Salinity is a major constraint to crop productivity worldwide. Piriformospora indica was shown to promote the growth and also enhance resistance/tolerance to biotic and abiotic stresses. This work was intended to study the potential of P. indica in enhancing growth and elevating salt resistance in barley (Hordeum vulgare L.). Physiological and morphological experiments were used to investigate the metabolome changes in inoculated plants. The seedlings were inoculated with P. indica and 2 weeks after inoculation were treated with three salt stress levels. Four weeks after inoculation, leaf samples were collected and metabolite was extracted from leaves of inoculated and noninoculated barley (cultivar Pallas) plants. The physiological results showed that P. indica increases the biomass of aerial parts of inoculated plants compared with control plants under ambient and stress condition. Also, fungus affected ion content in inoculated plants and increased the K+/Na+ and Ca2+/Na+ ratios. The metabolomic results revealed that P. indica increases the sugars and free amino acid content in inoculated plants compared with control plant under salt stress. According to the results, this fungus can be used to produce growth-stimulating agents and biological fertilizers for use in crop production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Salinity is one of the major physical parameters of an environment that determines the success or failure of plant establishment and growth. Mutualistic symbiosis with mycorrhizal and endophytic fungi can promote salt tolerance in plants and decrease yield losses. The most ancient symbioses are arbuscular mycorrhizal (AM) symbioses, which represent a widespread host. The endophytic fungus Piriformospora indica, a basidiomycete of the order Sebacinales, was isolated from the Indian Thar desert in 1997 [1]. P. indica is similar to AM fungi. The most important advantage of this fungus over AM fungus is that it is a facultative symbiont and can be easily grown on a variety of synthetic medium. P. indica has been shown to improve the growth of many plant species [2, 3].
Root colonization and association of fungal hyphae with root resulted in promotion of plant growth and seed yield especially under adverse conditions. The mechanism by which P. indica promotes the growth of plants is not yet very clear, but some studies have implicated various factors induced by it in plants that were responsible for its positive effects [4]. It has been shown that P. indica-infested plants are more resistant to salt stress compared with plants not inoculated with P. indica [5]. In order to elucidate the physiological and metabolic responses of P. indica colonized barley plants to salinization and compare them with noninoculated plants, we measured some physiological and morphological traits.
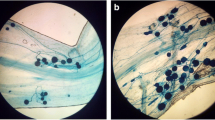
Microscopic inspection of roots in barley showed that the fungus enters roots and grows intercellularly in root cortex. A high degree of colonization was observed (more than 80%), and no colonization was observed in control plant roots.
We first characterized the interaction of P. indica with barley seedling. Plant inoculation with P. indica resulted in changes in total shoot fresh and dry weight. A growth-promoting effect could be observed during the growth and development of the plants. P. indica – inoculated plants grew faster and contained more leaves. Also, the overall habitus of inoculated plants looks bigger and stronger compared with control plants. After 4 weeks of barley development, the total shoot fresh and dry weights of inoculated plants were up to 26 and 24% higher than control plants under control (0 mM NaCl) conditions, respectively (Fig. 1).
To eliminate the effect of osmotic stress shock, plants were treated slowly. When inoculated and control plants were exposed for 2 weeks to moderate (100 mM) and severe (300 mM) salt stress, they showed reduced growth. The shoot fresh and dry weights were reduced 43 and 26%, respectively, in inoculated plants compared to 51 and 40% in control plants. Also, physiological experiment revealed that inoculated plants appear to be more resistant to stress in the greenhouse, so that in inoculated plants, shoot fresh and dry weights were up to 1.53 and 1.44 times higher compared with noninoculated plants under severe salt stress (300 mM) (Fig. 1).
Both drought and salinity stress disturb the mineral-nutrient relations in plants through their effects on nutrient availability, transport, and partitioning in plants. Additionally, salinity stress also causes ion deficiency or imbalance due to the competition of nutrients such as K+ and Ca2+ with the toxic ions Na+ and Cl– [6]. The major ions involved in salt stress signaling, include Na+, K+, and Ca2+. K+ is a critical mineral nutrient that protects plant from salt-induced damages. Impairment of the K+ nutritional status of plants by increased Na+ uptake is a well-known phenomenon in the tissues of many plant species [7]. Our results showed that salt stress increased Na+ content, whereas the K+ content was decreased under salt stress. Also, fungus slightly increased K+ content and decreased Na+ content in inoculated plants.
High salinity and high Na+ concentration result in increased cytosolic Ca2+ that is transported from the apoplast as well as the intracellular compartments [8]. This transient increase in cytosolic Ca2+ initiates stress signal transduction, leading to salt adaptation. Our results showed that salt stress increases Ca2+, especially under severe stress. P. indica significantly increased the Ca2+ under ambient and severe stress. Vadassery et al. reported the same results [9]. They concluded that Ca2+ is likely to be an early signalling component in the mutualistic interaction between P. indica and A. thaliana. It seems that Ca2+ has the same role in barley plants. For decades it has been shown that Ca2+ has a role in providing salt tolerance to plants [10]. In this report, P. indica had the greatest effect on Ca2+ content and likely influenced the stress signal transduction. Also, fungus increased the K+/Na+ and Ca2+/Na+ ratios in inoculated plants compared with control plants under stress condition. These ratios, especially the K+/Na+ ratio, are considered to be a reliable indicator of the severity of salt stress, or for screening plant genotypes for high Na+ tolerance. High K+/Na+ ratios will also improve the resistance of barley plant to salinity.
Our results showed that the Na+ content of control and inoculated plant leaves was markedly affected by salinity (Table 1). Under severe salt stress (300 mM NaCl), the Na+ content of control and inoculated plants increased up to 13 and 7 times, respectively. A slight decrease in K+ content was seen (7% and 2% in control and inoculated plants, respectively). The increase in Ca2+ content under stress was less pronounced and increased to 8 and 10% in control and inoculated plants, respectively. The amount of Ca2+ in inoculated plants was higher than in the control plant, especially under severe salt stress (27% higher). The K+/Na+ ratio was higher in inoculated plants compared with control plants under salt stress (2.13 in inoculated plants compared to 1.91 in control plants under severe salt stress). Similar results were seen on the Ca2+/Na+ ratio (0.52 in inoculated plants compared to 0.37 in control plants under severe salt stress).
To evaluate whether the accumulation of carbon-containing metabolites was reflected in the change in the carbon to nitrogen ratio, we measured the total contents of carbon and nitrogen. Parallel to further sugar accumulation, an increase in the carbon to nitrogen ratio was observed in inoculated plants under severe salt stress (Fig. 2).
Sugars are the substrates of carbon and energy metabolism and are used in the biosynthesis of polysaccharides like starch and cellulose in plants. Our results showed that P. indica increased the starch content in inoculated plants under stress conditions, but the starch content was decreased in inoculated plants under control conditions. Sherameti et al. reported that one of the major starch-degrading enzymes, glucan-water dikinase, is activated by the fungus in colonized root [11]. In our research, we observed the same results in inoculated plant leaves. It seems that the faster growth of colonized plants requires the breakdown of starch, which is deposited in the root and shoot amyloplasts.
Many environmental stresses like salinity lead to major changes in carbohydrate metabolism [12–14], and the sugar signaling pathways interact with stress pathways to modulate metabolism. Indirectly, the sugars play an important role during plant growth and development under abiotic stresses by regulating carbohydrate metabolism [15]. Our results showed that P. indica increases the content of sugars (glucose, fructose, and sucrose) in inoculated plants. This protected the plant under stress and also increased the growth rate of inoculated plants. Both C and N nutrients are essential for various cellular functions, and therefore adequate supply of these two nutrients are critical for plant growth, development, and response to a wide array of stresses [10]. The fungus increased the C and N content in the inoculated plants. Also, P. indica increased the C/N ratio. This ratio is likely involved in photosynthate partitioning and yield potential under stress [16]. It seems that the fungus promoted the vegetative growth under stress by increasing this ratio.
Levels of Starch and Soluble Sugars. The content of starch was decreased in both groups of plant under salt stress. It seems that salinity stress increases the activity the enzyme of β-amylase and reduces the content of starch. Inoculation of plants with P. indica increased the content of starch under moderate and severe salt stress.
The levels of soluble sugars, including glucose, fructose, and sucrose, were higher in inoculated plants compared with control plants under different levels of salt stress (except for glucose in 0 mM NaCl) (Fig. 3). Plants inoculated with P. indica partition a larger portion of biomass (i.e., fixed C) to vegetative tissues than control plants. The fungus increased the C/N ratio in inoculated plants under stress.
Amino Acids. One of the mechanisms that plants use to combat the detrimental effects of salt stress is to synthesize and accumulate compatible solutes, typically certain polyols, sugars, amino acids, betaines, and related compounds [17, 18].
In a recent study, salt stress increased a large number of free amino acids, especially proline, serine, asparagine, and glutamine. Fungus increased the content of some amino acids like aspartic acid, asparagines, arginine, alanine, and GABA in inoculated plants compared with control plants. These results were similar to those of other researchers. Mansour reported that amino acids (alanine, arginine, glycine, serine, leucine, and valine, together with the imino acid proline and the nonprotein amino acids citrulline and ornithine) accumulate in plants subjected to salt stress [19]. Fougere et al. reported that the concentration of asparagine frequently increases in response to stress [20]. In barley, NaCl stress enhanced the asparagine pool in roots, xylem sap, and leaf blades of seedlings, whereas glutamine increased only in roots and leaf blades [21]. Total free amino acids in the leaves have been reported to be higher in salt-tolerant than in salt-sensitive lines of sunflower [22], Eruca sativa [23], and Lens culinaris [24]. Simon-Sarkadi et al. reported that the total free amino acid content increased in all varieties during salt stress [25].
Two important amino acids (proline and GABA) were increased under salt stress. Proline, which occurs widely in higher plants and accumulates in larger amounts than other amino acids [26], regulates the accumulation of usable N. Proline is osmotically very active and contributes to membrane stability and mitigates the effect of NaCl on cell membrane disruption [27]. Although salt stress has a significant effect on proline content, fungus does not.
P. indica increased GABA significantly under moderate and severe salt stress. Some experiments showed that GABA is immensely and rapidly produced in response to biotic and abiotic stresses. It was hypothesized that the degradation of GABA could limit the accumulation of reactive oxygen intermediates under oxidative stress conditions [28]. GABA could be a signaling molecule in plants [29]. Based on our results, P. indica increased GABA amino acid in inoculated plants. Overall, the sugars and amino acid results showed that the endophyte fungus increased compatible solutes in inoculated plants, thus helping plants to protect their proteins and DNA from osmotic damages and the effect of reactive oxygen species. Moreover, some of these solutes like GABA might be involved in signaling pathways.
The determination of free amino acid contents in both groups of plants in the vegetative phase (fourth week) revealed that most of the free amino acid content in both groups (inoculated and control plants) increased with severity of salt stress, resulting in a maximum seventyfold increase in proline content under severe salt stress (Table 2). Of 20 amino acids, salt stress had a significant effect on 18 amino acids. Also, the fungus had a significant effect on 8 amino acids. Our results showed that salt stress increases the amount of GABA and proline. GABA was elevated by up to 1.8- and 1.7-fold in control and inoculated plants under severe salt stress, respectively. P. indica has a significant effect on GABA content and increased the amount of GABA in inoculated plants under moderate and severe salt stress. A remarkable increase in proline content was observed under severe salt stress. The level of proline was found to increase 3.5- and 4-fold in control and inoculated plants under moderate salt stress, respectively. Under severe salt stress, proline was found to increase 71- and 77-fold in control and inoculated plants, respectively. The level of proline was higher in inoculated plants compared with control plants under moderate and severe salt stress, but this increase was not significant.
Experimental
Fungal Experiment. P. indica was maintained on CM medium [30] at 24°C for 4 weeks. For solid medium, 15 g·L–1 agar was used.
Plant Culture and Co-Culture of Plants with P. indica . Barley (Hordeum vulgare L.) of the cultivar Pallas was grown in a 2:1 mixture of sand and perlite in a growth chamber at 22°C/18°C day/night cycle, 60% relative humidity, and a photoperiod of 16 h (220 μmol·m–2·s–1 photon flux density) and was irrigated by Wuxal Top N solution (Schering, N/P/K: 12/4/6). The experiment was conducted in a completely randomized design with two fungus treatment (inoculated and noninoculated) and three salt levels (0, 100, and 300 mM NaCl) in three independent replications. Barley kernels were sterilized with 1% sodium hypochloride, rinsed in distilled water 3–4 times, and germinated. For inoculation, seedling roots were immersed in an aqueous solution of 0.02% Tween-20 containing 5 × 105 spores mL–1. Control seedling roots (noninoculated) were immersed in the same solution without spores. Control and inoculated seedlings were transferred to pots and grown for 4 weeks. Before induction of stress, root samples were checked for P. indica infestation. Fourteen days after inoculation, plants were watered with double-distilled water containing 0, 100, and 300 mM NaCl at 2-day intervals. After 4 weeks of inoculation, leaf samples were collected from the control and inoculated plant under different salt stress condition. Three plants per pot (as one replication) were harvested for physiological analysis. The sample for metabolome analysis was immediately frozen in liquid N2.
Physiological Analysis. After harvesting, the total shoot fresh weight was obtained immediately, and then the dry weight was measured by drying in an oven at 70°C for 48 h. The Na+, K+, and Ca2+ contents were measured in six replicates using an ICP-OES machine after extraction with HNO3. Also, the K+/Na+ and Ca2+/Na+ ratios were measured.
Measurement of Carbon-to-Nitrogen Ratio. The relative content of total carbon and total nitrogen was measured according to [31] by an elemental analyzer (Vario EL; Elementaranalysen-systeme GmbH, Hanau, Germany) [31]. Each value represents by the mean of six replicates (three biological replicates and two technical replicates) ± SE.
Determination of Soluble Sugars and Starch. Fresh leaf (50 mg) was extracted in 80% ethanol and assayed for soluble sugars using a microtiter plate reader as described by [32]. Starch hydrolysis was carried out by incubating the aliquots in a buffer containing 50 mM sodium acetate, pH 5.2, and 7 units/mg of amyloglucosidase (Roche Diagnostics GmbH, Mannheim, Germany). Determination of the glucose produced was performed according to [33].
Determination of Amino Acids by Reverse-Phase HPLC. Amino acids were extracted as described in [31], and derivatized with fluorescence reagent 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate (ACQ) (AccQ-FluorTM Reagent Kit; Waters, http://www.waters.com). Probes were analyzed using the reverse-phase HPLC System Alliance 2795 (Waters): reverse-phase column (XBridge; 150 nm, 5 mL); excitation wavelength, 300 nm; detection wavelength, 400 nm. Eluents consist of buffers A [140 mM sodium acetate (Merck), 7 mM triethanolamine (Sigma-Aldrich), B [acetonitrile (Roth), and C [purified water (Millipore). The column was equilibrated with buffer A (0.6 mL per min). Separation was performed with gradient buffer B:1% at 0.5 min, 5% at 27 min, 9% at 28.5 min, 18% at 44.5 min, 60% at 47.5 min, and 0% at 50.5 min, at 37°C [34]. Chromatograms were analyzed by Empower Pro software (Waters, Milford, MA, USA).
The combination of metabolomics and physiological studies provided us with a better understanding of salt-tolerance mechanisms in P. indica-inoculated plants. Our results showed that P. indica promotes growth of barley plants and has an impact on plant stress response. The metabolomics results further revealed the complexity of compatibility mechanisms in host–microbe interactions and showed that inoculation of plants with P. indica changes the ion, starch, sugars, and amino acid contents. According to these results and because the fungus does not infest leaves, it is likely that P. indica might induce systemic resistance to salt stress by changing the metabolome of inoculated plants. P. indica has a clear advantage over the manipulation of individual components in salt (and other abiotic) stress tolerance, since it targets a large number of genes and even pathways simultaneously and has no obvious side effects for biotechnological application. P. indica may also serve as a model system to study molecular traits affecting abiotic resistance in cereals.
References
A. Varma, S. Verma, N. Sahay, B. Butehorn, and P. Franken, Appl. Environ. Microbiol., 65, 2741 (1999).
V. Rai, Biol. Plantarum, 45, 481 (2002).
M. Ghabooli, B. Khatabi, F. S. Ahmadi, M. Sepehri, M. Mirzaei, A. Amirkhani, J. V. Jorrin-Novo, and G. H. Salekdeh, J. Proteomics, 94, 289 (2013).
F. Waller, H. Achatz, H. Baltruschat, J. Fodor, M. Becker, M. Fischer, T. Heier, R. Huckelhoven, C. Neumann, and D. Von Wettstein, Proc. Natl. Acad. Sci. USA, 102, 13386 (2005).
X. Qiang, M. Weiss, K. H. Kogel, and P. Schafer, Mol. Plant Pathol., 13 (5), 508 (2012).
Y. Hu and U. Schmidhalter, J. Plant Nutr. Soil Sci., 168, 541 (2005).
J. Liu and J. K. Zhu, Proc. Nat. Acad. Sci. USA, 94, 14960 (1997).
H. Knight, J. Trewavas, and M. R. Knight, Plant J., 12, 1067 (1997).
J. Vadassery, S. Ranf, C. Drzewiecki, A. Mithofer, C. Mazars, D. Scheel, J. Lee, and R. Oelmuller, Plant J., 59, 193 (2009).
S. Mahajan and N. Tuteja, Arch. Biochem. Biophys., 444, 139 (2005).
I. Sherameti, B. Shahollari, Y. Venus, L. Altschmied, A. Varma, and R. Oelmuller, J. Biol. Chem., 280, 26241 (2005).
M. F. Thomashow, Annu. Rev. Plant Biol., 50, 571 (1999).
L. A. Wanner and O. Junttila, Plant Physiol., 120, 391 (1999).
S. Kaur, A. K. Gupta, and N. Kaur, Plant Growth Regul., 30, 61 (2000).
J. Price, A. Laxmi, S. Martin, and J. C. Jang, Plant Cell, 16, 2128 (2004).
L. Borras, G. A. Slafer, and M. E. Otegui, Field Crops Res., 86, 131 (2004).
H. J. Bohnert and R. G. Jensen, Trends Biotechnol., 14, 89 (1996).
S. Ramanjulu and D. Bartels, Plant, Cell Environ., 25, 141 (2002).
M. Mansour, Biol. Plantarum, 43, 491 (2000).
F. Fougere, D. Le Rudulier, and J. G. Streeter, Plant Physiol., 96, 1228 (1991).
T. Yamaya and H. Matsumoto, Berichte des Ohara Instituts fur Landwirtschaftliche Biologie, Okayama Universitat, 19, 181 (1989).
M. Ashraf and M. Tufail, J. Agron. Crop Sci., 174, 351 (1995).
M. Ashraf, Biol. Plantarum, 36, 255 (1994).
W. J. Hurkman, H. P. Tao, and C. K. Tanaka, Plant Physiol., 97, 366 (1991).
L. Simon-Sarkadi, G. Kocsy, and Z. Sebestyen, Acta Biol. Szeged., 46, 73 (2002).
E. Abraham, G. Rigo, G. Szekely, R. Nagy, C. Koncz, and L. Szabados, Plant Mol. Biol., 51, 363 (2003).
M. Mansour, Plant Physiol. Biochem., 36, 767 (1998).
N. Bouche and H. Fromm, Trends Plant Sci., 9, 110 (2004).
A. M. Kinnersley and F. J. Turano, Crit. Rev. Plant Sci., 19, 479 (2000).
G. H. Pham, R. Kumari, A. Singh, R. Malla, R. Prasad, M. Sachdev, M. Kaldorf, F. Buscot, R. Oelmuller, and R. Hampp, Plant Surface Microbiol., 593 (2004).
H. Rolletschek, M. R. Hajirezaei, U. Wobus, and H. Weber, Planta, 214, 954 (2002).
L. Voll, R. E. Hausler, R. Hecker, A. Weber, G. Weissenbock, G. Fiene, S. Waffenschmidt, and U. I. Flugge, Plant J., 36, 301 (2003).
M. R. Hajirezaei, Y. Takahata, R. N. Trethewey, L. Willmitzer, and U. Sonnewald, J. Exp. Bot., 51, 439 (2000).
V. B. Tognetti, M. D. Zurbriggen, E. N. Morandi, M. F. Fillat, E. M. Valle, M. R. Hajirezaei, and N. Carrillo, Proc. Nat. Acad. Sci., 104, 11495 (2007).
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 6, November–December, 2014, pp. 942–946.
Rights and permissions
About this article
Cite this article
Ghabooli, M. Effect of Piriformospora indica Inoculation on some Physiological Traits of Barley (Hordeum vulgare) Under Salt Stress. Chem Nat Compd 50, 1082–1087 (2014). https://doi.org/10.1007/s10600-014-1164-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-014-1164-9