCs transplanted into ARF animals promote the recovery of renal function and structure through the release of growth factors, such as Vascular endothelial growth factor (VEGF) [4]. huMSCs were stromal cells which were isolated from the fetal umbilical cord with Wharton’s Jelly [5]. huMSCs possess similar characteristics to BM-MSCs and also have a higher proliferative potency and lower immunogenicity. More importantly, these cells can be obtained without ethical disagreements [6]. huMSCs can be differentiated into osteoblast, cartilage cells, fat cells, pancreatic islet cells, nerve cells, myocardial cells, and reproductive cells, etc. [7]. huMSCs are expected to be the ideal cells for the therapy of various diseases including nervous system disorders, diabetes, cancer, as well as ARF [8, 9].
Epimedium brevicornum Maxim [10], an important traditional Chinese herbal medicine, has been widely used for “tonifying kidney and strengthening bone” for thousands of years. ICA (C33H40O15; molecular weight 676.65) is a flavonoid extracted from Epimedium brevicornum Maxim and is considered as the main pharmacological active constituent [11]. ICA can also promote BM-MSCs proliferation and differentiation, at the same time promoting the gene BMP − 7 expression and the recovery of renal function in injured renal [12]. Thus, we speculated that ICA-treated MSCs can enhance the effect of treatment for CRF compared with single treatment.
Our previous study showed that 100 μM ICA-treated huMSCs for 1 week can obviously up-regulate the expression of pluripotency gene Oct_4. As a result, ICA-treated huMSCs can accelerate the recovery of renal function by promoting huMSCs to differentiate into renal tubular epithelial cells in ARF mice. Nevertheless, these effects have no significant difference between the ICA-treated huMSCs group and huMSCs group. Therefore, in this study, we propose that ICA-treated huMSCs could reduce the oxidative damage and inflammatory responses through a paracrine effect, so as to accelerate the effect of treatment for CRF.
Materials and methods
huMSCs isolation and identification
Human umbilical cords were collected from full-term births after either cesarean section or normal vaginal delivery with the consent of parents. The fresh umbilical cords were collected at 4 °C. The tissue was disinfected with 2% Penicillin and streptomycin mixture and cleared extensively with PBS. The washing was repeated until they were cleaned from blood or blood clots. The cord vessels (arteries and vein) were removed from cord segments, and the exposed Wharton’s Jelly tissue was cut into 1 mm3 fragments. The tissue fragments were incubated with 0.1% collagenase type IV combined with 0.25% Trypsin for 30 min at 37 °C. Following digestion, the DMEM was supplemented with 10% fetal bovine serum to stop the reaction. Then the mixture was filtered by cell strainer where the undigested tissues were placed in culture bottles and cultured in DMEM supplemented with 10% fetal bovine serum (FBS), 1% l-glutamine, 1% penicillin, and streptomycin mixture. The culture bottle was placed in an incubator with saturated humidity at about 37 °C containing 5% CO2. The filtrate was centrifuged at 1000g for 5 min, and the collected cells were washed twice in PBS. Then the cells were suspended in medium and placed in the incubator with saturated humidity at about 37 °C containing 5% CO2. The medium was changed twice in 1 week. huMSCs were passaged at 80–90% confluency by 0.25% trypsin digestion. The fourth generation of cells was used in this study.
Icariin
Icariin (≥94% purity) was purchased from National Institutes for Food and Drug Control (Beijing, China). For the cell culture, Icariin was dissolved in dimethyl sulfoxide first and then added to complete medium. The chemical structure of ICA is shown in Fig. 1.
Flow cytometry
Once 80% confluence had been reached, adherent cells were analyzed using standard flow cytometry. Cells were incubated with monoclonal antibodies against CD29, CD31, CD34, CD44, CD45, CD90 (eBioscience) and analyzed on a BD FACSCalibur flow cytometer.
Cell labeling
huMSCs were pretreated with 100 uM ICA for 1 week, then 8 × 106 cells were labeled with the cross-linkable membrane dye, DiR. The labeled cells were re-suspended in 0.9% saline solution and prepared for transplantation. The normal huMSCs were labeled by the same method.
Adenine-induced chronic renal failure rat model
Adult male Wistar rats (outbred, 6–8 weeks of age, 240–260 g) were acquired from the Experiment Animal Center of Chinese Academy of Medical Science. Rats were raised in Animal House of Tianjin University of Traditional Chinese Medicine and access to rats formula feeds: containing 18.8% protein, 57% carbohydrate, 5.7% crude oil, 1.01% calcium, 0.65% phosphorus, 51 μg/kg vitamin D (BEIJING HFK BIOSCIENCE CO., LTD, Beijing, China), and water. The environment was kept at 22 ± 2 °C temperature, 50–60% humidity, and under a 12-h dark 12-h light cycle. All efforts were made to maintain a minimum of animal number and suffering. All animal experiment procedures were authorized by the Committee for Animal Care at Tianjin University of Traditional Chinese Medicine and coincided with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals.
Chronic renal failure models are induced by 2.5% adenine (Solarbio, Beijing, China) (200 mg/kg/d) administered by gavage for 4 weeks. Adenine was dissolved in sterile 0.9% saline solution [13, 14].
huMSCs transplantation
Rats were randomly divided into four groups: control group (non-adenine-treated rats receiving saline, n = 9), adenine group (adenine-treated rats receiving saline, n = 9), huMSCs group (adenine-treated rats receiving DiR-labeled huMSCs, n = 9), huMSCs + ICA group (labeled-treated rats receiving DiR-labeled ICA-treated huMSCs, n = 9). huMSCs (8 × 106) [15] suspended in 1 ml saline solution were injected via the tail vein after being adenine treated for 4 weeks. At 3, 7, and 14 days after being treated, the rats were anesthetized with 10% chloral hydrate of 4 ml/kg and blood was immediately collected from the angular vein. Subsequently, the rats were sacrificed by cervical vertebra dislocation and the kidneys of rats were excised for the following experiment. An optical molecular imaging system (NightOWL LB983 of Berthold, Germany) was used to observe the location of transfused huMSCs at different time points after the cells were treated.
Serum and tissue homogenate preparation
Rats were sacrificed at 3, 7, and 14 days after being treated; kidneys were removed for optical molecular imaging system analysis. The kidneys were washed in cold saline solution, and then ground in the pre-cooled 0.9% saline solution. The tissue homogenate was centrifuged at 3000 rpm for 15 min, and the supernatant was collected for MDA and SOD determination (Nanjing Jiancheng Bioengineering Institute, Jiangsu, China). Before the rats died, blood samples were collected and centrifuged at 3000 rpm for 15 min, and the supernatant was collected for BUN and creatinine Cr determination (Nanjing Jiancheng Bioengineering Institute, Jiangsu, China). The serum and tissue homogenate were stored at −80 °C until use.
Histopathology staining and elisa analysis
Renal tissues were fixed in 4% paraformaldehyde and Hematoxylin/Eosin (HE) staining was finished by the second people’s hospital of Tianjin in china. Part of the serum and tissue homogenate was used for Elisa analysis (Shanghai Baoman Biotechnology Co., Ltd, Shanghai, China) to detect the concentration of IL-10, IL-6, and TNF-α according to the kit instruction.
Quantitative RT-PCR
Total RNA was isolated from rat kidney tissues using an extract RNA kit according to the manufacturer’s instructions (TianGen Biotechnology Co., Ltd, Beijing, China). The expressions of GAPDH, bFGF, and BMP-7 were detected by quantitative RT-PCR (qRT-PCR). All samples were tested in triplicate by BIORAD iQ5. GAPDH was used as a loading control. The sequences of primers are listed in Table 1.
Statistical analysis
Data were shown as means ± SD. Statistical analysis was performed by ANOVA for multiple comparisons using SPSS 16.0. And cartograms were drawn by Prism software (Graph Pad, San Diego, USA). P < 0.05 was considered significant.
Results
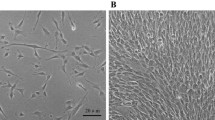
The characteristics of huMSCs
One week after the primary work, we can observe adherent cells with fibroblastic morphology as shown in Fig. 2a. After 3 weeks, cells reached 80% confluence and cells were passaged as shown in Fig. 2b. We analyzed the cells by flow cytometry at P4. As shown in Fig. 2c, huMSCs expressed CD29 (94.22%), CD44 (99.30%), CD90 (99.55%), but were negative for CD31 (0.02%), CD34 (0.03%), CD45 (0.22%).
ICA-treated huMSCs accelerate the recovery of renal function in chronic renal failure in rats
At 14 days after huMSCs transplantation with or without ICA treatment, we evaluated the body weight of rats and the results showed that body weight in the adenine group was significantly reduced compared with the control group (P < 0.01). And the body weight in huMSCs group and ICA-treated huMSCs group was significantly elevated than that in adenine group (P < 0.05). Moreover ICA-treated huMSCs group showed higher level than huMSCs group (P < 0.05) Fig. 3b. After 4 weeks of administration of adenine, the rats showed significant hair loss, increased urine output, and lack of energy and mortality of rats as high as 30%, but after transplanting huMSCs with or without ICA treatment into CRF rats for 14 days, those symptoms have been improved.
a Effects of ICA-treated huMSCs on rat renal function indexes at 3, 7, and 14 D (days) after huMSCs transplantation. Serum Cr and BUN levels in control group (non-adenine injection rats), adenine group (adenine-induced CRF rats), huMSC group (adenine-induced CRF rats receiving huMSCs), and huMSCs group + ICA (adenine-induced CRF rats receiving ICA-treated huMSCs, n = 3). b Body weight in different groups, n = 5. All data were expressed as mean ± SD. *P < 0.05 and **P < 0.01 versus control group. # P < 0.05 and ## P < 0.01 versus adenine group. ※ P < 0.05 and ※※ P < 0.01 versus huMSCs + ICA group
Transplantation of ICA-treated huMSCs protects the renal function by decreasing the levels of serum BUN and Cr. The levels of BUN and Cr in the adenine group markedly increased at 3, 7, and 14 days compared with the control group (both: P < 0.01 at 3 and 7 days; P < 0.05 at 14 days). At 3, 7, and 14 days after huMSCs transplantation with or without ICA treatment, the levels of BUN and Cr in the huMSCs group were reduced compared with the adenine group (both: P < 0.05 at 3 and 14 days, P < 0.01 at 7 days). More importantly, the levels of BUN and Cr in the ICA-treated huMSCs group reduced at 3, 7, and 14 days significantly compared with the huMSCs group (BUN: P < 0.05 at 14 days, P < 0.01 at 3 and 7 days; Cr: P < 0.05 at 7 and 14 days, P < 0.01 at 3 days) Fig. 3a.
We also observed the effect of ICA-treated huMSCs transplantation on the histology of CRF rats at 3, 7, and 14 days after adenine lavage. In the adenine group, there was notable damage in the renal tissues compared with the control group. Treatment with huMSCs and ICA-treated huMSCs alleviated renal injury as identified by dilated tubulars, preservation of the integrity of the tubules, and lower inflammatory cell accumulation. However, ICA-treated huMSCs resulted in improved amelioration of adenine-induced renal injury compared with the treatment with huMSCs at 14 days (Fig. 4).
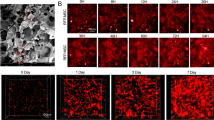
To trace the gathering of huMSCs, a fluorescence imaging system was used for kidneys of the CRF rats at days 3, 7, and 14 after transplantation. The 794 nm excitation and 810 nm emission filters were used. Visible red fluorescence was detected in the kidney region and the fluorescence intensity was higher in the huMSCs + ICA group at 3, 7, and 14 days (3 and 7 days: P < 0.01; 14 days: P < 0.05) (Fig. 5a). In order to more accurately quantify the number of huMSCs, after finishing the imaging, the kidneys were embedded in OCT compound (Tissue-Tek, Miles) for frozen section (Leica CM 1850, Leica Instruments), and sectioned at 20 μm thickness. Then sections were observed under a laser scanning confocal microscope (LSCM, Olympus FV1000, Japan). The fluorescent intensity was quantified by Image J software. The results showed that fluorescent intensity of DIR in huMSCs + ICA group was significantly higher than huMSCs at 14 days (Fig. 5b).
a Fluorescence imaging system for kidneys of CRF rats. Red fluorescence signals in the kidney could be clearly seen at 3, 7, and 14 D (days) after hucMSC transplantation. There are higher fluorescence signals in kidneys and fluorescence imaging showing a significant difference could be seen between the hucMSC group with hucMSC group + ICA group. b Fluorescent intensity of DIR in huMSCs + ICA group and huMSCs group at 14 days. (Scale bars: 200 µm). All data were expressed as mean ± SD, n = 3. ※ P < 0.05 and ※※ P < 0.01 versus huMSCs + ICA group
ICA-treated huMSCs transplantation alleviate renal oxidative damage of CRF rats
Adenine-induced CRF is associated with oxidative damage. We performed a SOD and MDA assay to evaluate the anti-oxidative damage effects of ICA-treated huMSCs on CRF rats at 3, 7, and 14 days after transplantation. Compared with the control group, the level of SOD is significantly reduced in renal tissue homogenate in the adenine group while being significantly increased in the huMSCs group at three time points (P < 0.05). Furthermore, the effect of the huMSCs + ICA group is better than the huMSCs group at 14 days (P < 0.05) Fig. 6a. For the MDA levels, compared with the adenine group, it is significantly reduced in huMSCs group at 14 days (P < 0.05), and huMSCs + ICA group is significantly reduced at both time points (P < 0.05) (Fig. 6b).
The concentration of IL-6, TNF-a, and IL-10 in serum and renal tissues at 3, 7, and 14 D (days) after cell transplantation was determined by ELISA. All data were expressed as mean ± SD, n = 3. *P < 0.05 and **P < 0.01 versus control group. # P < 0.05 and ## P < 0.01 versus adenine group. ※ P < 0.05 versus huMSCs + ICA group
ICA-treated huMSCs reduce inflammatory responses in adenine-induced CRF rats
IL-6, TNF-α, and IL-10, are three major inflammation-related cytokines. We analyzed these three indexes in serum and renal tissue homogenate by ELISA. Our results showed that the concentration of IL-6 and TNF-α in serum and tissue homogenate was significantly higher in the adenine group at both time points compared with the control group (P < 0.05). The concentration of both cytokines showed a downward trend in the huMSCs group and huMSCs + ICA group. Especially at 14 days, TNF-α and IL-6 in serum and tissue homogenate were significantly reduced in the huMSCs and huMSCs + ICA groups, moreover it was significantly lower in huMSCs + ICA group than that in huMSCs group (P < 0.05). Compared with the adenine group, the concentration of IL-10 in serum and tissue homogenate is significantly higher than that in the huMSCs group and huMSCs + ICA group (P < 0.05). Furthermore, IL-10 in the huMSCs + ICA group was significantly higher than that in the huMSCs group (P < 0.05) (Fig. 7).
ICA-treated huMSCs promote the expressions of BMP-7 and bFGF in adenine-induced CRF rats
The expression levels of BMP-7 and bFGF in renal tissue were analyzed by qRT-PCR and normalized to GAPDH. Our results showed that the expression levels of BMP-7 and bFGF were significantly higher in the huMSCs group and huMSCs + ICA group at 7 and 14 days after huMSCs transplantation than that in the adenine group (P < 0.01). Additionally, the huMSCs + ICA group has an obvious rising trend compared with the huMSCs group, and the expression of bFGF in huMSCs + ICA group was significantly higher than in huMSCs group at 14 days (Fig. 8).
Discussion
CRF is a slowly progressive renal damage and originating from diverse kidney diseases. CRF develops predominantly due to the injury and necrosis of renal proximal tubule cells as a result of ischemic or toxic insult. In recent years, researchers have found a variety of ways to cause CRF in rats such as renal resection, renal artery ligation, adenine, and adriamycin, etc. The advantage of the adenine method is simple and controllable as well as has a high success rate. The disadvantage is high animal mortality. Thus, we combined with the actual situation to choose adenine to cause CRF. The mechanisms of the adenine-induced CRF rat include azotemia, accumulation of uremic toxins, metabolic imbalances of amino acids and electrolytes [16], as well as complex inflammatory response [17]. Pathologically, renal tissue of adenine-fed rats shows lesions in kidney tubules and glomeruli [18]. Excess adenine is low solubility 2,8-dihydroxyadenine via the action of xanthine oxidase, which leads to its precipitation in kidney tubules and renal tubular damage. Our research is designed based on the above pathogenesis and aims to augment repair in CRF.
ICA is a Chinese medicine for “strengthening the kidney” that can promote bone marrow-derived MSCs proliferation and differentiation [19]. In recent years, researchers have found that bone marrow-derived MSCs can promote renal tubular epithelial cell regeneration, inhibiting fibrosis [20, 21]. Furthermore, ICA-modified bone marrow-derived MSCs can promote the growth factor expression of BMP-7 and bFGF [22, 23]. As huMSCs possess similar characteristics to bone marrow MSCs, this study was designed to identify possible effects of ICA-treated huMSCs for CRF. Our results demonstrate that ICA-treated huMSCs accelerate the recovery of renal function by reducing oxidative damage, inflammatory response, and the expression of growth factor.
For the process of CRF, we observed loss of a large number of renal tubular epithelial cells, renal interstitial fibrosis, BUN and Cr levels are significantly higher than normal at 4 weeks after being fed adenine. However, at 3, 7, and 14 days after ICA-treated huMSCs transplantation, we could trace the transplanted cells localized to the site of injury (kidney) and found that ICA-treated huMSCs can be more abundant in injured renal tissues compared with huMSCs. When huMSCs arrived at the damaged area, the structural recovery of the kidney and the regeneration of renal tubule cells can accelerate. These lines of evidence suggest that the transfused ICA-treated huMSCs could preferentially migrate to injured renal tissues and improve renal functions in CRF rats.
The mechanism of huMSCs transplantation for the treatment of CRF is related to oxidative damage, inflammation, and growth factor secretion [4]. SOD has been considered as one of the major indicators for oxidative damage. We found that the SOD levels significantly increased at 7 and 14 days after ICA-treated huMSCs transplantation. MSCs were reported to be involved in regulating the expression of pro-inflammatory cytokines (e.g., IL-6, TNF-a) and anti-inflammatory cytokines (e.g., IL-10) [24]. In our study, we found that ICA-treated huMSCs can reduce the inflammatory responses by decreasing the secretion of IL-6 and TNF-a, at the same time increasing the secretion of IL-10. Moreover, the growth factor BMP-7 and bFGF are beneficial to the renal tissue regeneration and repair [25, 26]. The result showed that ICA-treated huMSCs can up-regulate the expression levels of BMP-7 and bFGF in CRF rats. This was consistent with our finding that ICA-treated huMSCs have obvious therapeutic effects on renal function, thereby indicating that the above three mechanisms are effective.
There are several limitations in our study. Most importantly, although we showed that the transferred cells were localized at the injured kidney tissues, we did not delineate the interactions between transferred cells and renal cells. The precise mechanisms of how the transferred cells migrate to the damaged area should be evaluated in future experiments. It is with regret that we could not find all the significant differences between the ICA-treated huMSCs and huMSCs group concerning the mechanism of oxidative damage, inflammation, and growth factor secretion. For these reasons, we will improve our treatment of chronic renal failure in the future.
Conclusions
Our results demonstrate that the transfused ICA-treated huMSCs could preferentially migrate to injured renal tissues, promote renal cell regeneration and growth factor secretion, inhibit oxidative damage and inflammatory responses, and improve renal functions. These findings suggest that huMSCs combined with ICA may be a useful approach in the treatment of chronic renal failure.
References
NAHAS ME (2005) The global challenge of chronic kidney disease. Kidney Int 68:2918–2929
PLATT BSaJL (2001) Physiologic and immunologic hurdles to xenotransplantation. J Am Soc Nephrol 12:182–193
Morigi M (2004) Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J Am Soc Nephrol 15:1794–1804. doi:10.1097/01.asn.0000128974.07460.34
Togel F, Cohen A, Zhang P, Yang Y, Hu Z, Westenfelder C (2009) Autologous and allogeneic marrow stromal cells are safe and effective for the treatment of acute kidney injury. Stem Cells Dev 18:475–485. doi:10.1089/scd.2008.0092
Wang HS, Hung SC, Peng ST, Huang CC, Wei HM, Guo YJ, Fu YS, Lai MC, Chen CC (2004) Mesenchymal stem cells in the Wharton’s jelly of the human umbilical cord. Stem Cells 22:1330–1337. doi:10.1634/stemcells.2004-0013
Lund RD, Wang S, Lu B, Girman S, Holmes T, Sauve Y, Messina DJ, Harris IR, Kihm AJ, Harmon AM, Chin FY, Gosiewska A, Mistry SK (2007) Cells isolated from umbilical cord tissue rescue photoreceptors and visual functions in a rodent model of retinal disease. Stem Cells 25:602–611. doi:10.1634/stemcells.2006-0308
Chao KC, Chao KF, Fu YS, Liu SH (2008) Islet-like clusters derived from mesenchymal stem cells in Wharton’s Jelly of the human umbilical cord for transplantation to control type 1 diabetes. PLoS ONE 3:e1451. doi:10.1371/journal.pone.0001451
Rachakatla RS, Pyle MM, Ayuzawa R, Edwards SM, Marini FC, Weiss ML, Tamura M, Troyer D (2008) Combination treatment of human umbilical cord matrix stem cell-based interferon-beta gene therapy and 5-fluorouracil significantly reduces growth of metastatic human breast cancer in SCID mouse lungs. Cancer Invest 26:662–670. doi:10.1080/07357900701871134
Cao H, Qian H, Xu W, Zhu W, Zhang X, Chen Y, Wang M, Yan Y, Xie Y (2010) Mesenchymal stem cells derived from human umbilical cord ameliorate ischemia/reperfusion-induced acute renal failure in rats. Biotechnol Lett 32:725–732. doi:10.1007/s10529-010-0207-y
Velikago IibisP and sad. IS-Pb (1920) Trudy Imperatorskago S.-Peterburgskago botanicheskago sada. Acta Horti Petropolitani. Imperatorskiǐ S.-Peterburgskiǐ botanicheskiǐ sad., S.-Peterburg
Sun ZB, Wang JW, Xiao H, Zhang QS, Kan WS, Mo FB, Hu S, Ye SN (2015) Icariin may benefit the mesenchymal stem cells of patients with steroid-associated osteonecrosis by ABCB1-promoter demethylation: a preliminary study. Osteoporos Int 26:187–197. doi:10.1007/s00198-014-2809-z
Le ZLC, Tie Wu (2007) Effect of herba epimedii on renal bone morphogenetic protein-7 in male rats with kidney YANG in sufficiency. J Guangdong Med Coll 25:371–386.
HU An-kang ZX-r, YUAN Hong-hua (2011) Establishment of two rat models of chronic renal failure. Acta Lab Anim Sci Sin 19:34–38.
Wang J, Wang F, Yun H, Zhang H, Zhang Q (2012) Effect and mechanism of fucoidan derivatives from Laminaria japonica in experimental adenine-induced chronic kidney disease. J Ethnopharmacol 139:807–813. doi:10.1016/j.jep.2011.12.022
Behr L, Hekmati M, Lucchini A, Houcinet K, Faussat AM, Borenstein N, Noel LH, Lelievre-Pegorier M, Laborde K (2009) Evaluation of the effect of autologous mesenchymal stem cell injection in a large-animal model of bilateral kidney ischaemia reperfusion injury. Cell Prolif 42:284–297. doi:10.1111/j.1365-2184.2009.00591.x
Okabe C, Borges RL, de Almeida DC, Fanelli C, Barlette GP, Machado FG, Arias SC, Malheiros DM, Camara NO, Zatz R and Fujihara CK (2013) NF-kappaB activation mediates crystal translocation and interstitial inflammation in adenine overload nephropathy. Am J Physiol Renal Physiol 305:F155–F163. doi:10.1152/ajprenal.00491.2012
Ali BH, Al-Husseni I, Beegam S, Al-Shukaili A, Nemmar A, Schierling S, Queisser N, Schupp N (2013) Effect of gum arabic on oxidative stress and inflammation in adenine-induced chronic renal failure in rats. PLoS ONE 8:e55242. doi:10.1371/journal.pone.0055242
Susanne B Nicholas JY, Amin Aminzadeh, Keith C Norris, Albert Crum, Nosratola D Vaziri (2012) Salutary effects of a novel oxidative stress modulator on adenine-induced chronic progressive tubulointerstitial nephropathy. Am J Transl Res 4:257–268
Zhang JF, Li G, Chan CY, Meng CL, Lin MC, Chen YC, He ML, Leung PC and Kung HF (2010) Flavonoids of herba epimedii regulate osteogenesis of human mesenchymal stem cells through BMP and Wnt/beta-catenin signaling pathway. Mol Cell Endocrinol 314:70–74. doi:10.1016/j.mce.2009.08.012
Brodie JC, Humes HD (2005) Stem cell approaches for the treatment of renal failure. Pharmacol Rev 57:299–313. doi:10.1124/pr.57.3.3
Takahito ITO, Suzuki A Enyu Imai, Okabe Masaru, Hori Masatsugu (2001) Bone marrow is a reservoir of repopulating mesangial cells during glomerular remodeling. J Am Soc Nephrol 12:2625–2635
Zhai YK, Ge BF, Chen KM, Ma HP, Ming LG, Li ZF (2010) Comparative study on theosteogenic differentiation of rat bone marrow stromal cells effected by Icariin and IcarisideÒ. J Chin Med Mater 33:1896–1900.
Yang Li, Zhang Rong-hua, Zhu Xiao-feng, Cai Yu, Huang Feng (2010) Effect of icariin on the expression of transforming growth factor-beta 1 and bone morphogenetic protein-2 in the process of mesenchymal stem cells differentiation into osteoblasts. J Clin Rehabil Tissue Eng Res 14:3518–3522. doi:10.3969/j.issn.1673-8225.2010.19.020
Semedo P, Palasio CG, Oliveira CD, Feitoza CQ, Goncalves GM, Cenedeze MA, Wang PM, Teixeira VP, Reis MA, Pacheco-Silva A, Camara NO (2009) Early modulation of inflammation by mesenchymal stem cell after acute kidney injury. Int Immunopharmacol 9:677–682. doi:10.1016/j.intimp.2008.12.008
Borovecki F, Jelic M, Grgurevic L, Sampath KT, Bosukonda D, Vukicevic S (2004) Bone morphogenetic protein-7 from serum of pregnant mice is available to the fetus through placental transfer during early stages of development. Nephron Exp Nephrol 97:e26–e32. doi:10.1159/000077595
Gould SE, Day M, Jones SS, Dorai H (2002) BMP-7 regulates chemokine, cytokine, and hemodynamic gene expression in proximal tubule cells. Kidney Int 61:51–60. doi:10.1046/j.1523-1755.2002.00103.x
Acknowledgements
This work was performed at Tianjin University of Traditional Chinese Medicine, China, and was supported by a Grant from the Tianjin Research Program of Application Foundation and Advanced Technology (15JCYBJC26100) and Program for Changjiang Scholars and Innovative Research Team University (PCSIRT): IRT_14R41.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, W., Wang, L., Chu, X. et al. Icariin combined with human umbilical cord mesenchymal stem cells significantly improve the impaired kidney function in chronic renal failure. Mol Cell Biochem 428, 203–212 (2017). https://doi.org/10.1007/s11010-016-2930-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-016-2930-8