The marine derived fungus Alternaria alternata (Fr.) Keissl. was isolated from branch samples of Beibu Gulf, Qinzhou, Guangxi, China and identified by 16s rDNA. Chemical study of A. alternata led to the isolation of a new isomer compound named tricycloalternarene 18c and nine known compounds, including alternariol monomethyl ether, mannitol, cyclo(Gly-Ala), tenuazonic acid, allantoin, thymine, uracil, erythritol, and ergosterol. Their chemical structures were identified by extensive spectrum (IR, MS, 1D, and 2D NMR spectra) and compared with the reported literature data.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The genus Alternaria belongs to the family Dematiaceae and consists of more than 50 kinds of species. Phytochemical study of Alternaria genus has led to the isolation of pyrone, terpenoids, steroid, cyclodipeptides, quinones, and other compounds [1–5].
Alternaria alternata (Fr.) Keissl. is one of the common fungus in Alternaria genus; the main metabolites show anticancer properties against mammary cancer cells and hepatocellular carcinoma [6, 7] and other kinds of bioactivities such as antimicrobial, anti-inflammatory, bactericidal, and antioxidant [8–10]. Therefore, it is important to study its chemical composition more clearly.
The structures of 10 compounds were elucidated based on physical and spectroscopic data (UV, IR, 1D NMR, 2D NMR, and MS), and by comparison with reported literature data. The known compounds 2–10 were identified as alternariol monomethyl ether (2), mannitol (3), cyclo(Gly-Ala) (4), tenuazonic acid (5), allantoin (6), thymine (7), uracil (8), erythritol (9), and ergosterol (10) [11–19]. Compound 4 was isolated for the first time from Alternaria genus, and 6, 7, and 9 were separated from this species for the first time.
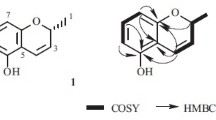
Compound 1 was isolated as a yellow oil and was assigned the molecular formula C19H26O5 on the basis of HR-ESI-MS data at m/z 335.1848 [M + H]+ (calcd for C19H26O5, 334.1780), indicating seven degrees of unsaturation. The UV spectrum of 1 showed absorption at λmax 263 nm, and the IR spectrum displayed absorption bands at 3364 (OH), 2973, 2900 (CH3), and 1653 cm–1 (C=O). From the UV, IR, 1H NMR, 13C NMR, HMBC, 1H–1H COSY, HSQC, and MS, the planar structure was postulated to be the same as that of tricycloalternarene 18b in the literature [1].

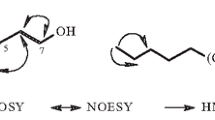
With respect to the NOESY data, differences in the absolute configuration were observed. The chemical shifts in the NMR spectrum at δ 70.34 (C-15) and 3.89 (J = 12.4, 5.1 Hz, H-15) in DMSO-d6 were nearly identical to that of tricycloalternarene 18b in the same position at δ 70.34 (C-15) and 4.02 (J = 12.6, 5.1 Hz, H-15) in CD3OD [1] instead of tricycloalternarene A in the same position at δ 74.05 (C-15) and 4.05 (J = 10.2, 4.2 Hz, H-15) in CDCl3 [20]. Hence 15-OH of compound 1 was identified to be in the α-configuration, similar to tricycloalternarene 18b, and H-15 was in the β-configuration. In the NOESY spectrum, H-15 (δ 3.89) correlated to H-10β (δ 2.05) and H-9 (δ 2.78) correlated to H-10α (δ 2.48), so H-9 was inferred to be in the α-configuration. Since CH3-8′ (δ 1.38) showed no correlations to H-9 (δ 2.78), it can be concluded H-9α was situated in a different plane from CH3β-8′. Hence, the absolute configuration of compound 1 was determined as 8R, 9R, 15R, which is different from that of tricycloalternarene 18b, and named tricycloalternarene 18c.
EXPERIMENTAL
General. The NMR spectra were recorded on a Bruker-600 spectrometer (Bruker, Switzerland), and HR-ESI-MS were recorded on a Thermo Q Exactive (Thermo Fisher Scientific, USA) system. Column chromatography was performed using silica gel, RP C-18, and Sephadex LH-20 as a stationary phase. PTLC was carried out using equipment manufactured by Beijing Tong Heng Innovation Technology Co., Ltd. (Beijing, China). IR data were recorded on a PerkinElmer Spectrum 100 (PerkinElmer, USA) system; UV data, on a UV-2700 spectrophotometer (Shimadzu, Kyoto, Japan). Melting points were recorded by an X-5 Micromelting point apparatus (UVTEC, Beijing, China).
Fungus Material. A. alternata was isolated from marine beach samples of Beibu Gulf, Qinzhou, Guangxi, China and identified as Alternaria alternata based on morphology and 16s rDNA sequence analysis. A voucher specimen (ZHS0220507) is deposited in the School of Pharmaceutical Science, Guangxi Medical University, and Guangxi Academy of Sciences, Nanning, Guangxi, China.
Extraction and Isolation. The fungus A. alternata was cultivated in 20 L MD liquid medium (add 5 g glucose, 2.5 g peptone, 0.5 g KH2PO4, and 0.25 g MgSO4·7H2O to 250 mL sterilized seawater, then pure water to 500 mL), and the incubator was shaken at 30°C and 200 rpm for 7 days. The culture medium was ultrasonically extracted with a quintuple volume of MeOH four times, and the extracts were concentrated. Then the concentrate was extracted with a quintuple volume n-BuOH to give 34.85 g of a crude extract. The crude extract was initially separated into enriched fractions using a silica gel (200 × 300 mesh) column and eluted with CHCl3–MeOH in a gradient manner (10:0–0:10) to generate five fractions (Frs. A–E). Fraction A (3.42 g) was applied to a Sephadex LH-20 column and eluted with CHCl3–MeOH (6:4) to get two subfractions (A1–A2). Subfraction A1 was recrystallized with MeOH to give compound 2 (2.5 mg). Subfraction A2 was subjected to the same separation method to get two subfractions (A2-1–A2-2). Compound 1 (16.7 mg) was isolated by preparative thin-layer chromatography (PTLC) and eluted with petroleum ether–EtOAc (1:1) from Subfr. A2-1. Compound 10 (6.8 mg) was isolated on an RP-C18 column and eluted with a gradient mixture of MeOH–H2O (4:6–10:0) from Subfr. A2-2. Fraction B (3.21 g) was separated into two subfractions (B1–B2) by the same method as Fr. A. Subfraction B1 was applied to a Sephadex LH-20 column and eluted with a gradient of MeOH–H2O (4:6–10:0) to give compounds 7 (24.7 mg) and 8 (16.1 mg). In the same way, compound 9 (30.0 mg) was isolated from Subfr. B2. Fraction C (9.98 g) was subjected to silica gel column chromatography and eluted with a gradient mixture of CHCl3–MeOH (10:0–0:10) to get five subfractions (C1–C5). Subfraction C2 was applied to an RP-C18 column, eluted with a gradient mixture of MeOH–H2O (4:6–10:0), and furthermore separated by a Sephadex LH-20 column and eluted with a gradient mixture of MeOH–H2O (4:6–10:0) to give compound 5 (13.7 mg). Fraction D (3.89 g) was subjected to RP-C18 column chromatography and eluted with a gradient mixture of MeOH–H2O (4:6–10:0), then recrystallized (MeOH) to get compound 4 (2.6 mg). Fraction E (11.97 g) was applied to a silica gel column and eluted with a gradient mixture of CH2Cl2–MeOH (10:0–0:10) to get four subfractions (E1–E4). Subfraction E1 was subjected to Sephadex LH-20 column chromatography and eluted with a gradient mixture of MeOH–H2O (4:6–10:0) to give compound 6 (21.6 mg). Meanwhile, compound 3 (22.2 mg) was isolated from Subfr. E2 using the same separation method.
Tricycloalternarene 18c (1). 1H NMR (600 MHz, DMSO-d6, δ, ppm, J/Hz): 0.93 (3H, d, J = 6.9, H-4′), 1.38 (3H, s, H-8′), 1.54 (1H, m, H-3b), 1.61 (1H, m, H-3a), 1.68 (1H, m, H-14b), 1.93 (1H, m, J = 7.0, H-4), 2.05 (1H, m, H-10b), 2.09 (1H, m, H-14a), 2.12 (1H, m, H-13b), 2.22 (2H, m, H-2), 2.42 (1H, m, H-7b), 2.48 (1H, m, H-10a), 2.48 (1H, m, H-13a), 2.54 (1H, m, H-7a), 2.78 (1H, dq, J = 7.0, 2.2, H-9), 3.53 (3H, s, OCH3), 3.89 (1H, dd, J = 12.4, 5.1, H-15), 5.35 (1H, s, H-6). 13C NMR (150 MHz, DMSO-d6, δ, ppm): 14.79 (C-10), 20.02 (C-4′), 22.81 (C-8′), 27.22 (C-13), 29.24 (C-14), 29.56 (C-3), 31.32 (C-2), 31.64 (C-4), 44.43 (C-7), 45.64 (C-9), 51.24 (OCH3), 70.34 (C-15), 87.71 (C-8), 104.88 (C-11), 120.35 (C-6), 148.70 (C-5), 170.56 (C-12), 173.24 (C-1), 197.15 (C-16).
Alternariol monomethyl ether (2), C15H12O5, light yellow powder, mp 268°C [11].
Mannitol (3), C6H14O6, colorless acicular crystals, mp 165.6°C [12].
Cyclo(Gly-Ala) (4), white powder, mp 235.5°C. HR-ESI-MS m/z 129.0658 [M + H]+. 1H NMR (600 MHz, CD3OD, δ, ppm, J/Hz): 1.43 (3H, d, J = 7.0, H-3), 3.92 (2H, m, H-2′), 4.01 (1H, q, J = 7.0, H-2). 13C NMR (150 MHz, CD3OD, δ, ppm): 19.42 (C-3), 45.44 (C-2′), 51.68 (C-2), 168.74 (C-1′), 171.63 (C-1) [13].
Tenuazonic acid (5), C10H15NO3, white powder, mp 133.5°C [14].
Allantoin (6), colorless crystals, mp 238.5°C. HR-ESI-MS m/z 157.0367 [M – H]–. 1H NMR (600 MHz, DMSO-d6, δ, ppm, J/Hz): 5.25 (1H, d, J = 8.2, H-4), 5.79 (2H, s, NH2-6), 6.89 (1H, d, J = 8.1, NH-CO-4), 8.06 (1H, m, NH-1), 10.54 (1H, s, NH-3). 13C NMR (150 MHz, DMSO-d6, δ, ppm): 62.42 (C-4), 156.76 (C-2), 157.35 (C-5), 173.60 (C-6) [15].
Thymine (7), white powder, mp 306.6°C. HR-ESI-MS m/z 127.0501 [M + H]+. 1H NMR (600 MHz, DMSO-d6, δ, ppm, J/Hz): 1.72 (3H, d, J = 1.3, H-7), 7.24 (1H, s, H-6), 10.58 (1H, s, NH-1), 11.00 (1H, s, NH-3). 13C NMR (150 MHz, DMSO-d6, δ, ppm): 11.85 (C-7), 107.71 (C-5), 137.76 (C-6), 151.53 (C-2), 164.96 (C-4) [16].
Uracil (8), C4H4N2O2, white powder, mp 314.6°C [17].
Erythritol (9), colorless crystals, mp 123.3°C. HR-ESI-MS m/z 123.0654 [M + H]+. 1H NMR (600 MHz, D2O, δ, ppm, J/Hz): 3.61 (4H, m, H-1, 4), 3.74 (2H, dd, J = 11.6, 2.4, H-2, 3). 13C NMR (150 MHz, D2O, δ, ppm): 62.58 (C-1, 4), 71.87 (C-2, 3) [18].
Ergosterol (10), C28H44O, colorless acicular crystals, mp 162.7°C [19]. Ten compounds were isolated from the fungus A. alternata, including a new isomer tricycloalternarene 18c and nine known compounds. Through extensive spectral data, the absolute configuration of tricycloalternarene 18c was identified, and all ten compounds will form the chemical basis for subsequent pharmacological experiments. Meanwhile, cyclo(Gly-Ala) (4) was isolated from Alternaria genus for the first time; allantoin (6), thymine (7), and erythritol (9) were isolated from A. alternata for the first time. Compounds of the tricycloalternarene series show significant bioactivity such as antimicrobial [21] and anticancer [22] activity. This work can provide the basis for more chemical compounds to explore new drug.
References
J. T. Wang, Z. H. Ma, G. K. Wang, F. Q. Xu, L. Chen, Y. Yu, G. Wang, and J. S. Liu, Phytochem. Lett., 31, 1 (2019).
Z. Z. Shi, F. P. Miao, S. T. Fang, X. H. Liu, X. L. Yin, and N. Y. Ji, J. Nat. Prod., 80, 2524 (2017).
O. Ozcinar, O. Tag, H. Yusufoglu, B. Kivcak, and E. Bedir, J. Nat. Prod., 81, 1357 (2018).
U. W. Hawas, S. El-Desouky, L. A. El-Kassem, and W. Elkhateeb, Appl. Biochem. Microbiol., 51, 579 (2015).
J. W. He, C. X. Wang, L. Yang, G. D. Chen, D. Hu, L. D. Guo, X. S. Yao, and H. Gao, Nat. Prod. Commun., 11, 829 (2016).
J. Hohenbichler, G. Aichinger, M. Rychlik, G. D. Favero, and D. Marko, Biomolecules, 10, 1018 (2020).
P. Palanichamy, S. Kannan, D. Murugan, P. Alagusundaram, and M. Marudhamuthu, J. Appl. Microbiol., 127, 1468 (2019).
X. L. Wu, S. Wang, C. Liu, C. F. Zhang, J. J. Guo, and X. Y. Shang, J. Nat. Med., 73, 620 (2019).
F. L. Li, W. G. Sun, J. K. Guan, Y. Y. Lu, S. Lin, S. T. Zhang, W. X. Gao, J. J. Liu, G. Du, J. P. Wang, H. C. Zhu, C. X. Qi, Z. X. Hu, and Y. H. Zhang, Org. Biomol. Chem., 16, 8751 (2018).
C. Sohini, G. Ranjan, M. Narayan Chandra, and V. Marie-Joelle, PloS One, 14, 1 (2019).
W. Gu, World J. Microbiol. Biotechnol., 25, 1677 (2009).
A. S. Awaad, H. M. Al-Zaylaee, S. I. Alqasoumi, M. E. Zain, E. M. Aloyan, A. M. Alafeefy, E. S. H. Awad, and R. M. El-Meligy, Phytother. Res., 28, 774 (2014).
Y. Y. Xu, L. Cheng, T. F. Yang, Q. L. Zhang, C. J. Chang, C. Feng, and W. Z. Liu, J. Chin. Med. Mater., 37, 2204 (2014).
M. Ashour, H. M. Yehia, and P. Proksch, J. Nat. Prod., 4, 108 (2011).
Y. Cai, Y. Lu, R. H. Chen, Q. L. Wei, and X. H. Lu, Phytomedicine, 18, 224 (2011).
A. S. Abdel-Razek, A. Hamed, M. Frese, N. Sewald, and M. Shaaban, Steroids, 138, 21 (2018).
E. M. C. Souza, E. L. Silva, A. M. R. Marinho, and P. S. B. Marinho, An. Acad. Bras. Cienc., 88, 29 (2016).
A. Liu, Z. M. Zou, L. Z. Xu, and S. L. Yang, J. Asian Nat. Prod. Res., 7, 861 (2005).
A. G. Boulis, A. A. Hamed, M. E. El-Awady, A. R. Mohamed, E. M. Eliwa, M. M. S. Asker, and M. Shaaban, Arch. Microbiol., 202, 1985 (2020).
L. Yuan, P. J. Zhao, J. Ma, G. H. Li, and Y. M. Shen, Helv. Chim. Acta, 91, 1588 (2008).
S. T. Fang, F. P. Miao, X. H. Liu, Y. P. Song, and N. Y. Ji, Phytochem. Lett., 23, 185 (2018).
L. Shen, S. J. Tian, H. L. Song, X. Chen, H. Guo, D. Wan, Y. R. Wang, F. W. Wang, and L. J. Liu, Mar. Drugs, 16, 402 (2018).
ACKNOWLEDGMENT
This research was funded by the Science and Technology Development Project of Guangxi, China (AB16380071), the Guangxi First-class Discipline Project for Pharmaceutical Sciences (GXFCDP-PS-2018), and the Guangxi Key Laboratory of Marine Natural Products and Combinatorial Biosynthesis Chemistry (GXMNP2018002).
Siyuan Liu and Meiqiong Liu contributed equally to this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 5, September–October, 2021, pp. 724–726.
Rights and permissions
About this article
Cite this article
Liu, S., Liu, M., Wu, H. et al. A New Isomer and Other Metabolites Isolated from Alternaria alternata. Chem Nat Compd 57, 844–847 (2021). https://doi.org/10.1007/s10600-021-03495-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-021-03495-8