Abstract
Piriformospora indica is known as a fungus that can easily colonize a wide range of plants and enhance host’s growth and tolerance to abiotic stresses, including salinity. The mechanistic basis behind this phenomenon remains poorly understood. This work was aimed to fill in this gap and reveal mechanisms enhancing salinity tolerance in maize roots colonised by P. indica. A range of agronomic and physiological characteristics were compared between inoculated and non-inoculated maize plants under 0/100/200 mM NaCl conditions. The impact of P. indica inoculation or root’s cytosolic K+ retention ability were also assessed using micro-electrode ion flux estimation technique. The results showed that inoculated plants had higher biomass, higher stomatal conductance, lower K+ efflux from roots and higher potassium content in shoots than non-inoculated plants under salt stress. Collectively, the results indicated that the beneficial effects of inoculation on plant performance under saline conditions were mainly attributed to the improved stomata operation associated with higher rate of K delivery into the shoots.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As a major agricultural problem, soil salinity affects most of arable land in arid regions. More than 800 million hectares of the total land surface suffers from soil salinization, significantly impacting on growth and productivity of agricultural crops (Shelden et al. 2016).
A number of approaches have been used to transform the saline–alkali land through fresh water irrigation and chemical modification, which however resulted in the huge cost and relatively minor beneficial effect. Attempts to improve the salt tolerance of crops through conventional breeding programmes have been also met with a rather limited success, due to physiological and genetic complexity of the salt tolerance trait. Also modest results were obtained from using an in vitro selection; marker-assisted breeding; the use of transgenic plants; interspecific hybridization; and using halophytes as alternative crops (Ismail and Horie 2017).
The naturally occurring plant growth-promoting rhizobacteria (PGPR) could result in a remarkable growth promotion and nutrient uptake under salinity stress in many plants (e.g. tomato, Balliu et al. 2015). Lately, the discovery of Piriformospora indica which belongs to the basidiomycete order Sebacinales suggested that this species could easily colonize a wide range of higher plants, increasing grain yield, and protecting their hosts from a large variety of abiotic stresses including drought (Zhang et al. 2017a, b), salinity (Ghaffari et al. 2016) and cold injury (Murphy et al. 2014). They have been also shown to be efficient in enhancing plant tolerance to biotic stresses (Waller et al. 2005), and assisting hosts in nutrients uptake (Achatz et al. 2010).
The mechanisms behind the above beneficial effects of P. indica on abiotic stress tolerance remain elusive. It was shown that P. indica affected the carbohydrate metabolism, nitrogen metabolism, and ethylene biosynthesis pathway in salt-affected plants; also, production of antioxidant enzymes that ameliorate damage of plants under salinity stress was enhanced (Waller et al. 2005; Ghaffari et al. 2016). It was also shown that P. indica could protect wheat plants from the detrimental effects of salinity by influencing the uptake of water, photosynthetic pigment contents and proline accumulation in seedlings (Zarea et al. 2012). Similarly, P. indica rescued growth diminution of rice seedlings under salt stress through enhanced photosynthetic pigment content and proline accumulation (Jogawat et al. 2013). Li et al. (2017) demonstrated that tolerance to salinity stress conferred by P. indica in Medicago truncatula was via accumulation of osmoprotectants, stimulating antioxidant enzymes and the expression of defence-related genes.
However, effects of salinity are broader than merely osmotic stress and increased ROS production. Specific ion toxicity becomes a dominant component after prolonged salinity exposures (Munns and Tester 2008), and disturbance to K+ homeostasis is also a key determinant of differential salinity stress tolerance, at both intra- and inter-specific level (Shabala et al. 2016; Shabala 2017). Ion toxicity caused by excessive Na+ and Cl− accumulation decrease the uptake of essential nutrients like phosphorus (P), potassium (K), nitrogen (N), and calcium (Ca) (Zhu 2001; Munns and Tester 2008). Moreover, Na+ toxicity is often associated with its competitive nature with K+ for binding to essential sites (Cuin et al. 2008). Also, salinity stress alters plant hormonal status and, specifically, ABA production. ABA is a major driving force behind stomatal closure that is essential in reduction of water evaporation under salt stress (Yang et al. 2014). ABA may also regulate other physiological and biochemical processes under salt stress, including those related to ionic homeostasis. It was shown, for example, that ABA contributes to a higher ratio of K+/Na+, by enhancing expression of genes encoding ion transporters and proton pumps which help in the Na+ compartmentation and selective absorption of K+ and thus maintains the normal K+/Na+ ratio (Janicka and Klobus 2007). However, not much is known about effects of P. indica on the functional expression and activity of transporters regulating plant Na+ and K+ homeostasis, and contributing mechanisms can be only speculated by making analogies with a mycorrhizal colonization.
Mycorrhizal colonization has also been shown to enhance K+ absorption and prevent Na+ translocation to shoot tissues, so mycorrhizal plants often have a higher K+/Na+ ratio and a lower shoot Na+ concentration under saline stress conditions (Sharifi et al. 2007). The only similar report for P. indica was a recent study by Abdelaziz et al. (2017) who showed that colonized Arabidopsis roots had lower Na+/K+ ratio and enhanced transcript levels of the genes encoding the high affinity potassium transporter 1 (HKT1) and the inward-rectifying K+ channels KAT1 and KAT2, compared to non-inoculated plants under salt stress.
In this work, we hypothesised that P. indica might regulate maize salt tolerance by similar mechanisms and aimed to gain a better understanding of how P. indica impacts plant ion homeostasis. Our specific focus was on root ion loading, one of the key traits conferring salinity stress tolerance in plants (Shabala et al. 2010; Bose et al. 2014).
Materials and methods
Plant materials and fungal culture
Grains of maize variety Huayu 11 were obtained from Hubei Jingchu Seed Industry Co., Ltd. Grains were surface sterilized with 2% NaClO for 10 min, and rinsed under running tap water for 1 h to remove any traces of NaClO. Grains were germinated on a tissue paper at 27 °C in a growth chamber under a dark condition for 2 days. P. indica was cultured on Aspergillus liquid media at 28 °C in the dark with shaking at 150 rpm for 12 days.
Root inoculation
The seedlings were inoculated by immersing them into 1% (w/v, 1 g filtered mycelium in 100 mL water) fungal mycelium; the control was inoculated with the autoclaved inoculum. All seedlings were planted into cylindrical pots (radius 14 cm, height 7 cm) filled with 1 kg sterilized sand (water content 25%) and grown in a growth chamber with watering 150 mL water every 2 days. A relative humidity of 55% and a temperature of 25 °C were provided, and daily illumination was 16 h. Roots of inoculated and non-inoculated plants were collected randomly from three different seedlings to check P. indica colonization after fortnight of inoculation. The extent of colonization was examined under a light microscope (Nikon CX41-72C02) as described by Jogawat et al. (2013). Briefly, washed root samples were soaked in 10% KOH solution overnight, acidified with 1 M hydrochloric acid for 20 min, and stained with 0.04% Trypan blue for 40 min, then examined under the microscope. The intracellular chlamydospores in roots were used as proof for colonization.
Experimental protocols
The experiment contained six treatments (2 × 3): inoculated/non-inoculated P. indica × 0/100/200 mM NaCl, which included: 0 mM NaCl + P. indica, 100 mM NaCl + P. indica, 200 mM NaCl + P. indica, 0 mM NaCl − P. indica, 100 mM NaCl − P. indica, 200 mM NaCl − P. indica (Fig. S1a).
Twelve plants of each treatment were grown, and the experiment was replicated three times. Seedlings were transplanted from sand into 1/2 Hoagland solution once colonization was examined. Two days after transplantation, salinity treatment has commenced by adding appropriate amounts of NaCl to Hoagland solution. The treatment lasted for 5 days. Agronomical and physiological characteristics were measured at the end of the treatments.
Agronomical assessment
The maize root surface area, root volume, total leaf area, fresh weight (FW) and dry weight (DW) of shoot and root of inoculated and non-inoculated plants were investigated after 5 d of salt treatments. Root surface area and root volume were assessed by WinRHIZO system (Regent Instruments, Canada). Leaf area was calculated by using 0.75 × length × maximum widths (Jin et al. 2016). The material was dried at 80 °C in an oven, to achieve a constant weight before the dry weight assessment.
Physiological assessment
The chlorophyll content (SPAD values) was measured daily after commencement of salinity treatment from the third leaf by using chlorophyll meter (SPAD-502, Konica, Japan). The malondialdehyde (MDA) content was determined at the end of the treatment as described elsewhere (Fan et al. 2015). Briefly, 0.3 g leaf tissue were ground with 5 mL trichloroacetic acid (TCA), then a mixture of 3 mL supernatant liquid and 3 mL thiobarbituric acid (TBA) was heated for 15 min by using a boiling water bath to complete the colour reaction. The absorbance was measured at 532 nm (A532), 600 nm (A600) and 450 nm (A450) using a spectrophotometer (Shimadzu UV-1800, Japan). The MDA concentration of the colour reaction product was calculated as CMDA (µmol/L) = 6.45(A532 − A600) − 0.56A450, and the MDA content of the sample was calculated using an equation: MDA (µmol/g FW) = ((CMDA × 6 mL/1000)/3 mL) × 5 mL/0.3 g. The gas exchange was measured by LI-6400 Portable Photosynthesis System (LI-COR, Lincoln, Nebraska, USA) from the same leaf as SPAD measurements. Measurements were conducted on a daily basis, between 10 am and 12 pm, under constant light conditions provided by the built-in lighting system.
Electrophysiology
After P. indica inoculation for 2 weeks, seedlings were transplanted from the sand into the aerated hydroponic solution (500 µM KCl and 100 µM CaCl2) and kept for 2 days. Then, net K+ fluxes were measured from mature root zone (20 mm from the root tip) in response to transient NaCl treatment using non-invasive microelectrode MIFE technique (UTas Innovation, Hobart, Australia), as described previously (Shabala et al. 2003). In brief, microelectrodes were pulled out using PE-22 puller (Narishige, Tokyo, Japan), dried in an oven and silanized with tributylchlorosilane (Cat 90794, Sigma-Aldrich, Australia). The prepared electrode blanks were backfilled with 200 mM KCl and electrode tips front-filled with potassium selective ionophore (Cat 99311, Sigma-Aldrich, Australia). Electrodes were mounted on a micromanipulator (MMT-5, Narishige, Tokyo, Japan) and calibrated in a set of three calibration solutions (250, 500 and 1000 µM KCl). For measurements, the electrode tip was positioned 45 µm away from the root surface. A computer-controlled stepper motor moved the electrode between two positions—45 µm (M1) and 85 µm (M2)—from the root surface, in a square-wave manner (half-cycle 6 s). The potential difference between the two positions were recorded and converted into electrochemical potential difference by CHART software (Newman 2001) using a calibrated Nernst slope. MIFEFLUX software was used to calculate net ion fluxes using cylindrical geometry profile (Newman 2001).
The ion flux measuring protocol was as described by Cuin et al. (2008). Briefly, a seedling was immobilised in a 35 mL Petri dish filled with 27 mL of Basic Salt Medium (BSM: 500 µM KCl and 100 µM CaCl2) solution and adapted for 1 h. The steady fluxes were measured in the BSM solution for 5 min. Then 3 mL of NaCl stock solution was applied to bring final NaCl concentration to either 100 mM or 200 mM, and transient K+ fluxes were recorded for a further 40 min. The first 60 s after NaCl stock addition to the measuring chamber were discarded to comply with non-stirred layer conditions required for MIFE measurements (Shabala and Hariadi 2005). This period appears as a gap in all figures.
Plant elemental analysis
The third leaves were harvested from 12 plants of each treatment and dried in an oven at 75 °C to obtain stable weight. For analysis, 0.5 g of dried leaf or root samples were powdered and digested in 5 mL H2SO4–H2O2, and then the reaction solutions were diluted to 100 mL. The potassium and sodium contents were detected using flame atomic absorption spectrometry (SuZhou FP640) modified from the method of Jiang et al. (2016). The sample ion content was calculated using a standard calibration curve taking into account a dilution factor.
Data analysis
The relative value (treatment value/control value) was calculated to estimate difference between inoculated (+ P. indica) and non-inoculated (− P. indica) plants under salinity stress. Data were analysed using two-way analysis of variance in SPSS version 17 (SPSS Inc., Chicago, USA).
Results
Inoculation examination
Chlamydospores of P. indica were observed in inoculated seedling’s lateral roots (Fig. S1c), and no colonization was observed in roots which were inoculated by autoclaved mycelium (Fig. S1b). The inoculation with life culture of P. indica had beneficial effects on both shoot (Fig. S2) and root growth (Fig. S3) of plants grown at extreme salinities (200 mM NaCl).
Growth parameters under salinity stress
The visual analysis showed that leaves of inoculated seedlings (+ P. indica) were bigger and more straight under salinity stress compared with non-inoculated plants (− P. indica). We also observed differences in roots with inoculated seedlings having more rootlets and longer roots under NaCl conditions compared with non-inoculated plants. Overall, all agronomical characteristics of inoculated plants such as fresh weight (FW), dry weight (DW) and root characteristics such as root volume were higher than in P. indica free plants under salinity stress (Fig. 1). This indicates that non-inoculated plants have more morphologically reduction than inoculated plants under NaCl condition.
Effect of P. indica on agronomical and root characteristics of Z. mays under salt treatment (expressed in relative units as percentage of control): a relative shoot fresh weight (FW), b relative shoot dry weight (DW), c relative plant fresh weight, d relative plant dry weight, e relative total leaf area, f relative root fresh weight (FW), g relative root dry weight (DW), h relative root volume, i relative root surface area. Mean ± SE (n = 5). *Significant at P < 0.05
Influence of P. indica on leaf MDA, SPAD content and gas exchange under salt condition
A mild salinity stress did not result in a substantial damage to membranes, with MDA content increasing only by approximately 10% in colonized plants (Fig. 2b). However, more severe 200 mM NaCl treatment led to the two-fold MDA increase compared with control. In both cases, the inoculated plants (+ P. indica) showed lower level of lipid peroxidation with MDA content in the P. indica colonized plants (+ P. indica) being lower than in P. indica free plants (− P. indica) (Fig. 2b). The above membrane damage was also reflected in the amount of the chlorophyll loss (Fig. 2a).
Effect of P. indica on leaf characteristics in Z. mays under salt treatment: a leaf chlorophyll content (SPAD values), b the relative malondialdehyde (MDA) content in leaves, c relative transpiration (Tr) (% control), d relative stomatal conductance (Gs) (% control), e relative intercellular CO2 concentration (Ci) (% control), f relative net photosynthetic rate (Pn) (% control). Mean ± SE (n = 5). *Significant at P < 0.05, **significant at P < 0.01
Salinity stress reduced leaf gas exchange characteristics in a dose-dependent manner (Fig. 2c–f). Specifically, relative stomatal conductance (Gs) and transpiration (Tr) of P. indica-free plants were 20% less than in P. indica-inoculated plants under 100 mM NaCl stress; however, the similar reduction was 40% when NaCl concentration increased to 200 mM (Fig. 2c, d). Significant (at P < 0.05) differences in Gs and Tr have been found between inoculated and non-inoculated plants under both 100 and 200 mM treatments, but colonized plants have always shown smaller reduction in Tr, Gs and intercellular CO2 concentration (Ci) than non-inoculated plants (Fig. 2c–e). As a result, the net CO2 assimilation rate in inoculated plants treated with 200 NaCl was 2.75-fold higher than in non-inoculated (Fig. 2f).
Elements and ion flux measurement
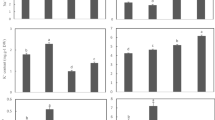
Salinity stress resulted in a massive accumulation of Na in both roots (Fig. 3a) and shoots (Fig. 3b). Root Na content was not dramatically different between 100 and 200 mM treatment while plant shoots treated with 200 NaCl accumulated three-fold more Na (Fig. 3b). Remarkably, plant inoculation with P. indica led to a reduction of Na accumulation in the root (Fig. 3a) but increased its content in the shoot (Fig. 3b), suggesting control of Na+ loading in root by P. indica.
Effect of P. indica on Na+/K+ content in Z. mays under salt treatment: a relative Na+ content in roots (% control), b relative Na+ content in shoots (% control), c relative K+ content in shoots (% control), d relative K+ content in roots (% control); ion fluxed measured from root mature zone surface. Transient net K+ fluxes of P. indica inoculated and non-inoculated Z. mays responses to e 100 mM NaCl, f 200 mM NaCl. Mean ± SE (n = 5). *Significant at P < 0.05, **significant at P < 0.01
The opposite scenario was observed for K+ content in shoots. Here, K content in roots exposed to 200 mM NaCl treatment was decreased by 4.5–5-fold compared with 100 mM treatment (Fig. 3d). The inoculated plants had less K+ in roots compared with their non-inoculated counterparts (Fig. 3d) under both 100 or 200 mM NaCl conditions but had much higher K content in the shoots (Fig. 3c).
We then checked whether lower K content in inoculated roots is related to their inability to keep K+ upon exposure to salinity (MIFE data Fig. 3e, f). This was not the case. An acute salinity stress induced root K+ efflux in a dose-dependent manner (Fig. 3e, f). However, the magnitude of K+ loss in inoculated plants was only half of that compared to non-inoculated counterparts. Taken all together (Fig. 3c, d), results suggested that inoculated plants are capable to regulate K loading in root and deliver sufficient quantities of K to the shoot (more than non-inoculated pants).
Discussion
The biggest advantage of Piriformospora indica compared with arbuscular mycorrhizal (AM) fungi is that P. indica can be easily cultured on various synthetic media, also it can colonize a very wide range of plants. Furthermore, P. indica can promote growth and ameliorate salt stress that caused damage of hosts as previously described (Ghaffari et al. 2016; Gill et al. 2016; Vahabi et al. 2016). The findings of this work are consistent with earlier reports and also demonstrate the beneficial effects of P. indica inoculation in maize. In addition, we demonstrate that maize seedlings colonized by P. indica had enhanced several vital parameters including growth, photosynthesis, and ion uptake under salinity stress compared with non-inoculated plants.
Chlorophyll content (SPAD data, Fig. 2a) was affected by salinity in inoculated plants only at the very severe stress while the difference in the gas exchange characteristics (Gs and Tr, Fig. 2c, d) was observed even at a mild stress level. Specifically, the SPAD values of non-inoculated plant leaf was only half of those in inoculated plants after 200 mM NaCl treatment for 5 days. Impacts to Gs and Tr were even higher with values in the non-inoculated plants being 60% lower under 200 mM NaCl and 13–20% lower under 100 mM NaCl than in inoculated plants. These indicate that the beneficial effects of inoculation on plant performance (biomass) were mainly attributed to the improved stomata operation. Indeed, both Gs and Tr of inoculated plants were significantly different from those in non-inoculated plants (P < 0.01) under salinity stress, which means the P. indica-colonized plants can still maintain higher stomatal conductance and transpiration rate when exposed to salinity.
Salinity stress reduces water availability leading to decline in both Gs and net photosynthetic rate (Pn) (Choudhury et al. 2017), with stomatal closure considered as the primary factor to limit photosynthesis. The decrease in stomatal conductance can be induced by the incapability of guard cells to fully adjust stomatal apertures due to the lack of the fully functional osmotic adjustment (Misra et al. 2015). Salinity stress also results in a significant increase in ABA level (Han et al. 2015) that acts as a signal to the stomata closure. At the early stage of salinity stress, changes in stomatal conductance are also matched by changes in the root hydraulic conductivity. It was shown that tolerant plants have some aquaporin genes up regulated to release the reduction of hydraulic conductance, which can help to maintain high stomatal conductance and photosynthesis to improve plant salt resistance (Liu et al. 2015).
The osmotic adjustment can be achieved by either de novo synthesis of compatible solutes (organic osmolytes) or by increased uptake of inorganic ions (K, Na and Cl). As the energy cost of biosynthesis of organic osmolytes may be 10-fold higher than for uptake of inorganic ions (Oren 1999), it was argued that the latter strategy is more efficient (Shabala et al. 2010). However, Na+ accumulation in the cytosol results in Na+ toxicity and should be avoided by an efficient Na+ sequestration in vacuoles, which is not always the case. Thus, relying on K+ for the osmotic adjustment in the shoot is the most preferred option.
In this study, the Na+ content in inoculated maize roots was 17% less than in non-inoculated plants, and the shoot K+ content in inoculated plants was 25% higher than in non-inoculated plants. This indicates that the inoculated plants were capable to reduce Na+ load in the roots but at the same time transported much more K+ to the shoots. As potassium is a key ion mediating stomata movement (Kollist et al. 2014), that may explain 2.5-fold higher Gs values in the inoculated plants treated with 200 mM NaCl compared with the non-inoculated plants. The higher K+ availability may be thus instrumental for the fully functional stomatal operation, allowing plants to avoid toxicity of Na and, at the same time, conserve a pool of available ATP that otherwise is used for the (expensive) process of de novo synthesis of organic osmolytes.
To avoid toxicity of Na+ to shoots, plants need to reduce its delivery to photosynthetic tissues. This can be achieved by either increased rate of re-translocation of Na+ back to roots via the phloem (Kobayashi et al. 2017), or by controlling the amount of Na+ loaded into the xylem (Shabala et al. 2013; Zhu et al. 2016). As the former option represents a “futile cycle”, an efficient control of xylem Na+ loading should be preferred. Several ion transporters may impact on xylem Na+ content. This includes Na+/H+ antiporter (SOS) (Shi et al. 2002) and high-affinity Na+/K+ permeable transporter (HKT) (Zhang et al. 2017a, b). The most likely candidates for xylem K+ loading are HKT, high-affinity K+ transporter (HAK) (Wang et al. 2015) and stellar K+ outward rectifying channel (SKOR) (Hu et al. 2016). Which of them may be affected by P. indica inoculation remain to be studied.
ABA is considered as a stress hormone, Roberts and Snowman (2000) suggested that ABA can induce accumulation of K+ through K+ channels, which is consistent with our results showing that the roots of inoculated plants had less K+ efflux compared with non-inoculated plants (Fig. 3e, f), thus indicating that under salinity condition plants inoculated with P. indica have higher ability to keep potassium than non-inoculated plants.
As discussed above, maintenance of shoot K+ content is important for plants in order to retain growth and development under saline condition. Also, K+ supply to a shoot requires xylem loading. Furthermore, accumulation of K+ in the xylem vessels of maize and sunflower roots can be induced by ABA (Collins and Kerrigan 1974; Founier et al. 1987). Research conducted on barely and Arabidopsis showed that ABA levels in inoculated roots could be higher than in non-inoculated roots that were attributed to P. indica-induced ABA signalling to affect initial host defence (Peskan-Berghofer et al. 2015).
Based on the above results, a testable model is suggested (Fig. 4). Salinity stress increases ABA levels in shoots, leading to stomata closure, in an attempt to save water although causing yield penalties. At the same time, increased ABA level in roots controls xylem parenchyma-based K+ and Na+ loading thus controlling their delivery to the shoot, to be used for osmotic adjustment. Plants inoculated with P. indica were shown to have elevated ABA levels (Peskan-Berghofer et al. 2015) and thus have an advantage of being more capable in sending K for osmotic adjustment (and stomata re-opening). ABA also restricts Na delivery to the shoot by controlling its loading into the xylem. This model may be used as a guide for the future studies. Also, the molecular identity of these transporters remains a subject for the future work.
Abbreviations
- ABA:
-
Abscisic acid
- AM:
-
Arbuscular mycorrhizal
- BSM:
-
Basic salt medium
- Ci:
-
Intercellular CO2 concentration
- DW:
-
Dry weight
- FW:
-
Fresh weight
- Gs:
-
Stomatal conductance
- MDA:
-
Malondialdehyde
- MIFE:
-
Micro-electrode ion flux estimation
- PGPR:
-
Plant growth-promoting rhizobacteria
- P. indica :
-
Piriformospora indica
- Pn:
-
Net photosynthetic rate
- TBA:
-
Thiobarbituric acid
- TCA:
-
Trichloroacetic acid
- Tr:
-
Transpiration
References
Abdelaziz ME, Kim D, Ali S, Fedoroff NV, Al-Babili S (2017) The endophytic fungus Piriformospora indica enhances Arabidopsis thaliana growth and modulates Na+/K+ homeostasis under salt stress conditions. Plant Sci 263:107–115
Achatz B, Rüden SV, Andrade D, Neumann E, Pons-Kühnemann J, Kogel KH, Franken P, Waller F (2010) Root colonization by Piriformospora indica, enhances grain yield in barley under diverse nutrient regimes by accelerating plant development. Plant Soil 333:59–70
Balliu A, Sallaku G, Rewald B (2015) AMF inoculation enhances growth and improves the nutrient uptake rates of transplanted, salt-stressed tomato seedlings. Sustainability 7:15967–15981
Bose J, Rodrigo A, Shabala S (2014) ROS homeostasis in halophytes in the context of salinity stress tolerance. J Exp Bot 65:1241–1257
Choudhury FK, Rivero RM, Blumwald E, Mittler R (2017) Reactive oxygen species, abiotic stress and stress combination. Plant J 90:856–867
Collins JC, Kerrigan AP (1974) The effect of kinetin and abscisic acid on water and ion transport in isolated maize roots. New Phytol 73:309–314
Cuin TA, Betts SA, Chalmandrier R, Shabala S (2008) A root’s ability to retain K+ correlates with salt tolerance in wheat. J Exp Bot 59:2697–2706
Fan H, Li T, Sun X, Sun XZ, Zheng CS (2015) Effects of humic acid derived from sediments on the postharvest vase life extension in cut chrysanthemum flowers. Postharvest Biol Technol 101:82–87
Founier JM, Benlloch M, Díaz de la Guardia M (1987) Effect of abscisic acid on exudation of sunflower roots as affected by nutrient status, glucose level and aeration. Physiol Plant 69:675–679
Ghaffari MR, Ghabooli M, Khatabi B, Hajirezaei MR, Schweizer P, Salekdeh GH (2016) Metabolic and transcriptional response of central metabolism affected by root endophytic fungus Piriformospora indica under salinity in barley. Plant Mol Biol 90:699–717
Gill SS, Gill R, Trivedi DK, Anjum NA, Sharma KK, Ansari MW, Ansari AA, Johri AK, Prasad R, Pereira E, Varma A, Narendra Tuteja N (2016) Piriformospora indica: potential and significance in plant stress tolerance. Front Microbiol 7:332
Han Y, Yin SY, Huang L (2015) Towards plant salinity tolerance-implications from ion transporters and biochemical regulation. Plant Growth Regul 76:13–23
Hu J, Ma Q, Kumar T, Duan HR, Zhang JL, Yuan HJ, Wang Q, Khan SA, Wang P, Wang SM (2016) ZxSKOR is important for salinity and drought tolerance of Zygophyllum xanthoxylum by maintaining K+ homeostasis. Plant Growth Regul 80:195–205
Ismail AM, Horie T (2017) Genomics, physiology, and molecular breeding approaches for improving salt tolerance. Annu Rev Plant Biol 68:405–434
Janicka RM, Klobus G (2007) Modification of plasma membrane and vacuolar H+-ATPases in response to NaCl and ABA. J Plant Physiol 164:295–302
Jiang C, Cui Q, Feng K, Xu D, Li C, Zheng Q (2016) Melatonin improves antioxidant capacity and ion homeostasis and enhances salt tolerance in maize seedlings. Acta Physiol Plant 38:1–9
Jin H, Li A, Wang J, Bo Y (2016) Improvement of spatially and temporally continuous crop leaf area index by integration of CERES-Maize model and MODIS data. Eur J Agron 78:1–12
Jogawat A, Saha S, Bakshi M, Dayaman V, Kumar M, Dua M, Varma A, Oelmüller R, Tuteja N, Johri AK (2013) Piriformospora indica rescues growth diminution of rice seedlings during high salt stress. Plant Signal Behav 8:e26891
Kobayashi N, Yamaji N, Yamamoto H, Okubo K, Ueno H, Costa A, Tanoi K, Matsumura H, Kashino M, Horiuchi T, Nayef MA, Shabala S, An G, Ma JF, Horie T (2017) OsHKT1;5 mediates Na+ exclusion in the vasculature to protect leaf blades and reproductive tissues from salt toxicity in rice. Plant J 91:657–670
Kollist H, Nuhkat M, Roelfsema MR (2014) Closing gaps: linking elements that control stomatal movement. New Phytol 203:44–62
Li L, Li L, Wang XY, Zhu PY, Wu HQ, Qi ST (2017) Plant growth-promoting endophyte Piriformospora indica alleviates salinity stress in Medicago truncatula. Plant Physiol Biochem 119:211–223
Liu P, Yin L, Wang S, Zhang M, Deng X, Zhang S, Tanaka K (2015) Enhanced root hydraulic conductance by aquaporin regulation accounts for silicon alleviated salt-induced osmotic stress in Sorghum bicolor L. Environ Exp Bot 111:42–51
Misra BB, Acharya BR, Granot D, Assmann SM, Chen S (2015) The guard cell metabolome: functions in stomatal movement and global food security. Front Plant Sci 6:334
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Murphy BR, Doohan FM, Hodkinson TR (2014) Yield increase induced by the fungal root endophyte Piriformospora indica in barley grown at low temperature is nutrient limited. Symbiosis 62:29–39
Newman IA (2001) Ion transport in roots: measurement of fluxes using ion-selective microelectrodes to characterize transporter function. Plant Cell Environ 24:1–14
Oren A (1999) Bioenergetic aspects of halophilism. Microbiol Mol Biol Rev 63:334–348
Peskan-Berghofer T, Vilches-Barro A, Muller TM, Glawischnig E, Reichelt M, Gershenzon J, Rausch T (2015) Sustained exposure to abscisic acid enhances the colonization potential of the mutualist fungus Piriformospora indica on Arabidopsis thaliana roots. New Phytol 208:873–886
Roberts SK, Snowman BN (2000) The effects of ABA on channel-mediated K+ transport across higher plant roots. J Exp Bot 51:1585–1594
Shabala S (2017) Signalling by potassium: another second messenger to add to the list? J Exp Bot 68:4003–4007
Shabala S, Hariadi Y (2005) Effects of magnesium availability on the activity of plasma membrane ion transporters and light-induced responses from broad bean leaf mesophyll. Planta 221:56–65
Shabala S, Shabala L, Van Volkenburgh E (2003) Effect of calcium on root development and root ion fluxes in salinised barley seedlings. Funct Plant Biol 30:507–514
Shabala S, Shabala S, Cuin T, Pang J, Percey W, Chen Z, Conn S, Eing C, Wegner L (2010) Xylem ionic relations and salinity tolerance in barley. Plant J 61:839–853
Shabala S, Hariadi Y, Jacobsen SE (2013) Genotypic difference in salinity tolerance in quinoa is determined by differential control of xylem Na+ loading and stomatal density. J Plant Physiol 170:906–914
Shabala S, White RG, Djordjevic MA, Ruan YL, Mathesius U (2016) Root-to-shoot signalling: integration of diverse molecules, pathways and functions. Funct Plant Biol 43:87–104
Sharifi M, Ghorbanli M, Ebrahimzadeh H (2007) Improved growth of salinity-stressed soybean after inoculation with salt pre-treated mycorrhizal fungi. J Plant Physiol 164:1144–1151
Shelden MC, Dias DA, Jayasinghe NS, Bacic A, Roessner U (2016) Root spatial metabolite profiling of two genotypes of barley (Hordeum vulgare L.) reveals differences in response to short-term salt stress. J Exp Bot 67:3731–3745
Shi HZ, Quintero FJ, Pardo JM, Zhu JK (2002) The putative plasma membrane Na+/H+ antiporter SOS1 controls long-distance Na+ transport in plants. Plant Cell 14:465–477
Vahabi K, Dorcheh SK, Monajembashi S, Westmann M, Reichelt M, Falkenberg D, Hemmerich P, Sherameti I, Oelmüller R (2016) Stress promotes Arabidopsis—Piriformospora indica interaction. Plant Signal Behav 11:e1136763
Waller F, Achatz B, Baltruschat H, Fodor J, Becker K, Fischer M, Heier T, Hückelhoven R, Neumann C, Wettstein DV, Franken P, Kogel K (2005) The endophytic fungus Piriformospora indica reprograms barley to salt-stress tolerance, disease resistance, and higher yield. Proc Natl Acad Sci USA 102:13386–13391
Wang Q, Guan C, Wang P, Lv ML, Ma Q, Wu GQ, Bao AK, Zhang JL, Wang SM (2015) AtHKT1; 1 and AtHAK5 mediate low-affinity Na+ uptake in Arabidopsis thaliana under mild salt stress. Plant Growth Regul 75:615–623
Yang R, Yang T, Zhang H, Qi Y, Xing Y, Zhang N, Li R, Weeda S, Ren S, Ouyang B, Guo YD (2014) Hormone profiling and transcription analysis reveal a major role of ABA in tomato salt tolerance. Plant Physiol Biochem 77:23–34
Zarea MJ, Hajinia S, Karimi N, Mohammadi GE, Rejali F, Varma A (2012) Effect of Piriformospora indica and Azospirillum strains from saline or non-saline soil on mitigation of the effects of NaCl. Soil Biol Biochem 45:139–146
Zhang M, Cao Y, Wang Z, Shi J, Liang X, Song W, Chen Q, Lai J, Jiang C (2017a) A retrotransposon in an HKT1 family sodium transporter causes variation of leaf Na+ exclusion and salt tolerance in maize. New Phytol 217:1161–1176
Zhang WY, Wang J, Xu L, Wang AA, Huang L, Du HW, Qiu LJ, Oelmüller R (2017b) Drought stress responses in maize are diminished by Piriformospora indica. Plant Signal Behav 13:e1414121
Zhu JK (2001) Plant salt tolerance. Trends Plant Sci 6:66–71
Zhu M, Shabala L, Cuin T, Huang X, Zhou MX, Munns R, Shabala S (2016) Nax loci affect SOS1-like Na+/H+ exchanger expression and activity in wheat. J Exp Bot 67:835–844
Acknowledgements
The financial support was provided by Engineering Research Centre of Ecology and Agricultural Use of Wetland, Ministry of Education (KF201605), and the open fund of Hubei Collaborative Innovation Centre for Grain Industry (LXT-16-10).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10725_2018_431_MOESM1_ESM.doc
Fig. S1 Experimental protocols and inoculation examination: (a) A schematic diagram of 23 the experimental protocols employed in this study. b, c - P. indica colonization of maize roots. (b) Control, (c) Root segments stained with trypan blue showing colonization of Z. mays by P.indica (arrow). Fig. S2 Photos of plants taken 5 days after onset of salt stress: (a) 0 mM NaCl +P. indica, (b) 100 mM NaCl +P. indica, (c) 200 mM NaCl +P. indica, (d) 0 mM NaCl -P. indica, (e) 100 mM NaCl -P. indica, (f) 200 mM NaCl -P. indica. Fig. S3 Photos of roots taken 5 days after onset of salt stress. (a) 0 mM NaCl +P. indica, (b) 100 mM NaCl +P. indica, (c) 200 mM NaCl +P. indica, (d) 0 mM NaCl -P. indica, (e) 100 mM NaCl -P. indica, (f) 200 mM NaCl -P. indica. (DOC 1451 KB)
Rights and permissions
About this article
Cite this article
Yun, P., Xu, L., Wang, SS. et al. Piriformospora indica improves salinity stress tolerance in Zea mays L. plants by regulating Na+ and K+ loading in root and allocating K+ in shoot. Plant Growth Regul 86, 323–331 (2018). https://doi.org/10.1007/s10725-018-0431-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-018-0431-3