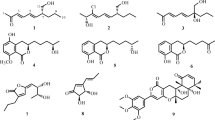
A new coumarin, 7-(γ,γ-dimethylallyloxy)-6-hydroxy-4-methylcoumarin (1), was isolated from the mixed fermentation broth of two mangrove fungi (strain No. K38 and E33) isolated from the South China Sea coast. The structure of 1 was determined by comprehensive spectroscopic methods, especially 2D NMR techniques. Primary bioassays showed that compound 1 at 0.25 mM has no inhibitory activity against Fusarium graminearum, Gloeosporium musae, Rhizoctonia solani, and Phytophthora sojae.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Mixed fermentation has been used in the food industry to enhance enzyme production [1–3]. Recently, it also has been used as a strategy for natural product chemistry research, which can result in increased antibiotic activity in the crude extract, increased yields of previously described or undetected metabolites, analogues of known metabolites resulting from combined pathways, and induction of previously unexpected pathways for bioactive constituents [4]. To date, at least 11 new compounds, including pestalone [2], libertellenones A–D [5], emericellamides A and B [6], marinamides A and B [7], aspergicin [8], 8-hydroxy-3-methyl-9-oxo-9H-xanthene-1-carboxylic acid methyl ether [9], and (–)-byssochlamic acid bisdiimide [10], were isolated from the mixed cultured marine-derived microorganisms.
In the study, we describe the isolation and identification of a new coumarin derivate, 7-(γ,γ-dimethylallyloxy)-6-hydroxy-4-methylcoumarin, obtained from the mixed fermentation broth of mangrove fungi E33 and K38. Its antifungal effects against four plant pathogenic fungi were measured in vitro.
A 200 L fermentation broth was concentrated and extracted with ethyl acetate. The extract was repeatedly chromatographed on silica gel to obtain compound 1 from the 25% ethyl acetate–petroleum fraction as colorless needles.
Compound 1 has the molecular formula C15H16O4 determined by HR-ESI-MS ([M]+, m/z 260.1044; calcd 260.1043). Strong UV peaks at λmax 228, 290, and 345 nm and an IR band at νmax 1694 cm–1 suggested a 7-oxygenated coumarin skeleton [11, 12].
In the 1H NMR spectra (Table 1), the characteristic signals of a trisubstituted coumarin with three singlets at δ 6.15, 7.08, and 6.82, and a methyl signal at δ 2.36 (3H, s), as well as a hydroxyl signal at δ 5.69 (1H, br.s), were observed. Additionally, the 1H NMR spectrum of 1 also revealed a set of peaks at δ 5.46 (1H, t, J = 7.2 Hz), 4.65 (2H, d, J = 7.2 Hz), 1.81 (3H, s), and 1.77 (3H, s) that were characteristic signals of a prenyloxy moiety [13, 14]. The HMBC correlations from the hydroxyl proton at δ 5.69 (6-OH) to carbons at δ 108.1 (C-5), 142.8 (C-6), and 149.1 (C-7), the methyl protons at δ 2.36 (4-CH3) to carbons at δ 112.5 (C-3), 152.5 (C-4), and 113.3 (C-10), and the methene protons at δ 4.65 (H-1′) to carbon at δ 149.1 (C-7) indicated that the hydroxyl moiety, the methyl group, and the prenyloxy moiety were connected to the carbons at δ 142.8 (C-6), 152.5 (C-4), and 149.1 (C-7), respectively (Fig. 1). Therefore, the structure of compound 1 was elucidated as 7-(γ,γ-dimethylallyloxy)-6-hydroxy-4-methylcoumarin.
In our present study, four kinds of plant pathogenic fungi were examined. The inhibitory zone diameters of compound 1 against Fusarium graminearum, Gloeosporium musae, Rhizoctonia solani Kuhn, and Phytophthora sojae (Kaufmann & Gerdemann) were all below 6.00 mm at 0.25 mM concentration. This suggested that compound 1 has no inhibitory activity against these plant pathogens.
Experimental
UV absorptions were measured in MeOH on a Shimadzu UV-2450 spectrophotometer (Shimadzu corporation, Kyoto, Japan). IR spectra were obtained on a Nicolet 5DX-FTIR spectrophotometer (Thermo Electron corporation, Madison, USA). NMR data were recorded on a Bruker AV III 600 MHz NMR spectrometer (Bruker BioSpin GmbH company, Rheinstetten, Germany), using CD3COCD3 as solvent and TMS as internal standard, and coupling constants (J) are in Hz. EI-MS and HR-EI-MS were acquired on Agilent 1100 Series LC/MSD Trap and Agilent TOF MSD 1946D mass spectrometers, respectively. Chromatography was carried out on silica gel column (200–300 mesh; Qingdao Haiyang Chemicals Co., Ltd., Qingdao, China). All other reagents used were analytical grade.
Fungus Material and Culture Conditions. The fungi E33 and K38 were isolated from the South China Sea coast. They are both apospory and their general species have not been identified. Starter cultures were maintained on cornmeal seawater agar. Plugs of agar supporting mycelial growth were cut and transferred aseptically to a 250 mL Erlenmeyer flask containing 100 mL of liquid medium (glucose 10 g/L, peptone 2 g/L, yeast extract 1 g/L, NaCl 30 g/L). The flask was incubated at 30C on a rotary shaker for 5–7 days. The mycelium was aseptically transferred to 500 mL Erlenmeyer flasks containing culture liquid (200 mL). The flasks were then incubated at 30C for 25 days.
Extraction and Separation of Metabolites. The cultures (200 L) were filtered through cheesecloth. The filtrate was concentrated to 5 L in vacuo below 50C and extracted five times by shaking with an equal volume of ethyl acetate. The combined extracts were chromatographed repeatedly on a silica gel column using gradient elution from petroleum to ethyl acetate to obtain 7-(γ,γ-dimethylallyloxy)-6-hydroxy-4-methylcoumarin (1) from the ethyl acetate–petroleum ether (25:75) fraction.
7-( γ , γ -Dimethylallyloxy)-6-hydroxy-4-methylcoumarin (1). C15H16O4, colorless needles. UV (MeOH, λmax, nm): 228, 290, 345. IR (KBr, νmax, cm–1): 3421, 2925, 1694, 1611, 1570, 1450, 1291, 1208, 975, 865. For 1H NMR (600 MHz, CD3COCD3, δ, ppm, J/Hz) and 13C NMR (150 MHz, CD3COCD3, δ, ppm) data, see Table 1. EI-MS m/z 260 [M]+, HR-EI-MS m/z 260.1044 [M]+; calcd 260.1043.
Fungicidal Acivity in vitro. The antimicrobial activity of the pure compound against four fungal pathogens Fusarium graminearum, Gloeosporium musae, Rhizoctonia solani Kuhn, and Phytophthora sojae (Kaufmann & Gerdemann) were determined by the paper disc-agar diffusion assay [15]. A paper disk of 6.00 mm diameter was impregnated with the pure compounds at 0.25 mM concentration and then placed on PDA medium freshly seeded with a microconidial suspension of the tested fungal pathogens. The inhibitory zone diameters were measured after incubation at 28C for 48 h. All treatments consisted of three replicates.
References
I. Wiesel, H. J. Rehm, and B. Bisping, Appl. Microbiol. Biotechnol., 47, 218 (1997).
M. Cueto, P. R. Jensen, C. Kauffman, W. Fenical, E. Lobkovsky, and J. Clardy, J. Nat. Prod., 64, 1444 (2001).
M. Gutierrez-Correa and R. P. Tengerdy, Biotechnol. Lett., 20, 45 (1998).
R. K. Pettit, Appl. Microbiol. Biotechnol., 83, 19 (2009).
D. C. Oh, P. R. Jensen, C. A. Kauffman, and W. Fenical, Bioorg. Med. Chem., 13, 5267 (2005).
D. C. Oh, C. A. Kauffman, P. R. Jensen, and W. Fenical, J. Nat. Prod., 70, 515 (2007).
F. Zhu, Chin. Sci. Bull., 51, 1426 (2006).
F. Zhu, G. Chen, X. Chen, M. Huang, and X. Wan, Chem. Nat. Compd., 47, 767 (2011).
C. Y. Li, J. Zhang, C. L. Shao, W. J. Ding, Z. G. She, and Y. C. Lin, Chem. Nat. Compd., 47, 382 (2011).
C.Y. Li, W. J. Ding, C. L. Shao, Z. G. She, and Y. C. Lin, J. Asian Nat. Prod. Res., 12, 809 (2010).
S. W. Hwang, J. R. Lee, J. Lee, H. S. Kwon, M. S. Yang, and K. H. Park, Heterocycles, 65, 1963 (2005).
T. T. Dao, T. L. Tran, J. Kim, P. H. Nguyen, E. H. Lee, J. Park, I. S. Jang, and W. K. Oh, J. Nat. Prod., 75, 1332 (2012).
H. Saglam, T. Gozler, and B. Gozler, Fitoterapia, 74, 564 (2003).
C. C. Shen, H. C. Yang, R. L. Huang, J. C. Chen, and C. C. Chen, J. Chin. Med., 16, 57 (2005).
A. W. Bauer, W. M. M. Kirby, J. C. Sherris, and M. Turck, Am. J. Clin. Pathol., 36, 493 (1966).
Acknowledgment
This work was supported by the National Natural Science Foundation of China (21102049), the Natural Science Foundation of Guangdong Province (9451064201003751), the Science and Technology Project of Guangzhou City (11C12100771), and the Key Academic Program of the 3rd Phase “211 Project” of South China Agricultural University (2009B010100001), and the national scholarship fund for studying abroad of China (file No. 201208440268).
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 2, March–April, 2015, pp. 213–214.
Rights and permissions
About this article
Cite this article
Wang, J., Huang, S., Li, C. et al. A New Coumarin Produced by Mixed Fermentation of Two Marine Fungi. Chem Nat Compd 51, 239–241 (2015). https://doi.org/10.1007/s10600-015-1252-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-015-1252-5