From the seeds of the traditional medicinal plant Ziziphus jujuba growing in Uzbekistan, the fungal endophyte Alternaria sp. was isolated. Extracts of this fungus grown on solid rice and liquid Wickerham culture media yielded 21 natural products. These natural products include the new phthalide natural product (±)7-methoxyphthalide-3-acetic acid (1) in addition to 20 known compounds including 19 polyketides and one alkaloid. Moreover, the OSMAC (One Strain MAny Compounds) approach is applied in this study. The unambiguous structure of the new compound was elucidated on the basis of one- and two-dimensional NMR spectroscopy in addition to HR-ESI-MS. Compounds 2, 3, 4, and 7 exhibited a cytotoxic activity against mouse lymphoma cell lines (L5178Y) with IC50 values of 1.7, 7.8, 6.8, and 6.2 μM, respectively when compared with the positive control kahalalide F (IC50 4.3 μM).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Endophytic fungi are a highly diverse polyphyletic group including primarily ascomycetous fungi that occur ubiquitously in plants [1]. They reside in the intercellular spaces of stems, petioles, roots, leaves, and even seeds of plants without causing any obvious negative impact [2]. The relationship between the endophyte and its host plant may range from latent phytopathogenesis to mutualistic symbiosis [3]. Once inside their host plant, endophytic fungi usually assume a quiescent state and enhance host plant resistance to biotic and/or abiotic stresses, and in many instances this is accomplished by secondary metabolites production [4]. Functional metabolites of these fungi have already demonstrated a considerable potential to impact the pharmaceutical arena [5,6,7,8,9,10].
It is worth mentioning that most plant species that have been previously studied host at least one endophytic organism [11, 12]. There are over 300,000 plant species inhabiting our planet, and it can be assumed that each individual plant hosts from a few to hundreds of endophytic fungi distributed in different plants tissues [11, 12]. Therefore, these subtle inhabitants should be screened in order to investigate further bioactive natural compounds that can be used for human health [13, 16].
The fungal strain Alternaria sp. was investigated in this study. This fungus was isolated from the fresh healthy seeds of the traditional medicinal plant Ziziphus jujuba growing in Uzbekistan. The seeds of Ziziphus jujuba are known to cure eye diseases and are also useful in leucorrhoea. In addition, they have been used in traditional medicine as a sedative. They are also known to depress activity of the central nervous system, which reduces anxiety and induces sleep [14].
The crude EtOAc extract of Alternaria sp., grown on solid rice medium, was partitioned between n-hexane and 90% aqueous MeOH. Column chromatography of the hydromethanolic fraction on Sephadex LH-20 followed by semipreparative HPLC separation yielded the new natural phthalide derivative 7-methoxyphthalide-3-acetic acid (1).
In addition, 15 known compounds (2–16) were co-isolated. Similarly, the 90% MeOH-soluble fraction from the liquid Wickerham cultures of Alternaria sp. was subjected to Sephadex LH-20 and then semipreparative reversed-phase HPLC, affording five known isocoumarin derivatives (17–21).
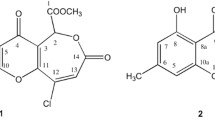
Compound 1 was isolated as a yellowish brown solid, displaying a UV spectrum that had a close similarity to that of 10. The HR-ESI-MS of compound 1 showed a pseudomolecular ion at m/z 223.0604 [M + H]+, indicative of the molecular formula C11H10O5 and with 14 amu higher than 10, hinting a possible presence of an extra methyl group in 1 relative to 10. Structural elucidation of 1 was based on results of 1D and 2D NMR spectral analysis including 1H NMR, 1H–1H COSY, and HMBC spectra. The 1H NMR spectrum displayed three aromatic protons at δ 7.01 (dd, J = 8.0, 1.2 Hz, H-4), 7.55 (t, J = 8.0 Hz, H-5), and 6.91 (d, J = 8.0, 1.2 Hz, H-6), methylene protons at δ 3.08 (dd, J = 16.6, 4.6 Hz, H-1′A) and 2.79 (dd, J = 16.6, 8.0 Hz, H-1′B), and a methine proton at δ 5.82 (dd, J = 8.0, 4.6 Hz, H-3) (Table 1). The 13C NMR spectrum of 1 showed a total of 11 carbons (Table 1). Detailed analysis of the 2D NMR spectra (including COSY, HSQC, and HMBC) allowed us to establish the planar structure of 1. The trisubstituted benzene ring of the phthalide moiety was supported by the COSY spin system observed from H2-1′ to H-6, including the long-range correlation CH(3)CH(4), in addition to the HMBC correlations from H-6 to C-4 (δ 112.8), C-5 (δ 114.2), C-7 (δ 159.4), and C-7a (δ 115.3). Moreover, the phthalide skeleton was confirmed by the HMBC correlations from H-4 to C-3 (δ 78.6), C-5 (δ 114.2), and C-6 (δ 117.3). Furthermore, HMBC correlations from H2-1′ to C-3, C-4a (δ 153.2), and C-2′ (δ 172.1) were also observed. The methoxyl group (δH 3.71, s, 7-OCH3) was confirmed to be attached to C-7 from a clear HMBC correlation of its protons to C-7 (δ 159.4). Thus, the planar structure of 1 was elucidated as depicted (Fig. 1). Compound 1 was identified as a new natural product for the first time. This new natural product has been reported as a synthetic intermediate in the synthesis of isoochracinic acid (10) [15, 16]. Moreover, only a tentative 1H NMR analysis had been reported for this synthetic intermediate in the literature [15, 16]. Herein, a full set of NMR data including 1D, 2D NMR and HR-ESI-MS is presented for this new natural product for the first time. This compound is assumed to be a biogenetic precursor of isoochracinic acid (10). This was evidenced by the co-existence of them in the original HPLC chromatogram of the above titled fungus, which was confirmed by LC-MS analysis. The biogenesis of such phthalide derivatives is of interest because the substation at C-3 is rare [15]. It is worth mentioning that in the present study, both 1 and 10 were obtained as racemates (±) as indicated by their zero optical rotations, and this is confirmed from the literature [15].
Based on a comparison of 1H, 13C NMR and mass spectroscopic data with published data, the known compounds (2–21) were identified as alternariol (2) [17], alternariol-5-O-methyl ether (3) [18], altenusin (4) [19], tenuazonic acid (5) [19], stemphytriol (6) [20], altertoxin II (7) [21], altenuene (8) [23], 4′-epialtenuene (9) [23], isoochracinic acid (10) [22], talaroflavone (11) [19], alteric acid (12) [19], alterlactone (13) [19], 2,5-dimethyl-7-hydroxychromone (14) [19], altechromone B (15) [23], ferulic acid (16) [24], citreoisocoumarinol (17) [25], 6-methylcitreoisocoumarin (18) [26], citreoisocoumarin (19) [25], orthosporin (20) [27], and diaportinol (21) [28].
It is known that fermentation of fungi on different culturing media can provide different fungal metabolites to be used for biochemical evaluation [29], and this technique is called OSMAC (One Strain Many Compounds) [30]. The chemical screening in the present study indicated a difference between Alternaria extracts obtained from liquid Wickerham medium and solid rice cultures. The extract obtained from solid cultures revealed altenusin (4) and alternariol (2) as the main components, whereas liquid cultures mainly produced isocoumarin derivatives citreoisocoumarinol (17), 6-methylcitreoisocoumarin (18), citreoisocoumarin (19), orthosporin (20) and diaportinol (21).
All compounds were subjected to a cytotoxicity assay against L5178Y mouse lymphoma cells. Among them, alternariol (2), alternariol-5-O-methyl ether (3), altenusin (4), and altertoxin II (7) showed significant cytotoxic activity with IC50 values of 1.7, 7.8, 6.8, and 6.2 μM, respectively comparable with the positive control kahalalide F (IC50 4.3 μM).
Experimental
General. The optical rotations were recorded on a PerkinElmer-241 MC polarimeter. NMR spectra were recorded on Bruker ARX 500 or Avance DMX 600 NMR spectrometers. Mass spectroscopic data were recorded on an LC-MS HP1100 Agilent Finnigan LCQ Deca XP Thermoquest mass spectrometer, while HR-ESI-MS was recorded on a FTHRMS-Orbitrap (Thermo Finnigan) mass spectrometer. HPLC was obtained with a Dionex P580 system coupled to a photodiode array detector (UVD340S). The analytical column (125 × 4 mm, L × i.d.) was prefilled with Europhere 10 C18 (Knauer, Germany). For TLC and preparative TLC, precoated silica gel 60 F254 plates (Merck) were used followed by observation under UV 254 and 366 nm. Column chromatography was performed using Merck MN silica gel 60 M (0.04–0.063 mm) or Sephadex LH-20 as stationary phases. Solvents were predistilled before use, and a spectral grade of solvents was used for spectroscopic measurements. Further HPLC separation was performed on a semipreparative HPLC system from Lachrom-Merck Hitachi (pump L7100; UV detector L7400; column Europhere 100 C18, 300 × 8 mm, Knauer, Germany) with a flow rate of 5.0 mL/min.
Fungal Material. The fungus Alternaria sp. (EK10.4.5 W) was isolated under sterile conditions from healthy fresh seeds of Ziziphus jujuba Lam. collected in Uzbekistan in September 2010 according to the procedure described by [29]. The fungus was identified by molecular biological protocol, and the sequence was deposited in the GenBank under accession number (KR347485). The fungal strain was cultivated on both static liquid Wickerham and solid rice media. Fermentation was performed in five flasks for 40 days at room temperature.
Extraction and Isolation. The extraction was performed with ethyl acetate (2 _ 600 mL). The EtOAc extracts from rice medium (10.0 g) or liquid Wickerham medium (2.0 g) were partitioned between n-hexane and 90% MeOH in water. The hydromethanolic fraction was chromatographed over Sephadex LH-20 using MeOH as eluting solvent to yield alternariol 2 (389 mg), alternariol-5-O-methyl ether 3 (1.00 g), altenusin 4 (489.7 mg), and tenuazonic acid 5 (835 mg). Further purification of the remaining fractions was achieved by semipreparative HPLC (Merck Hitachi L7100) using MeOH–H2O as the mobile phase to yield 7-methoxyphthalide-3-acetic acid 1 (1.9 mg), stemphytriol 6 (2.0 mg), altertoxin II 7 (3.5 mg), altenuene 8 (1.3 mg), 4′-epialtenuene 9 (1.5 mg), isoochracinic acid 10 (4.2 mg), talaroflavone 11 (3.5 mg), alteric acid 12 (4.4 mg), alterlactone 13 (1.9 mg), 2,5-dimethyl-7-hydroxychromone 14 (6.4 mg), altechromone B 15 (3.6 mg), and ferulic acid 16 (2.0 mg) from rice cultures, and citreoisocoumarinol 17 (1.9 mg), 6-methylcitreoisocoumarin 18 (2.2 mg), citreoisocoumarin 19 (5.3 mg), orthosporin 20 (1.1 mg), and diaportinol 21 (1.6 mg) from liquid Wickerham medium.
(±)7-Methoxyphthalide-3-acetic Acid (1). Yellowish brown solid; \( {\left[\upalpha \right]}_{\mathrm{D}}^{20} \) 0° (c 0.3; MeOH). UV (λmax, nm) (PDA): 206.3, 235.4, 299.9. For 1H and 13C NMR in MeOH-d4, see Table 1. ESI-MS positive m/z 223.1 [M + H]+; HR-ESI-MS m/z 223.0604 [M + H]+ (calcd for C11H11O5, 223.0601).
Cytotoxicity Assay. Cytotoxicity was tested against L5178Y mouse lymphoma cells using the microculture tetrazolium (MTT) assay and compared to that of untreated controls [31]. As negative controls, 0.1% EGMME–DMSO containing medium was used in the assay. The potent cytotoxic agent depsipeptide kahalalide F, obtained from Elysia grandifolia, was used as a positive control.
References
K. D. Hyde and K. Soytong, Fungal Divers., 33, 163 (2008).
C. W. Bacon and J. F. White, Microbial Endophytes, Marcel Dekker, New York, 2000.
G. A. Strobel and D. M. Long, ASM News, 64, 263 (1998).
T. N. Sieber, Fungal Biol. Rev., 21, 75 (2007).
R. X. Tan and W. X. Zhou, Nat. Prod. Rep., 18, 448 (2001).
A. A. L. Gunatilaka, J. Nat. Prod., 69, 509 (2006).
H. W. Zhang, Y. C. Song, and R. X. Tan, Nat. Prod. Rep., 23, 753 (2006).
V. C. Verma, R. N. Kharwar, and G. A. Strobel, Nat. Prod. Commun., 4, 1511 (2009).
R. N. Kharwar, A. Mishra, S. K. Gond, A. Stierle, and D. B. Stierle, Nat. Prod. Rep., 28, 1208 (2011).
A. H. Aly, A. Debbab, and P. Proksch, Pharmazie, 68, 499 (2013).
G. Strobel and B. Daisy, Microbiol. Mol. Biol. Rev., 67, 491 (2003).
W. Y. Huang, Y. Cai, K. D. Hyde, H. Corke, and M. Sun, World J. Microbiol. Biotechnol., 23, 1253 (2007).
M. A. Gamboa, S. Laureano, and P. Bayman, Mycopathologia, 156, 41 (2002).
R. T. Mahajan and M. Z. Chopda, Pharmacogn. Rev., 3, 320 (2009).
D. W. Knight and D. A. Portas, Tetrahedron Lett., 51, 4543 (1977).
S. O. Silva, J. N. Reed, R. J. Billedeau, X. Wang, D. J. Norris, and V. Snieckus, Tetrahedron, 48, 4863 (1992).
P. A. Onocha, D. A. Okorie, J. D. Connolly, and D. S. Roycroft, Phytochemistry, 40, 1183 (1995).
E. Stinson, W. B. Wise, R. A. Moreau, A. J. Jurewicz, and P. E. Pfeffer, Can. J. Chem., 64, 1590 (1986).
A. Hassan, Novel Natural Products from Endophytic Fungi of Egyptian Medicinal Plants, Chemical and Biological Characterization, Dissertation, Heinrich-Heine-University, 2007.
K. Krohn, M. John, H. J. Aust, S. Draeger, and B. Schulz, Nat. Prod. Lett., 14, 31 (1999).
A. Arnone, G. Nasini, L. Merlini, and G. Assante, J. Chem. Soc. Perkin Trans., 1, 525 (1986).
K. Kameda and M. Namiki, Chem. Lett., 3, 1491 (1974).
Y. Kimura, T. Mizuno, H. Nakajima, and T. Hamasaki, Biosci. Biotechnol. Biochem., 56, 1664 (1992).
S. E. Sajjadi, Y. Shokoohinia, and N. Moayedi, J. Nat. Pharm. Prod., 7, 159 (2012).
S. Lai, Y. Shizuri, S. Yamamura, K. Kawai, and H. Furukawa, Heterocycles, 32, 297 (1991).
G. A. Ellestad, F. M. Lovell, N. A. Perkinson, R. T. Hargreaves, and W. J. McGahren, J. Org. Chem., 43, 2339 (1978).
A. Ichihara, M. Hashimoto, T. Hirai, I. Takeda, Y. Sasamura, S. Sakamura, R. Sato, and A. Tajimi, Chem. Lett., 14, 95 (1989).
T. O. Larsen and J. Breinholt, J. Nat. Prod., 62, 1182 (1999).
J. Kjer, A. Debbab, A. H. Aly, and P. Proksch, Nat. Protoc., 5, 479 (2010).
H-J. Schiewe and A. Zeeck, J. Antibiot., 52, 635 (1999).
M. Ashour, R. Edrada, R. Ebel, V. Wray, W. Watjen, K. Padmakumar, W. E. G. Muller, W.- H. Lin, and P. Proksch, J. Nat. Prod., 69, 1547 (2006).
Acknowledgment
The KSU are gratefully acknowledged for (R. S. O). We are indebted to Prof. W. E. G. Muller (Johannes Gutenberg University, Mainz, Germany) for performing the cytotoxicity assay.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 6, November–December, 2017, pp. 878–880.
Rights and permissions
About this article
Cite this article
Orfali, R.S., Ebrahim, W. & El-Shafae, A.M. Secondary Metabolites from Alternaria sp., a Fungal Endophyte Isolated from the Seeds of Ziziphus jujuba . Chem Nat Compd 53, 1031–1034 (2017). https://doi.org/10.1007/s10600-017-2195-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-017-2195-9