Methyl (3-hydroxy-4-(2-hydroxy-6-methylheptan-2-yl)benzoyl)glycinate (1), a new sydonic acid derivative with glycinate, together with sydonic acid (2), sydowic acid (3), and 7-deoxy-7,14-didehydrosydonic acid (4), were isolated from a marine-derived Aspergillus sydowii strain CUGB-F126. Their structures were elucidated by spectroscopic analysis, including high-resolution mass spectroscopy, and 1D and 2D NMR techniques. All of these compounds did not show inhibitory activity against Staphylococcus aureus and Candida albicans.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
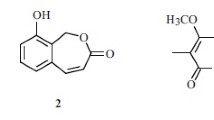
Aspergillus sydowii has been described as a pathogen for humans that causes aspergillosis and onychomycosis [1, 2]. It was also identified as the causative agent of aspergillosis for sea fan corals [3, 4]. Chemical investigations on this species led to a series of bioactive secondary metabolites most of which were related to the sydonic acid sydowinin and diketopeperazine [5,6,7,8,9]. During our systematic search for new secondary metabolites from marine-derived fungi isolated from the sediment of Bohai Sea, a marine-derived Aspergillus sydowii strain was investigated for its chemical constituents, which led to the identification of a new sydonic acid derivative with glycinate (1), together with sydonic acid (2) [6], sydowic acid (3) [5], and 7-deoxy-7,14-didehydrosydonic acid (4) [10]. Herein, we report the isolation and structure elucidation of the new compound.
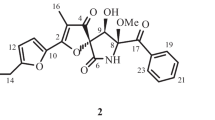
Compound 1 was obtained as a colorless amorphous powder. The HR-ESI(+)MS data for 1 revealed a pseudo-molecular ion ([M + Na]+) consistent with the molecular formula C18H27NO5(∆mmu –0.6) requiring six degrees of unsaturation. The 1H NMR spectrum of 1 in DMSO-d6 (Table 1) revealed resonances for a 1,3,4-trisubstituted benzene ring at δ 7.21 (1H, d, J = 1.8 Hz) for H-2, 7.34 (1H, d, J = 8.4 Hz) for H-5, and 7.25 (1H, dd, J = 8.4, 1.8 Hz) for H-6, four methylenes at δ 1.93 (1H, td, J = 13.2, 4.2 Hz) and 1.65 (1H, td, J = 13.2, 4.2 Hz) for H2-9, 1.27 (1H, m) and 1.00 (1H, m) for H2-10, 1.06 (2H, q, J = 7.2 Hz) for H2-11, 3.97 (2H, d, J = 6.0 Hz) for H2-16, one methine at δ 1.43 (1H, m) for H-12, three methyl groups at δ 0.77 (3H, d, J = 7.2 Hz) for H3-13, 0.78 (3H, d, J = 7.2 Hz) for H3-14, and 1.50 (3H, s) for H3-15, as well as one methoxy group at δ 3.65 (3H, s) for H3-18. The protonated carbons and their corresponding protons were assigned by the HSQC experiment. The 13C NMR (Table 1) and DEPT spectra gave 18 signals, which confirmed the presence of a 1,3,4-trisubstituted benzene ring at δ 133.0 (s, C-1), 115.0 (d, C-2), 154.5 (s, C-3), 135.9 (s, C-4), 126.7 (d, C-5), and 117.2 (d, C-6), four methylenes at δ 41.5 (t, C-9), 21.3 (t, C-10), 38.8 (t, C-11), and 41.1 (t, C-16), one methine at δ 27.3 (d, C-12), three methyl groups at δ 22.4 (q, C-13 and C-14) and 28.4 (q, C-15), and one methoxy at δ 51.6 (q, C-18), as well as two carbonyls at δ 166.4 (s, C-7) and 170.4 (s, C-17).

The aliphatic chain from C-9 to C-14 was confirmed by the H–H COSY signals. The HMBC correlations from H3-15 to C-4, C-8, and C-9 and from H-5 to C-8 revealed that the aliphatic chain was attached to C-4 through C-8. The HMBC signals from H-OH to C-2, C-3, and C-4 indicated that the hydroxyl was attached to C-3. The carbonyl (C-7) at C-1 was confirmed by the HMBC signals from H-2 and H-6 to C-7. The HMBC correlations from H2-16 to C-7 and C-17 indicated that the glycine moiety was attached to C-7 and form an amide bond. The methoxy group was confirmed by the HMBC signal from H3-18 to C-17. Thus, the planar structure of 1 was identified by the above-mentioned correlations. The absolute configuration was assigned as the same as that of sydonic acid (2).
The isolated compounds were tested against Staphylococcus aureus and Candida albicans. All the tested compounds did not exhibited inhibitory activity at 100 μg/mL.
Experimental
NMR spectra were recorded on a Bruker 600 MHz spectrometer at 600 MHz for 1H and 150 MHz for 13C in DMSO-d6 using solvent signals (DMSO, δH 2.50/δC 39.5) as reference, and the coupling constants are in Hz. Optical rotation was recorded on a PerkinElmer 241 spectropolarimeter. HR-ESI-MS data were measured with an APEX II FT-ICR-MS spectrometer (Bruker Daltonics, Inc. USA). Column chromatography was performed with silica gel (200–300 mesh, Qingdao Marine Chemical Inc. China) and Sephadex LH-20 (Pharmacia Biotech AB, Uppsala, Sweden). HPLC separation was performed on an Agilent 1100 series system with a Zorbax C18 reversed-phase semipreparative column (5 μm 250 × 9.4 mm).
Microorganism. The marine fungus Aspergillus sydowii CUGB-F126 was isolated from seawater collected from the Bohai Sea, Tianjin in October 2007. The fungus was cultured and kept on PDA prepared in natural seawater. It was identified according to its morphological characteristics.
Fermentation. The fungus was cultured and kept on a potato dextrose agar (PDA) plate prepared in natural seawater at 28°C for 7 days. A small spoon of spores growing on the plate was inoculated into a 250 mL conical flask containing 40 mL of liquid medium composed of glucose (1.0%), maltose (1.0%), and yeast extract (0.3%), mannitol (2.0%), monosodium glutamate (1.0%), KH2PO4 (0.05%), MgSO4 (0.03%), corn germ plasm (0.1%), and natural seawater, its pH adjusted to 7.2, and cultured at 28°C for 3 days on a rotary shaker at 160 rpm. Then 5 mL of the resultant seed culture was inoculated into 500 mL conical flasks each containing 50 g rice and 30 mL natural seawater, and incubated without shaking for 15 days.
Extraction and Isolation. The culture whole broth was extracted with EtOAc exhaustively, and the solvent removed under reduced pressure at < 50°C to yield a dark residue. The EtOAc extract (2.8 g) was subjected to a normal-phase silica gel (200–300 mesh) column chromatography and eluted with a gradient of increasing Me2CO (0–100%) in petroleum ether. The fraction eluted by 10% Me2CO in petroleum ether was rechromatographed over Sephadex LH-20 using petroleum ether–CHCl3–MeOH (5:5:1) to afford eight subfractions. The seventh subfraction was further separated by reversed-phase semipreparative HPLC using MeOH–H2O (65:35) as mobile phase to yield compound 1 (1.2 mg). The fraction eluted by 8% Me2CO in petroleum ether was rechromatographed over Sephadex LH-20 using petroleum ether–CHCl3–MeOH (5:5:1) to afford seven subfractions. The fifth subfraction was further separated by reversed-phase semipreparative HPLC using MeOH–H2O (70:30) as mobile phase to yield compounds 2 (2.2 mg), 3 (2.5), and 4 (3.3 mg).
Methyl ( S )-(3-Hydroxy-4-(2-hydroxy-6-methylheptan-2-yl)benzoyl)glycinate (1), colorless amorphous powder; \( {\left[\upalpha \right]}_D^{25} \) +35° (c 0.01, MeOH). ESI-MS m/z 338 [M + H]+; HR-ESI-MS m/z 360.1787 (calcd for C18H27NO5, 360.1781). For 1H and 13C NMR data, see Table 1.
References
T. F. Patterson, W. R. Kirkpatrick, M. White, J. W. Hiemenz, J. R. Wingard, B. Dupont, M. G. Rinaldi, D. A. Stevens, and J. R. Graybill, Medicine (Baltimore), 79, 250 (2000).
A. Yamada, H. Noguchi, H. Sakae, T. Sugita, M. Hiruma, and M. Hiruma, Med. Mycol. J., 53, 205 (2012).
G. W. Smith, L. D. Ives, I. A. Nagelkerken, and K. B. Richie, Nature, 383, 487 (1996).
K. Kim and C. D. Harvell, Am. Nat., 164, S52 (2004).
T. Hamasaki, Y. Sato, and Y. Hatsuda, Agric. Biol. Chem., 39, 2337 (1975).
T. Hamasaki, K. Nagayama, and Y. Hatsuda, Agric. Biol. Chem., 42, 40 (1978).
K. Trisuwan, V. Rukachaisirikul, M. Kaewpet, S. Phongpaichit, N. Hutadilok-Towatana, S. Preedanon, and J. Sakayaroj, J. Nat. Prod., 74, 1663 (2011).
X. Liu, F. Song, L. Ma, C. Chen, X. Xiao, B. Ren, X. Liu, H. Dai, A. M. Piggott, Y. Av-Gay, L. Zhang, and R. J. Capon, Tetrahedron Lett., 54, 6081 (2013).
A. Kaur, H. A. Raja, B. A. Darveaux, W. L. Chen, S. M. Swanson, C. J. Pearce, and N. H. Oberlies, Magn. Reson. Chem., 53, 616 (2015).
M. Y. Wei, C. Y. Wang, Q. A. Liu, C. L. Shao, Z. G. She, and Y. C. Lin, Mar. Drugs, 8, 941 (2010).
Acknowledgment
This work was supported by the Fundamental Research Funds for the Central Universities (2-9-2015-165) and key lab of marine bioactive substance and modern analytical technique, SOA (MBSMAT-2016-04).
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 6, November–December, 2017, pp. 898–899.
Rights and permissions
About this article
Cite this article
Xu, X., Zhao, S., Yin, L. et al. A New Sydonic Acid Derivative From a Marine Derived-Fungus Aspergillus sydowii . Chem Nat Compd 53, 1056–1058 (2017). https://doi.org/10.1007/s10600-017-2200-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-017-2200-3