The neuropharmacological activity of two (–)-cytisine derivatives with adamantyl fragments was studied. It was shown that N-1-adamantylcytisine-12-thiocarbamide exhibited in tests in vivo a pronounced mnestic effect, increased the lifespan of laboratory animals under hypoxic conditions, and also enhanced in vitro binding of transcription factors NFAT and NF-κB to the DNA sequences corresponding to them.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Adamantane derivatives, e.g., preparations of amantadine and memantine, represent a separate group of drugs that are antagonists of NMDA-receptors and are used to treat neurodegenerative diseases [1–5]. The structural features of adamantane that lead to the high membranotropic activity of its derivatives are responsible for their various pharmacological properties. Thus, synthetic modifications and discovery research are directed simultaneously toward the creation of antiviral, antiparasite, and antitumor agents [6–9].
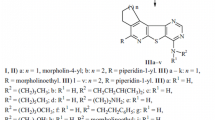
We found recently that cytisine-containing ureas obtained via the reaction of (–)-cytisine and isocyanates exhibited high nootropic activity in tests in vivo [10]. N-Phenylcytisine-12-carbamide (2) demonstrated the greatest (Mt = 92%) mnestic activity among the studied compounds. (–)-Cytisine (1) was reacted with the corresponding 1-adamantylisothio- and 1-adamantylisocyanate in benzene at 80°C to afford N-adamantylcytisine-12-carbamide (3) and N-adamantylcytisine-12-thiocarbamide (4) in order to study the structure activity relationship.

The structure of 3 was confirmed by NMR spectroscopy. The structure of 4 was established from an X-ray crystal structure analysis (XSA) (Fig. 1).
The specific nootropic activity of 3 and 4 was studied using a basic conditioned passive-avoidance reflex (CPAR) model [10, 11]. The reference drug was piracetam. Antihypoxic activity used normobaric hypoxia with hypercapnia as a model [11]. Table 1 presents the results of the performed studies.
According to the results, changing the phenyl in 2 to adamantyl reduced markedly the mnestic activity (Mt). This indicator for 3 was only 15.3%. On the other hand, antihypoxic properties of 3 increased. Whereas carbamide 2 did not affect the lifespan of laboratory animals relative to the control, adamantyl derivative 3 increased it by 23.8%.
Introducing a thiocarbonyl function into the structure had the most favorable effect. Activity Mt of 4 increased by 77.4%; the lifespan under hypoxia conditions, by 30.5%.
The mechanisms of action of the synthesized compounds were studied by comparing the influence of them on the DNA-binding activity of transcription factors (TF) in a system in vitro . Pharmacologically significant biotargets such as TF, which are involved in the pathogenesis of neurodegenerative diseases, include NFAT, NF-κB, STAT1, and HIF1α (a factor inducing hypoxia).
NFAT is known to regulate gene expression of pro- and anti-inflammatory cytokines and their receptors, thereby controlling various stages of the immune response, inflammation, and oncogenesis [12]. TF STAT1 is a component of the JAK/STAT-pathway, which transmits signals of cytokines, hormones, and growth factors into the cell nucleus [13]. The family of protein TF NF-κB is a part of signal pathways that play a key role in regulation of the immune response, cell proliferation, and neuroprotection [14]. The factor inducing hypoxia (HIF1α) mediates the cell response to hypoxia by controlling the expression of genes involved in angiogenesis, proliferation, erythropoiesis, glucose metabolism, pH maintenance, and migration [15].
According to the results, replacing the phenyl in 2 by adamantyl was accompanied by a slight reduction of the activity of 3 and 4 on the binding activity of TF STAT1. However, the presence of the thiocarbonyl in 4 gave a more pronounced effect (Fig. 2a).
Introduction of the adamantyl group in 3 increased HIF1α-regulated luciferase activity relative to 2, which contained a phenyl group (Fig. 2b). Replacing the carbamide group in adamantyl derivative 3 by thiocarbamide in 4 reduced slightly the DNA-binding activity of HIF1α.
In our opinion, the similar nature of the action of the studied compounds on the DNA-binding capability of NFAT and NF-κB was interesting. In particular, N-phenylcytisine-12-carbamide (2) and N-adamantylcytisine-12-thiocarbamide (4) enhanced the binding of NFAT and NF-κB with their recognition sequences whereas N-adamantylcytisine-12-carbamide (3) had practically no effect on this parameter (Fig. 2c and 2d).
Thus, the capability of 2, 3, and 4 to modulate the DNA-binding activity of biotargets, i.e., TF STAT1, HIF1α, NFAT, and NF-κB, was demonstrated in this series of experiments.
The results allow the mechanisms of the found nootropic [10], antihypoxic, and anti-inflammatory [7] activities of several synthetic (–)-cytisine derivatives to be explained rather reliably.
Experimental
We used commercially available (–)-cytisine (CAS 485-35-8), 1-adamantylisocyanate (CAS 4411-25-0), 1-adamantylisothiocyanate (CAS 4411-26-1), and benzene [9072-35-9] as starting materials. The course of reactions was monitored by TLC on Alugram® plates with an aluminum support coated by a layer (0.2 mm) of standard silica gel 60 (Macherey-Nagel, Germany). Melting points of crystalline compounds were determined on a Boetius apparatus (PHMK 05 VEB Wagetechnik Rapido, Radebeul). Optical rotation angles were measured on a PerkinElmer 341 LC polarimeter (Na lamp, wavelength 589 nm).
PMR and 13C and 15N NMR spectra of the synthesized compounds were recorded relative to TMS internal standard on Bruker Avance III pulsed spectrometers at operating frequencies 500.13 for 1H and 125.47 MHz for 13C. Two-dimensional 1H–1H COSY, 1H–13C HSQC, 1H–13C HMBC, and 1H–1H NOESY spectra were recorded in standard multi-pulse sequence modes of the instrument software.
The XSA experiment used a dataset of 12,823 reflections that were obtained on a Bruker Smart Apex2 CCD diffractometer at 100 K (λ Mo Kα-radiation, 2θmax = 64°). The starting set of measured intensities was processed using SAINT and SADABS programs included in the Apex2 programming [16]. The structure was solved by direct methods and refined by anisotropic full-matrix least-squares methods for non-hydrogen atoms over \( F_{\mathrm{hkl}}^2 \). H atoms were placed in geometrically calculated positions with the exception of the amide H atom, the position of which was located in a difference electron-density synthesis. Then, the N–H distance was normalized to the value 0.90 Å. H atoms were refined using a rider model [U iso (H) = nU eq (C,N), where n = 1.2]. A total of 5,986 independent reflections (R int = 0.0258) was used for the refinement. The convergence of the refinement over all independent reflections was wR2 = 0.0823 [R1 = 0.0342 over 5.652 reflections with I > 2σ(I)]. All calculations were performed on an IBM PC using the SHELXTL program set [17]. Atomic coordinates and thermal factors were deposited in the Cambridge Crystallographic Data Centre (CCDC) No. 926834; http://www.ccdc.cam.ac.uk/products/csd/request/.
Synthesis of Thio- and Carbamide of (–)-Cytisine 3 and 4. A mixture of (–)-cytisine (1, 1.8 mmol) and the corresponding adamantylisothio- or adamantylisocyanate (1.8 mmol) was refluxed until the starting alkaloid disappeared completely (TLC monitoring) and filtered. The solid was washed with benzene to afford 3 and 4 in quantitative yields.
N -1-Adamantylcytisine-12-carbamide (3); mp 195–197°C, \( \left[ \alpha \right]_{\mathrm{D}}^{20 } \)–78.0° (CHCl3). HR-MS (EI): m/z calcd for C22H29N3O2 [M+] 367.2254, found 367.1994.
1H NMR spectrum (CDCl3, δ, ppm, J/Hz): 1.60 (3H, m, HAd-δ), 1.8 (3H, m, HAd-β), 1.92 (1H, ddt, 2J = 12.7, 3J8anti-7 = 3.2, 3J8anti-9 = 3.2, 4J8anti -10endo = 1.1, Hanti-8), 1.99 (1H, dtt, 2J = 12.7, 3J8syn-7 = 3.5, 3J8syn-9 = 3.5, 4J8syn-11endo = 1.6, 4J8syn-13endo = 1.8, Hsyn-8), 2.0 (3H, m, HAd-γ), 2.47 (1H, m, H-9), 3.03 (1H, m, H-7), 3.04 (1H, dd, 2J = 13.4, 3J13exo-9 = 2.5, Hexo-13), 3.06 (1H, ddd, 2J = 11.2, 3J11exo-9 = 2.5, 4J11exo-10exo= 1.0, Hexo-11), 3.85 (1H, ddd, 2J = 15.6, 3J10exo-9 = 6.6, 4J10exo-11exo= 1.0, Hexo-10), 3.95 (1H, ddt, 2J = 11.2, 3J11exo-9 = 3.3, 4J11endo-13endo= 1.7, 4J11endo-8syn= 1.6, Hendo-11), 4.00 (1H, ddt, 2J = 13.4, 3J13endo-7 = 3.1, 4J13endo-11endo = 1.7, 4J13endo-8syn = 1.7, Hendo-13), 4.04 (1H, m, H-15), 4.08 (1H, dt, 2J = 15.6, 3J10endo-9 = 1.1, 4J10endo-8anti = 1.1, Hendo-10), 6.07 (1H, dd, 3J5-4 = 7.0, 4J5-3 = 1.5, H-5), 6.47 (1H, dd, 3J3-4 = 9.1, 4J3-5 = 1.5, H-3), 7.29 (1H, dd, 3J4-3 = 9.1, 3J4-5 = 7.0, H-4). 15 N NMR spectrum (CDCl3, δ, ppm): 105.74 (s, N15); 174.43 (s, N1).
13C NMR spectrum (CDCl3, δ, ppm): 26.0 (C-8), 27.60 (C-9), 29.50 (γ-CAd), 34.7 (C-7), 36.38 (δ-CAd), 42.03 (β-CAd), 49.17 (α-CAd), 50.8 (C-10), 51.23 (C-11), 51.42 (C-13), 105.28 (C-5), 117.32 (C-3), 138.83 (C-4), 149.28 (C-6), 156.39 (C-14), 163.44 (C-2).
N -1-Adamantylcytisine-12-thiocarbamide (4); mp 174–175°C. Colorless single crystals of 4 were obtained by slow evaporation of solvent from a solution of 4 in benzene. Crystals of C22H29N3OS (MW = 383.54) at 100 K were monoclinic, a = = 6.6439 (11), b = 11.868 (2), c = 12.114 (2) Å, β = 97.220 (3)°, V = 947.6 (3) Å3, spase group P21 Z = 2, d calcd = 1.344 mg/m3. Diazabicyclononane was situated in the chair–half-chair conformation. The C14-N12 [1.363(2) Å] and C14-N15 [1.352(2) Å] distances were slightly greater than the statistical average for thiourea (1.346 Å) [18]. This indicated that this fragment was not conjugated. The presence of an acidic proton on N15 and a proton-accepting carbonyl led to the formation of an intermolecular H-bond [N15-H15…O1, N…O 3.285(2) Å, H…O 2.42 Å, <NHO 161°]. However, it was rather weak because of the presence of the bulky adamantyl substituent.
Specific nootropic activity of the synthesized compounds was studied by the literature method [11] using a basic CPAR model. The study procedure was described in detail before [10]. The experiment was carried out on Wistar white rats (180–200 g). Distilled H2O (in the control) and the synthesized compounds were administered per os in equal volumes at a dose of 50 μmol/kg 60 min before training.
Antihypoxic properties of the synthesized compounds were studied in mongrel white mice of both sexes (20–25 g). Distilled H2O (in the control) and the synthesized compounds were administered per os at a dose of 50 μmol/kg 60 min before the start of the experiment. Hypoxia with hypercapnia [11] was modeled in sealed chambers (200 cm3 volume). A laboratory animal was placed into a glass container of this volume in order to reproduce this type of hypoxia. The container was hermetically sealed. The oxygen content was gradually reduced and the CO2 pressure in the air was increased due to breathing of the animal. The lifespan of an animal in minutes under hypoxia conditions was recorded. The effectiveness of the studied compounds was estimated using the formula [(tt – tc)/tc]∙100%, where tt is the average lifespan of animals that received the test compound and tc, the average lifespan of control animals.
Cell vitality after incubation with a compound was determined using the PrestoBlue test. For this, HEK293 cells (human fetal kidney cells, Russian collection of cell cultures (RCCC), Institute of Cytology (IC), Russian Academy of Sciences (RAS), St. Petersburg) were seeded into 96-well plates at 10,000 cells per well. The medium in the wells was changed after 24 h. The cells were treated with solutions of the compounds in DMSO (100 μM) to final concentrations of 1, 10, and 100 μM. The cells were incubated in the presence of the compounds for another 2 d under the same conditions. When the incubation was finished, the medium was changed and the cells were treated with a commercial solution PrestoBlueTM (Invitrogen, USA) in the amount recommended by the manufacturer (1/10 of the culture volume). The fluorescence of the dye (degree of dye reduction) was measured at wavelength 590 nm using a 2300 EnSpire® Multimode Plate Reader multiplate analyzer (PerkinElmer, USA).
Results were represented as the number of living cells relative to a control. The number of cells in the control was taken as 100%, where the cells were incubated without the compounds but with DMSO solvent (0.1%). The IC50 values, concentrations of a compound at which death of 50% of the cells was observed, are not given because the compounds did not exhibit cytotoxic activity.
To study the effect of compounds on DNA-binding activity of the target transcription factors (STAT-1 HIF-1; NFAT; NF-κB) we used pTL-Luc - based plasmids (Luciferase Reporter Vector pTL-Luc; Panomics, Inc., Fremont), bearing specific transcription factors - binding enhancer elements. Transient transfection of HEK293 cell line (Institute of Cytology, Russian Academy of Science, St-Petersburg) with TF-specific reporter vectors was performed with Lipofectamine 2000 (Invitrogen, Carlsbad) according to the manufacturer’s protocol. Briefly, HEK293 were seeded at a density of 40∙103 cells/well in 96-well culture plates. Next day single TF-Luc reporter plasmid was co-transfected with pRL-TK Renilla luciferase plasmid (as an internal control, in the ratio 10:1). After transfection cells were incubated with compounds (10 μM) for 24 h. The luciferase activity of the cell lysates was measured according to the Dual-Luciferase® Reporter Assay System manual (Promega, Madison) on the EnSpire® Multimode Plate Readers (PerkinElmer, Waltham).
Data are presented as mean ± SEM. One-way ANOVA followed by Student´s t-test was performed to statistical analysis. A level of p<0.05 was considered as statistically significant. All experiments were repeated three times.
References
F. J. E. Vajda, J. Clin. Neurosci., 9, 4 (2002).
M. F. Beal, Ann. Neurol., 38, 357 (1995).
I. V. Damulin, Russ. Med. Zh., 9, No. 25, 1178 (2001).
W. Danysz, C. G. Parsons, and G. Quack, Amino Acids, 19, 167 (2000).
W. Danysz, C. G. Parsons, H.-J. Mobius, A. Stoffler, and G. Quack, Neurotoxic. Res., 2, 85 (2000).
G. Stamatiou, A. Kolocouris, N. Kolocouris, G. Fytas, G. B. Foscolos, J. Neyts, and E. De Clercq, Bioorg. Med. Chem. Lett., 11, 2137 (2001).
M.-J. Perez, J. Balzarini, M. Hosoya, E. De Clercq, and M.-J. Camarasa, Bioorg. Med. Chem. Lett., 2, 647 (1992).
N. Kolocouris, G. Zoidis, G. B. Foscolos, G. Fytas, S. R. Prathalingham, J. M. Kelly, L. Naesens, and E. De Clercq, Bioorg. Med. Chem. Lett., 17, 4358 (2007).
O. N. Zefirova, E. V. Nurieva, H. Lemcke, A. A. Ivanov, D. V. Shishov, D. G. Weiss, S. A. Kuznetsov, and N. S. Zefirov, Bioorg. Med. Chem. Lett., 18, 5091 (2008).
I. P. Tsypysheva, A. V. Kovalskaya, N. S. Makara, A. N. Lobov, I. A. Petrenko, E. G. Galkin, T. A. Sapozhnikova, F. S. Zarudii, and M. S. Yunuysov, Khim. Prir. Soedin., 565 (2012).
Handbook of Experimental (Preclinical) Study of New Drugs [in Russian], Moscow, 2005, 827 pp.
M. R. Muller and A. Rao, Nat. Rev. Immunol., 10, 645 (2010).
I. A. Kostanyan, A. V. Vonarshenko, and V. M. Lipkin, Bioorg. Khim., 36, 15 (2010).
M. S. Hayden and S. Ghosh, Genes Dev., 18, 2195 (2004).
G. L. Wang, B. H. Jiang, E. A. Rue, and G. L. Semenza, Proc. Natl. Acad. Sci. USA, 92, 5510 (1995).
APEX2 and SAINT, Bruker AXS Inc., Madison, Wisconsin, USA, 2005.
G. M. Sheldrick, Acta Crystallogr. Sect. A: Found. Crystallogr., 64, 112 (2008).
F. H. Allen, O. Kennard, D. G. Watson, L. Brammer, A. G. Orpen, and R. Taylor, J. Chem. Soc. Perkin Trans. 2, No. 2, 1 (1987).
Acknowledgment
The work was supported by a grant of the RFBR No. 12-03-00724-a, FTP “Science and Science-Pedagogical Departments of Innovation Russia” (Contract No. 8046) and the Basic Research Program of the RAS Presidium “Fundamental Sciences – Medicine” (Project Design and Synthesis of Potential Clinical Candidates for Treating Neurodegenerative Diseases, Injuries, and Neurotrauma Among (–)-Cytisine Derivatives) for 2013.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Prirodnykh Soedinenii, No. 4, July–August, 2013, pp. 606–609.
Rights and permissions
About this article
Cite this article
Tsypysheva, I.P., Koval′skaya, A.V., Lobov, A.N. et al. Synthesis and neuropharmacological activity of N-1-adamantylcytisine-12-carbamide and its 12-thiocarbonyl analog. Chem Nat Compd 49, 707–711 (2013). https://doi.org/10.1007/s10600-013-0713-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-013-0713-y