A new chlorinated amino acid derivative with a rare 3-chloro-4-hydroxyphenyl unit named aspergamide A (1), along with three known compounds, xylarinol A (2), nectriapyrone (3), and asperisocoumarin A (4), were isolated from the sponge-derived fungus Aspergillus sp. LS53. Their structures were determined by combining detailed spectroscopic analysis and literature data. Compounds 1, 3, and 4 showed weak Vibrio harveyi inhibition with MIC values ranging from 16 to 64 μg/mL, whereas 2 was found to be inactive.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Marine fungi have been recognized as an abundant source of halogenated natural products with an impressive array of biological properties ranging from antimicrobial to enzyme-inhibitory [1]. However, chlorinated fungal metabolites containing the 3-chloro-4-hydroxyphenyl subunit are very rare. Only five halogenated compounds containing the 3-chloro-4-hydroxyphenyl moiety have so far been isolated from nature, including coniothyriomycin [2], 3-chloro-4-hydroxybenzeneethanol [3], 3-chloro-4-hydroxyphenylacetic acid [4, 5], 3-chloro-4-hydroxyphenylacetamide [5, 6], and (+)-xylariamide A [5, 7]. In the course of our ongoing investigation to find novel fungal bioactive compounds, a new aspergamide A (1) containing a 3-chloro-4-hydroxyl phenyl skeleton, together with three known compounds xylarinol A (2), nectriapyrone (3), and asperisocoumarin A (4), were isolated from a sponge-derived Aspergillus sp. LS53. The isolation, structure elucidation, and biological activities of these isolates are presented here.

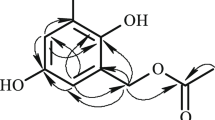
Compound 1 was obtained as a yellow powder. Its molecular formula was determined as C15H16NO6Cl by a combination of HR-ESI-MS and NMR data with seven degrees of unsaturation. The HR-ESI-MS peaks at m/z 342.0795 [M + H]+ and 344.0688 [M + H]+ in the ratio of 3:1 provided additional support for the presence of one chlorine atom in 1. The 1H NMR spectrum of 1 indicated the presence of three aromatic methine protons at δ 7.04 (d, J = 1.7 Hz, H-2), 6.93 (d, J = 8.3 Hz, H-5), and 6.88 (dd, J = 8.3, 1.7 Hz, H-6), implying a 1,3,4-trisubstituted benzene ring (Table 1). In combination with HR-ESI-MS data and the chemical shifts in the benzene ring, it can be deduced that compound 1 contains the 3-chloro-4-hydroxyphenyl subunit. Analysis of the 13C NMR and HSQC spectra suggested the presence of one amide carboxyl carbonate δ 163.1 (C-10); two ester carbonyls at δ 171.5 (C-9) and 165.9 (C-13) (Table 1). The HMBC correlations of H2-7/C-1 and C-9, H-8/C-9, H-15/C-9, and H-2 and H-6/C-7, as well as the COSY correlations between H2-7 and H-8, established a 3-chloro-4-hydroxy-substituted phenylalanine methyl ester moiety. The additional HMBC correlations of H-11/C-10, C-12, and C-13 and H-12/C-10, C-11, and C-13 together with the large coupling constants (15.4 Hz) between H-11 and H-12 showed the presence of a fumaric acid moiety.
Furthermore, the HMBC correlation of H3-14/C-13 revealed that a methoxyl group was linked to the fumaric acid unit, constituting a fumaric acid methyl ester moiety. The linkage between the two units in 1 was examined based on the HMBC cross-peak of H-8/C-10 (Fig. 1), which suggested that the fumaric acid methyl ester moiety was connected to the C-8 position of 3-chloro-4-hydroxy-substituted phenylalanine methyl ester moiety via an amide bond, leading to a planar structure assignment as shown for 1. The absolute configuration at C-8 in 1 was assigned an R configuration based on comparison with literature [7]. Thus, the complete structure of 1 was established and named as aspergamide A.
The structure of three known compounds identified as xylarinol A (2) [8], nectriapyrone (3) [9], and asperisocoumarin A (4) [10] were deduced by comparison of their spectroscopic data with those reported in the literature. Their antimicrobialactivity was tested against an aquatic pathogen, Vibrio harveyi, which commonly infects fish. Compounds 1, 3, and 4 displayed weak antibacterial activity with MIC values of 16, 64, and 32 μg/mL against V. harveyi, respectively. However, compound 2 was appreciably inactive up to a concentration of 128 μg/mL [11].
Experimental
General Experimental Procedures. NMR data were recorded on an Agilent 600 MHz NMR system, with TMS as internal standard. Optical rotation was recorded on a Perkin-Elmer model 341 polarimeter. The HR-ESI-MS spectrum was obtained on an Agilent 6224 TOF-MS mass spectrometer. Medium-pressure liquid chromatography (MPLC) was performed on an ODS column (15 μm). Vacuum liquid chromatography (VLC) was carried out with silica gel (200–300 mesh). Compound purification was carried out by semi-preparative HPLC separation on a Waters HPLC instrument (Alliance 2695, Milford, MA, USA) equipped with a Waters 2996 detector and a C18 column (250 × 20 mm ID, 5 μm; YMC Co. Ltd., Tokyo, Japan).
Fungal Material. The fungus Aspergillus sp. LS53 was isolated from the tissue of the sponge Haliclona sp. collected from Linshui, Sanya City, China. The fungus was identified by rDNA amplification and sequence analysis of the ITS region. The strain specimen in PDA medium was deposited at the College of Food and Pharmaceutical Sciences, Ningbo University, China.
Culture, Extraction and Isolation. The strain was cultivated in 250 mL Erlenmeyer flasks, each containing 100 mL PDB medium (potato 200 g/L, glucose 20 g/L, sea salt 100 g/L, H2O 1L). After 2 days of incubation at 25°C with 180 rpm agitation, the 10% concentration of the cultures were used to inoculate Erlenmeyer flasks (250 mL), each containing 80 g rice solid medium (rice 80 g, sea salt 35 g, H2O 120 mL) [12]. Fermentation under static cultivation was carried out at room temperature for 40 days. The fermentation products were extracted three times with ethyl acetate. The extracting solutions were merged by boiling away solvent under reduced pressure to afford 23 g of oily crude extract.
Then the crude extract was subjected to VLC on a silica gel column (6 × 15 cm, 200–300 mesh) using petroleum ether–ethyl acetate as gradient eluent (from 15:1 to 0:1), affording five fractions (Frs. 1–5). Fraction 3 (3.75 g) was applied to MPLC using MeOH–H2O gradients (20–80% MeOH, UV detection at 254 nm) to give three subfractions Subfrs.3.A–3.C. Subfraction 3.A (116 mg) was further separated by semipreparative HPLC (40% MeOH–H2O) to yield 1 (3.0 mg), 2 (5.7 mg), and 3 (8.9 mg). Subfraction 3.B (97 mg) was further purified by semipreparative HPLC (55% MeOH–H2O) to yield 4 (12.5 mg).
Aspergamide A (1). C15H17NO6Cl, yellow amorphous powder, \( {\left[\alpha \right]}_{\mathrm{D}}^{25} \) –12° (c 0.10, MeOH). HR-ESI-MS, m/z 342.0795/344.0688 [M + H]+ (calcd for C15H18NO6Cl, 342.0744/344.0715 in 3:1-intensity). For 1H and 13C NMR, see Table 1.
Xylarinol A (2). C10H8O3, white solid. 1H NMR (600 MHz, CD3OD, δ, ppm, J/Hz): 7.30 (1H, d, J = 12.1), 7.28 (1H, t, J = 7.9), 6.99 (1H, d, J = 7.9), 6.97 (1H, d, J = 7.9), 6.31 (1H, d, J = 12.1), 5.22 (2H, s). 13C NMR (150 MHz, CD3OD, δ, ppm): 62.5 (C-1), 170.8 (C-3), 122.9 (C-4), 142.9 (C-5), 138.5 (C-5a), 121.9 (C-6), 131.3 (C-7), 118.3 (C-8), 156.0 (C-9), 122.9 (C-9a).
Nectriapyrone (3). C11H14O3, colorless oil. 1H NMR (600 MHz, DMSO-d6, δ, ppm, J/Hz): 6.69 (1H, d, J = 7.2), 6.09 (1H, s), 3.90 (3H, s), 1.93 (3H, s), 1.84 (3H, s), 1.83 (3H, d, J = 7.2). 13C NMR (150 MHz, DMSO-d6, δ, ppm): 165.2 (C-2), 103.0 (C-3), 166.1 (C-4), 91.6 (C-5), 160.2 (C-6), 127.0 (C-7), 129.9 (C-8), 14.4 (C-9), 8.7 (C-10), 56.2 (C-11), 12.3 (C-12).
Asperisocoumarin A (4). C15H12O4, yellow solid. 1H NMR (600 MHz, CDCl3, δ, ppm, J/Hz): 7.91 (1H, d, J = 8.1), 7.23 (1H, d, J = 8.1), 6.59 (1H, s), 2.36 (1H, s), 2.30 (1H, s), 2.19 (1H, s). 13C NMR (125 MHz, CDCl3, δ, ppm): 146.9 (C-2), 181.7 (C-3), 123.4 (C-3a), 130.5 (C-4), 120.4 (C-5), 146.0 (C-5a), 104.5 (C-6), 160.1 (C-7), 157.9 (C-9), 106.3 (C-9a), 165.7 (C-9b), 20.2 (C-10), 134.3 (C-11), 19.9 (C-12), 17.1 (C-13).
References
K. Y. He, C. Zhang, Y. R. Duan, G. L. Huang, C. Y. Yang, X. R. Lu, C. J. Zheng, and G. Y. Chen, J. Antibiot., 70 (7), 823 (2017).
K. Krohn, C. Franke, P. G. Jones, H. J. Aust, S. Draeger, and B. Schulz, Eur. J. Org. Chem., 1992 (8), 789 (1992).
M. C. R. Franssen, M. A. Posthumus, and H. C. V. D. Plas, Phytochemistry, 27 (4), 1093 (1988).
W. B. Turner and D. C. Aldridge, Fungal Metabolites II, Academic Press, New York, 1983.
K. D. Beattie, N. Ellwood, R. Kumar, X. Yang, P. C. Healy, V. Choomuenwai, R. J. Quinn, A. G. Elliott, J. X. Huang, and J. L. Chitty, Phytochemistry, 124, 79 (2016).
R. A. Davis, D. Watters, and P. C. Healy, Tetrahedron Lett., 46 (6), 919 (2005).
R. A. Davis, J. Nat. Prod., 68 (5), 769 (2005).
J. Wang, X. Wei, X. Lu, F. Xu, J. Wan, X. Lin, X. Zhou, S. Liao, B. Yang, and Z. Tu, Tetrahedron, 70 (51), 9695 (2014).
A. G. Avent, J. R. Hanson, and A.Truneh, Phytochemistry, 31 (3), 1065 (1992).
Z. Xiao, S. Chen, R. Cai, S. Lin, K. Hong, and Z. She, Beilstein J. Org. Chem., 12, 2077 (1992).
W. Li, L. Ding, N. Wang, J. Xu, W. Zhang, B. Zhang, and H. Jin, Marine Drugs, 17 (5), 283 (2019).
I. Srm, H. M. Abdallah, E. S. Elkhayat, N. M. Musayeib, H. Z. Asfour, M. F. Zayed, and G. A. Mohamed, J. Asian Nat. Prod. Res., 20 (1), 75 (2017).
Acknowledgment
We gratefully acknowledge financial support from the National Key Research and Development Program of China (2018YFC0310900), the National Natural Science Foundation of China (41776168, 41706167), the Ningbo Public Service Platform for High-Value Utilization of Marine Biological Resources (NBHY-2017-P2), the Zhejiang Provincial Public Welfare Technology Program (LGC19B020002), the Natural Science Foundation of Ningbo (2018A610320), the Ningbo Sci. & Tech. Projects for Common Wealth (2017C10016), the National 111 Project of China (D16013), the Li Dak Sum Yip Yio Chin Kenneth Li Marine Biopharmaceutical Development Fund, and the K. C. Wong Magna Fund in Ningbo University.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 1, January–February, 2020, pp. 97–99.
Rights and permissions
About this article
Cite this article
Zhang, L., Qiu, P., Ding, L. et al. A New Antibacterial Chlorinated Amino Acid Derivative from the Sponge-Derived Fungus Aspergillus sp. LS53. Chem Nat Compd 56, 109–111 (2020). https://doi.org/10.1007/s10600-020-02955-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-020-02955-x