A new isoflavan, (3S)-1′,4′-dihydroxy-6,7,8,2′,3′-pentamethoxyisoflavan (1), together with six known compounds, abruquinone G (2), abruquinone B (3), isoliquiritigenin (4), gallic acid (5), ethyl gallate (6), and 1-O-galloyl-β-D-glucose (7) were isolated from Abrus precatorius. Their structures were elucidated on the basis of physical and spectral analysis, respectively. Compounds 1, 4, and 7 were isolated from this plant for the first time.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Abrus precatorius L., popularly known as wild liquorice, is a wild plant native to China belonging to the family Leguminosae. A. precatorius is considered as a “poisonous plant” due to the acute toxicity of its seeds. The seeds are highly toxic due to abrin and have been used as an insecticide and for the treatment of some skin diseases in China since ancient times [1]. However, the leaves and roots are used in folk medicine to treat different conditions like hepatitis and bronchitis. Several kinds of secondary metabolites have been identified from A. precatorius, including alkaloids, steroids, triterpenoids, and isoflavanquinones [2,3,4,5,6,7]. In our research, the aqueous EtOH extract of the aerial parts was separated by repeated chromatography to give seven compounds. Their structures were elucidated as (3S)-1′,4′-dihydroxy-6,7,8,2′,3′-pentamethoxyisoflavan (1), abruquinone G (2) [8], abruquinone B (3) [8], isoliquiritigenin (4) [9], gallic acid (5) [10], ethyl gallate (6) [11], and 1-O-galloyl-β-D-glucose (7) [12]. Compounds 1, 4, and 7 were isolated from this plant for the first time. In this paper, we report the isolation and structural elucidation of the new compound 1.
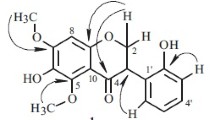
For compound 1, the molecular formula C20H24O8 was deduced from HR-ESI-MS m/z: 393.1557 [M + H]+, (calcd 393.1544), 415.1381 [M + Na]+, this being supported by its 1H and 13C NMR data. The 13C NMR spectrum showed 20 signals, 12 of which were found in the region between δC 100 and 150. This was indicative of a highly substituted aromatic ring system. The remaining carbon resonances were attributed to an oxygenated methylene (δ 69.44), five methoxys (δ 61.30, 61.23, 60.82, 60.77, 56.41), one methine (δ 32.11), and one methylene (δ 30.76). The 1H NMR spectrum displayed signals indicative of two aromatic protons (δ 6.47 s, 6.37 s) and five signals of aromatic methoxy groups δ 3.94, 3.91, 3.90, 3.89, 3.80, in accordance with five carbon signals at δ 60.77, 60.82, 61.23, 61.30, and 56.41. Additionally, five aliphatic protons were observed at δ 4.40 (1H, m), 4.01 (1H, dd, J = 6, 10 Hz), 3.01 (1H, dd, J = 10.5, 16 Hz), 2.91 (1H, m), and 3.57 (1H, m), suggesting an isoflavan scaffold. Unambiguous assignment of 1H and 13C NMR data was achieved by a combination of COSY, HMBC, HSQC, and NOESY experiments and by comparison with reported data of isoflavanquinones [13]. The connectivity of the rings as well as the position of the methoxy groups was determined with the aid of HMBC correlations. The connectivity of rings A and C was confirmed by the 3JC–-H coupling between H-5 (δ 6.37, ring A) and C-4 (δ 30.76, ring C). An HMBC correlation between H-3 (δ 3.57, ring C) and C-6′ (δ 122.34, ring B) and H-5′ (δ 6.47, ring B) and C-3 (δ 32.11, ring C) corroborated the connectivity between rings B and C (Fig. 1). These signals appeared similar to the 1H and 13C NMR spectroscopic data of abruquinone G2 isolated from the whole plant of A. precatorius [8]. The only major difference was the substituent at C-4 (no substituent group in compound 1 and hydroxyl in 2).
The absolute configuration of 1 was established by comparing its experimental CD spectrum with reported data. The CD curves of compound 1 exhibited a negative Cotton effect in the 260–300 nm regions. Our CD data and the sign of optical rotation were in good agreement with those of (3S)-5,7,3′,4′-tetramethoxyisoflavan [14]. Comparing additionally the UV spectra of this compound, we conclude that isoflavan 1 has the 3S-configuration.
Experimental
General. NMR spectra were recorded on a Bruker-AV-500 MHz spectrometer equipped with a 5 mm BBO probe operating at 500 MHz (1H) and 125 MHz (13C). Chemical shifts are reported as δ values (ppm) with the residual solvent signal as internal reference, J in Hz. Standard pulse sequences from Topspin 2.1 software package were used. HR-MS data were measured with a LCQDECA XP HPLC/MSn mass spectrometer, and UV spectrum was obtained on a Beckman DU-640 UV spectrophotometer. CD was measured with an Applied photophysics Chirascan spectrometer. For column chromatography (CC), silica gel (200–300 mesh) and GF254 for TLC were obtained from Qingdao Marine Chemical Factory, Qingdao, China.
Plant Material. Samples of the whole plant of Abrus precatorius L. were collected at Luhuitou, Hainan Province of China during October 2013. The plant was identified as Abrus precatorius L. by S. Zhang and a voucher specimen was deposited at the South China Sea Institute of Oceanology.
Extraction and Isolation. The whole plant of Abrus precatorius (1.2 kg) was dried in the air. The dried plant material was ground to a coarse powder using a grinder. The powder was extracted with EtOH (95%) three times (7 days each time) at room temperature. The combined alcohol extracts were concentrated in vacuo. The residue was suspended in H2O and extracted with petroleum ether, EtOAc, and n-BuOH successively. The EtOAc layers were concentrated to afford 4.76 g residues. The EtOAc extract was subjected to CC (SiO2, CHCl3–EtOAc, TLC monitoring) to give 10 fractions A–J. Fractions C and D (1.42 g) were fractionated in CC using the solvent system petroleum ether–acetone under gradient conditions. These fractions were combined based on TLC. Then ware purified by Sephadex LH-20 (CHCl3–MeOH 1:1) to give 1 (5 mg), 2 (8 mg), 3 (6 mg), 4 (10 mg), 5 (8 mg), 6 (7 mg), and 7 (7 mg).
Compound 1. PMR spectrum (500 MHz, Py-d5, δ, ppm, J/Hz): 6.47 (1H, s, H-5′), 6.37 (1H, s, H-5), 4.40 (1H, m, H-2), 4.01 (1H, dd, J = 6, 10, H-2), 3.01 (1H, dd, J = 10.5, 16, H-4), 2.91 (1H, m, H-4), 3.57 (1H, m, H-3), 3.90 (3H, s, 6-OCH3), 3.91 (3H, s, 3′-OCH3), 3.94 (3H, s, 2′-OCH3), 3.80 (3H, s, 7-OCH3), 3.89 (3H, s, 8-OCH3). 13C NMR spectrum (125 MHz, Py-d5, δ, ppm): 146.79 (s, C-6), 145.19 (s, C-9), 141.40 (s, C-8), 137.93 (s, C-4′), 139.18 (s, C-7), 142.11 (s, C-3′), 142.16 (s, C-2′), 140.41 (s, C-1′), 122.34 (s, C-6′), 117.09 (s, C-10), 108.01 (d, C-5′), 107.31 (d, C-5), 69.44 (t, C-2), 61.30 (q, 8-OCH3), 61.23 (q, 6-OCH3), 60.82 (q, 3′-OCH3), 60.77 (q, 2′-OCH3), 56.41 (q, 7-OCH3), 32.11 (d, C-3), 30.76 (t, C-4).
References
C. M. Ma, N. Nakamura, and M. Hattori, Chem. Pharm. Bull., 46, 982 (1998).
S. C. Qing and H. Z. Bi, Acta Bot. Sin., 40, 734 (1998).
R. M. Kenneth, J. W. Wallace, Y. N. Babu, V. K. Murty, and M. G. Rao, Phytochemistry, 28, 299 (1989).
M. Anam, Ind. J. Chem., 42B, 386 (2003).
S. C. Kuo, S. C. Chen, L. H. Chen, J. B. Wu, J. P. Wang, and C. M. Teng, Planta Med., 61, 307 (1995).
N.-C. Kim, D. Kim, and A. D. Kinghorn, Nat. Prod. Lett., 16, 261 (2002).
E. M. Anam, Phytomedicine, 8, 24 (2001).
Y. Hata, M. Raith, S. N. Ebrahimi, S. Zimmermann, T. Mokoka, D. Naldoo, G. Fouche, V. Maharaj, M. Kaiser, R. Brun, and M. Hamburger, Plata Med., 79, 492 (2013).
X. Y. Tian, B. Y. Han, C. J. Xiao, and B. Jiang, Nat. Prod. Res. Dev., 26, 188 (2014).
L. G. Li, Q. B. Liu, X. X. Huang, Z. X. Liu, Y. Peng, and S. J. Song, J. Shenyang Pharm. Univ., 32, 343 (2015).
J. Xian, X. F. Xian, Yu Tao, Y. Xu, C. Hu, and Q. B. Liu, J. Shenyang Pharm. Univ., 31, 262 (2014).
A. Q. Wang, L. L. Jun, and Z. Z. Wu, Chin. Trad. Herb. Drugs, 34, 685 (2003).
Y. Hata, S. N. Ebrahimi, M. D. Mieri, S. Zimmermann, T. Mokoka, D. Naidoo, G. Fouche, V. Maharaj, M. Kaiser, R. Brun, O. Potterat, and M. Hamburger, Fitoterapia, 93, 81 (2014).
K. Kurosawa, W. D. Ollist, B. T. Redman, I. O. Sutherland, H. M. Alves, and O. R. Gottlieb, Phytochemistry, 17, 1423 (1978).
Acknowledgment
This research was supported by the National Natural Science Foundation of China (No. 11-41306147-1) and Functional Development Project of Chinese Academy of Sciences (No. Y3YQ041). We thank Yun Zhang, Aijun Sun, and Chuanrong Li for providing mass and NMR spectral measurements in the Public Service Center of Instrument and Equipment, South China Sea Institute of Oceanology, Chinese Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 2, March–April, 2017, pp. 220–221.
Rights and permissions
About this article
Cite this article
Xiao, Z., Tao, S., Yang, Y. et al. A New Isoflavan from Abrus precatorius . Chem Nat Compd 53, 257–259 (2017). https://doi.org/10.1007/s10600-017-1965-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-017-1965-8