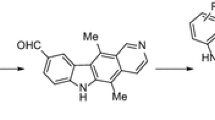
The present review aims at surveying literature on the synthesis, biological and photophysical activities, especially anticancer properties, of tetracyclic natural compound ellipticine (5,11-dimethyl-6H-pyrido[4,3-b]carbazole) since its first isolation and synthesis in 1959.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In 1959, ellipticine (5,11-dimethyl-6H-pyrido[4,3-b]-carbazole, 1) was isolated by Goodwin et al. from the leaf material of an Australian small tropical evergreen tree, Ochrosia elliptica Labill (belonging to the Apocynaceae family) (Fig. 1).1,2,3,4,5,6 This plant was harvested in Florida (USDA plant introduction station). Ellipticine (1) was also available from other plants of the same family (Apocynaceae), such as Ochrosia vieillardii, Ochrosia moorei, and Ochrosia acuminata. Strychnos dinklagei of the Loganiaceae family was another source of this tetracyclic natural product.7,8,9 A few of its derivatives were obtained from various other species of the genera Aspidosperma and Tabernaemontana. Since its inception, the synthesis of ellipticine and its derivatives has generated wide interest amongst the research community owing to its promising anticancer activity.5, 7, 8 The research group of Woodward was the first to report the synthesis of ellipticine (1) in the same year of its first isolation, i.e. in 1959.9, 10 In those early days, the research community focused on the synthesis of ellipticine (1) only. However, a few years later, it was observed that the presence of a simple hydroxy or methoxy group at the C-9 carbon enhanced its cytotoxic activity. Hence, 9-hydroxyellipticine (2) and 9-methoxyellipticine (3) gradually became attractive derivatives (Fig. 1).11, 12 It is worthwhile to mention that 9-methoxyellipticine (3) was also isolated from plants of the Apocynaceae family.
The structural architecture of ellipticine (1) has aroused a considerable interest among researchers, as the molecule is composed of a carbazole moiety condensed to a pyridine ring. Over the years, compounds containing the carbazole skeleton have shown multifarious biological activities.13,14,15 Among them, antimicrobial, anti-inflammatory, antioxidative, neuroprotective, antidiarrhoeal, analgesic, and pancreatic lipase inhibitory activities are the most noteworthy. In addition, carbazole derivatives have found substantial applications in light emitting diodes, photoinduced electron sensors, probable photosensitive biological units, and fluorescent markers in the field of biology.13, 16
On the other hand, pyridine, an important aromatic heterocycle, is abundant in natural compounds such as alkaloids, vitamins, and coenzymes. The unique features like stability, solubility in water, small molecular dimension, capability to form hydrogen bond, and, obviously, basicity have made this moiety useful in drug design.17,18,19
The planar polycyclic structure of ellipticine (1) is responsible for its interaction with DNA through intercalation, exhibiting a high DNA binding affinity ~106 M–1.20,21,22,23,24 Compared to other molecules which are capable of intercalating DNA, the distinguishing ability of ellipticine (1) is due to the presence of nitrogen atoms in the aromatic ring which can easily be protonated. Under physiological conditions, both versions of ellipticine (1), monocationic and neutral, are observed and these forms are responsible for DNA binding, oxidative bioactivation, and interactions with membrane barriers. The ability of ellipticine (1) to modify the functioning of enzymes like topoisomerase II and telomerase is also noteworthy.22,23,24
The structural facets of ellipticine (1), along with its promising antitumor and pharmacological activities, have prompted the synthetic organic chemists to develop new synthetic routes to the pyrido[4,3-b]carbazole skeleton and to perform various structural modifications on it with the purpose of obtaining more reactive derivatives.11, 25,26,27
A few reviews on the synthesis of ellipticine (1) have already been published.1, 12 However, no single report encompassing the synthesis, biological activities, and photophysical responses since discovery of this compound in 1959 has been made until now. This review aims at portraying the details related to the synthesis, photophysical studies, and biological importance of this "wonder molecule" to the scientific community.
Synthesis of ellipticine and its derivatives
In 1959, the structure of ellipticine (1) was definitively assigned by Woodward et al. with the help of its first total synthesis.9 Woodward et al. carried out condensation of indole with 3-acetylpyridine in AcOH medium in the presence of ZnCl2 to furnish 1,1-bis(indol-3-yl)-1-(3-pyridyl)-ethane (4). This condensation product on reduction with Zn and Ac2O under reflux yielded 1,4-diacetyldihydropyridine derivative 5. Pyrolysis of compound 5 at 200°C in vacuo produced a distillate from which ellipticine (1) was easily separated (Scheme 1).
The simplicity of Woodward's original route attracted many researchers in those times although the overall yield of the ellipticine (1) through the route proposed by Woodward et al. was minuscule (~2%). In 1987, Zee and Su made an attempt to modify the above-mentioned methodology to overcome the problematic reaction steps and low yields (Scheme 2).28 They performed the condensation of indole and 3-acetylpyridine using HCl as catalyst instead of ZnCl2. A considerably improved yield of bisindolyl derivative 4 was observed. Vacuum pyrolysis of compound 4 yielded product 6. The latter was hydrogenated to form intermediate 7. During the course of reductive acetylation, when treated with Zn and Ac2O, the expected 1,4-diacetylpyridine derivative was not isolated. Instead, 2-acetyl-1,2-dihydroellipticine (8) was obtained through intramolecular condensation in moderate yield. Product 8 was hydrolyzed using 10% H2SO4 followed by aromatization to generate ellipticine (1) in considerable yield.
Researchers across the globe have synthesized ellipticine skeleton by forming B, C, and D rings (Fig. 1). The first synthesis of ellipticine (1) through the formation of B ring was carried out by Stillwell and Woodward in 1964.12, 29, 30 They used the concept of the Fischer indole cyclization to close the pyrrole ring. (Z)-Pent-3-en-2-one (9) on condensation with 1-methyl-4-piperidone (10) in the presence of NaH yielded isoquinolinone 11 (Scheme 3). Upon reductive Stork alkylation, using MeI in the presence of Li and liquid NH3, compound 11 was converted to 2,5,8-trimethyloctahydroisoquinolin-6(2H)-one (12) which under treatment with phenylhydrazine produced the corresponding hydrazone 13, the Fischer indole cyclization of which yielded partly hydrogenated ellipticine 14. The latter underwent aromatization with Pd/C yielding ellipticine (1) in ~ 0.3% yield.
In 1987, analogous synthetic strategy was implemented by Archer et al. to obtain 5,11-demethylellipticines (Scheme 4).31 Hexahydroisoquinolin-6-one 15 upon catalytic hydrogenation yielded a mixture of cis-isomer 16a and trans-isomer 16b. The mixture of both isomers was separated by chromatography, and trans-isomer 16b was used for the subsequent steps. Initially, condensation reaction between ketone 16b and p-methoxyphenylhydrazine (17) produced hydrazone 18, which, on Fischer indole cyclization, afforded carbazole derivative 19, which, upon treatment with Pd/C, underwent dehydrogenation and debenzylation to produce 9-methoxy-6H-pyrido[4,3-b]carbazole (20), which, upon treatment with pyridine hydrochloride, afforded 6H-pyrido-[4,3-b]carbazol-9-ol (21) in ~52% yield.
Using N-(2,5-dimethylphenyl)acetamide as starting material, Miller et al. in 1989 synthesized 7-bromo-5,8-dimethylisoquinolin-6-amine (22)32 in four steps. Compound 22 underwent Suzuki coupling33 with phenylboronic acid to produce phenyl derivative 23. The latter was converted to the corresponding azide 24, which on heating with dodecane yielded ellipticine (1) through nitrene insertion in considerably good yield (~42% starting from N-(2,5-dimethylphenyl) acetamide) (Scheme 5).
Another nitrene insertion reaction was proposed by Liu and Knochel in 2007.34 They synthesized triazenes 26a,b starting from 2-iodoanilines 25a,b. Negishi coupling35 with 7-bromo-5,8-dimethylisoquinoline (27) gave 7-aryl-quinolines 28a,b. Synthetic conversion of the latter to the corresponding azides 29a,b followed by refluxing in mesitylene yielded ellipticine (1) or 9-methoxyellipticine (3), respectively (Scheme 6).
In 1983, Miller et al. proposed a methodology where benzotriazole intermediate was employed in the synthesis of ellipticines 1, 3 (Scheme 7).36 o-Nitroaniline 30a and its 4-methoxy derivative 30b underwent the Goldberg coupling with 6-bromo-5,8-dimethylisoquinoline (31) yielding diarylamines 32a,b.36, 37 Reduction of the nitro functionality produced aromatic amines 33a,b, which underwent diazotization to obtain benzotriazoles 34a,b. Pyrolysis of the latter at 500°C yielded ellipticine (1) (~69% yield) and 9-methoxyellipticine (3) (~62% yield).
In 1992, Marsais et al. synthesized a trifunctional pyridine derivative for C-3 coupling with an indole to obtain 1-fluoroellipticine.38 Initially, 2-fluoropyridine (35) was brominated to generate 3-bromo-2-fluoropyridine (36) which was lithiated and isomerized to 4-bromo-2-fluoro-3-lithiopyridine intermediate which, on immediate addition of acetaldehyde at low temperature, produced trisubstituted pyridine 37. Compound 37 was treated with thionyl chloride, and the resulting chloro derivative 38 reacted with 1-indolylmagnesium iodide. The reaction of indolyl adduct 39 with tributyl(vinyl)stannane 40 yielded vinyl ether 41 which under acidic conditions readily underwent cyclization to afford 1-fluoroellipticine (42a) in a moderate yield (Scheme 8).
May et al. in 1988 developed a synthetic precursor to 3,4-didehydropyridine (3,4-pyridyne, I) and explored its use in synthesis of ellipticine (1) via aza-Diels–Alder reaction involving 1,4-dimethylpyrano[3,4-b]indol-3-one (43).39, 40 Initially, indole on treatment with lactic acid in presence of KOH in a sealed vessel at 250°C produced α-methylindole-3-acetic acid (44) which, on further treatment with acetic anhydride solution and boron trifluoride–diethyl ether complex, produced 1,4-dimethylpyrano[3,4-b]-indol-3-one (43) (Scheme 9). 3-(3,3-Dimethyltriazen-1-yl)-pyridine-4-carboxylic acid (45) prepared in two steps from 3-aminopyridine-4-carboxylic acid (46) was used as the precursor to 3,4-pyridyne (I). Refluxing compounds 45 and 46 in MeCN produced nearly equal amounts of ellipticine (1) and isoellipticine (47).
Pyranoindole 48 containing a benzenesulfonyl group on the indole nitrogen atom underwent a cycloaddition reaction with pyridyne intermediate II in a regioselective way to deliver the sole product 49.12 Deprotection of the benzenesulfonyl group on the nitrogen center produced 1-chloroellipticine (42b) which was hydrogenated on Pd affording ellipticine (1) (Scheme 10).
In 1962, Cranwell and Saxton published another synthetic route to ellipticine.41 In this route (Scheme 11), 1,4-dimethylcarbazole (50a) was initially prepared using indole and hexane-2,5-dione under acidic conditions. Vilsmeier–Haack formylation of compound 50a led to the formation of 3-formyl-1,4-dimethylcarbazole (51). Condensation reaction of the latter with 2,2-diethoxyethylamine produced imine 52. This was reduced and further transformed into ellipticine (1) via the Pomeranz–Fritsch synthesis.42
In 2010, Konakahara et al. employed the strategy of Suzuki–Miyaura coupling to synthesize biphenyl compounds which was followed by double N-arylation to synthesize carbazoles (Scheme 12).43 Initially, 2,5-dimethylphenol (53) was formylated followed by iodination yielding aryl iodide 54. The latter underwent Suzuki–Miyaura coupling with (2-hydroxyphenyl)boronic acid and subsequent activation of the hydroxyl groups resulting in ditriflate 55. The latter underwent a double N-arylation in presence of tert-butyl carbamate and produced 3-formyl-1,4-dimethylcarbazole (51). The aldehyde functionality of compound 51 upon condensation with α-aminoacetaldehyde diethyl acetal followed by reduction with NaBH4 and treatment with nosyl chloride gave compound 56 which on treatment with 6 M HCl in 1,4-dioxane yielded ellipticine (1).
An important synthetic route toward ellipticine (1) was proposed by Bäckvall and Plobeck in 1990.44 Cycloaddition reaction of indolylmagnesium iodide with 3-(phenylsulfonyl) hexane-2,4-diene (57) yielded tetrahydrocarbazole 58. The lithium salt of acetonitrile underwent the Michael addition followed by the elimination of the sulfone group using sodium amalgam produced nitrile 59. Aromatization of the latter using chloranil in xylene afforded carbazole derivative 60. The reduction of the cyano functionality followed by formylation produced formamide 61. A Bischler–Napieralski cyclization of compound 61, followed by aromatization, produced ellipticine (1) in a considerable yield (Scheme 13). A route toward ellipticine (1) using Diels–Alder cycloaddition to 4H-furo[3,4-b]indoles was developed by Gribble et al.45
Another synthetic route toward ellipticine (1) was proposed by Nagarajan et al. in 2014 (Scheme 14).46 They initiated their work using 1,4-dimethylcarbazole derivatives 50a,b. N-Benzylation reaction of compounds 50a,b afforded the respective protected derivatives 62a,b. Vilsmeier–Haack formylation reaction of compounds 62a,b using DMF and POCl3 at 70°C produced aromatic aldehydes 63a,b. This was followed by Pinnick oxidation, using NaClO=, 30% aqueous H2O2, and KH2PO4 in THF–H2O, 2:1. Carboxylic acids 64a,b, on treatment with SOCl2 followed by amidation of the resulted acid chlorides with 2-aminoethanol, yielded amides 65a,b. H3PO4-mediated Friedel–Crafts cyclodehydration at 150°C furnished dihydropyridocarbazolones 66a,b. The reductive amination of the latter using mild reagents like Tf2O and Et3SiH produced N-benzylellipticines 67a,b. Treatment of the latter with Pd/C resulted in removal of the N-benzyl group and the formation of ellipticine (1) and 9-methoxyellipticine (3), respectively.
In 2006, Ho et al. proposed a synthetic route to ellipticine (1) starting from 4,7-dimethyl-1H-inden-2(3H)-one (68) (Scheme 15).47 Reduction of compound 68 with NaBH4, followed by iodination and acetylation yielded compound 69 which underwent the Suzuki coupling with 2-nitrobenzeneboronic acid to generate biaryl compound 70. Cyclization of the latter in the presence of P(OEt)3 afforded 1,2,3,5-tetrahydrocyclopenta-[b]carbazole derivative 71. The regioselective oxidation with DDQ at the benzylic methylene group at position 9 followed by the hydrolysis of the ester group and reduction generated diol 72 as a mixture of cis- and trans-isomers. Compound 72 underwent oxidative ring cleavage with NaIO4 with subsequent recyclization with AcONH4 resulting in the formation of ellipticine (1).
In 1996 and 2001, Miki et al. proposed a synthetic route toward ellipticine (1) via coupling reaction of N-protected indole-2,3-dicarboxylic anhydride 73 with (3-bromo-4-pyridyl)triisopropoxytitanium (74).48, 49 At low temperature, these two compounds reacted to yield coupling product 75. Treatment with 20% HClO4 ensured both deprotection and decarboxylation of compound 75 in high yield. The Wittig reaction of compound 76 produced methylidene derivative 77 which underwent hydrogenation on PtO2, followed by the Stille coupling of the reduction product with tributyl(1-ethoxyvinyl) tin in presence of tetrakis(triphenylphosphine)palladium(0). The resulting acrylate 78 was converted to ellipticine (1) in high yield upon treatment with 10% HCl (Scheme 16).
Synthesis of compounds with modified ellipticine core
Structural modifications of the ellipticine heterocyclic skeleton or 5- and 11-methyl groups were performed by several researchers to overcome the mild adverse properties of the natural ellipticine core. For example, Vann et al. in 2017 reported the synthesis of two new ellipticine derivatives, 5-demethyl-N-methylellipticine (79) and 5-demethyl-N,2-dimethylellipticinium iodide (80) (Scheme 17).50 It was observed through DNA decatenation and cleavage assays that both of these two ellipticine derivatives played the role of catalytic inhibitors of human topoisomerase IIα and, most importantly, both of them were found to be more potent than the parent compound 1.51
Initially, 4,9-dimethyl-9H-carbazole-3-carbaldehyde (81) was synthesized from Hagemann's ester (ethyl 2-methyl-4-oxocyclohex-2-enecarboxylate) using the methodology proposed by Ergün et al. in 1998.52 Aldehyde 81, on treatment with aminoacetaldehyde diethyl acetal yielded imine 82 under solvent-free conditions. Imine 82 was reduced to amine 83 with NaBH4. The treatment of amine 83 with benzenesulfonyl chloride produced the corresponding sulfonamide 84. The latter, on treatment with 6 N HCl, underwent cyclization to 5-demethyl-N-methylellipticine (79) which was treated with methyl iodide in DMF to afford 5-demethyl-N,2-dimethylellipticinium iodide (80) (Scheme 17).50
In 1991, a wide range of 11-amino-11-demethylellipticines were synthesized by Bisagni et al. via C-2 coupling route (Scheme 18).53 They initiated the synthesis using the concept of lithiation at C-2 atom of the indole ring in 5-methoxy-1-(phenylsulfonyl)-1H-indole (85) and treated the lithiated form with 4-acetyl-N,N-diisopropylnicotinamide to afford the coupled product 86 which was converted to the corresponding lactone 87. The latter was reduced using activated Zn powder in AcOH followed by treatment with HCl to produce carboxylic acid 88. The reaction of acid 88, activated by 1,1'-carbonyldiimidazole (CDI), with primary amines, cyclization using POCl3, and subsequent removal of the protecting group using Raney nickel yielded a series of 11-aminoellipticines 89a–c.
In 1985, Gribble et al. proposed a synthetic route toward the formation of ellipticine skeleton where 3,4-pyridinedicarboxylic anhydride (90) was converted to the corresponding monomethyl ester 91 on treatment with NaOMe followed by acidic workup.54 The free carboxylic acid functionality present in monomethyl ester 91 on treatment with SOCl2 produced the corresponding acid chloride 92.
The latter, on treatment with 1-phenylsulfonylindole and under the Friedel–Crafts acylation conditions, produced keto ester 93 which upon cyclization using Comins' methodology produced ellipticinequinone 94a (Scheme 19).
Another methodology toward the synthesis of ellipticinequinone was published by Bennasar et al. in 2005.55 They treated N-benzyl-2,3-disubstituted indole 95 with 3-pyridylmagnesium bromide to afford adduct 96 which, on reduction with triethylsilane, followed by hydrolysis and phenylselenation produced acyl selenide 97. The major step of this synthetic pathway was the cyclization of acyl selenide 97 with hexabutylditin to produce N-benzylellipticinequinone 94b in moderate yield (Scheme 20).
An efficient route toward the synthesis of ellipticinequinone 94a was proposed by Nagarajan et al. in 2014 starting from a simpe molecule isatin (98) (Scheme 21).56 Reaction of sodium 2-(2-aminophenyl)-2-oxoacetate (99) and 2-bromo-1-(pyridin-4-yl)ethanone hydrobromide (100) in DMF medium at 70°C produced isatin adduct 101 which underwent a rearrangement to produce indole-3-carboxylic acid 102. Esterification of the latter by treatment with SOCl2 in EtOH followed by ortho lithiation using LiHMDS–TMEDA and cyclization lead to ellipticinequinone 94a.
Another concise synthetic route toward ellipticinequinone 94a was proposed by the same research group in 2014 (Scheme 22).57 Ethyl 1H-indole-2-carboxylate (103) was treated with pyridine-3-carboxaldehyde (nicotinaldehyde, 104) in the presence of AlCl3 to produce carbinol 105 which was not isolated from the reaction medium. Instead, it was immediately oxidized using 2-iodoxybenzoic acid (IBX) in DMSO to yield ketone 106 which underwent ortho lithiation with lithium tetramethylpiperidide (LiTMP) leading to ellipticinequinone 94a.
Moody et al. proposed a synthetic route (Scheme 23) to produce 1-chloro-9-methoxy-5-azaellipticine (1-chloro-9-methoxy-11-methyl-6H-indolo[2,3-b][1,6]naphthyridine, 107), an important ellipticine derivative. Sonogashira coupling of 2-iodo-4-methoxyaniline (25b) with propyne yielded alkyne 108 which, on treatment with triphosgene, was converted to isocyanate 109.58 The aza-Wittig reaction59 of compound 109 with iminophosphorane 110a followed in situ by intramolecular radical cycloaddition reaction yielded 1-chloro-9-methoxy-5-azaellipticine (107). The chlorine atom present in the molecule was substituted with various amines to produce different 5-azaellipticine derivatives 111a–c (yields not given).
Zhang et al. in 2000 proposed the synthesis of 5-aza analogs of ellipticine (Scheme 24).60 According to their methodology, methyl 2-iodobenzoate (112) in cross-coupling reaction with propyne using a Pd catalyst produced alkyne 113. The ester group of compound 113 was hydrolyzed in alkaline medium to generate the corresponding carboxylic acid 114 which in reaction with diphenylphosphoryl azide produced isocyanate 115. The aza-Wittig reaction of compound 115 with iminophosphorane 110b followed in situ by cyclization of carbodiimide intermediate III afforded 5-azaellipticine 116.
Biological activity of ellipticine and its derivatives: a brief account
Several anticancer agents have been discovered from various natural sources. The knowledge of cancer biology and molecular genetics were useful for the development of such anticancer agents. Ellipticine (1), a weakly basic polycyclic molecule and an important plant alkaloid has received considerable attention owing to its anticancer activity since the 1960s. The ability to intercalate DNA, inhibition of topoisomerase II, the capability of inhibiting DNA replication and RNA transcription made ellipticine scaffold an important structure for development of anticancer agents.24, 61,62,63,64
The dimensions of ellipticine molecule are similar to that of proflavine (117) (Fig. 2). In a way similar to proflavine, ellipticine (1) binds to helical DNA preferentially by intercalation though the extent of binding is higher in case of ellipticine (1). According to Kohn et al. in 1975, the evidence for DNA intercalation was based on effects on the sedimentation and viscosity of sheared DNA fragments.65 Other evidence included electric dichroism measurements and the removal and reversal of the supercoiling of closed circular DNA. The distinguishing behavior of ellipticine (1) compared to proflavine and other related compounds was its uncharged character at neutral pH and protonation under weakly acidic conditions.
It was predicted that ellipticine (1) binds to DNA probably through the protonated form. As a rule, compounds having cytotoxic anticancer behavior interact either directly or indirectly with nuclear DNA.66 Usually, both the direct and the indirect action at the DNA level are responsible for biological activity. Ellipticine (1) displayed cytotoxic activity against tumor cells due to its direct binding ability to DNA. Several compounds in the ellipticine series exhibited multimodal mechanism of action: binding via covalent bonding, interfering with the topoisomerase II activity and generating oxidizing species.67, 68 Ellipticine (1) induced the cleavage of protein-associated DNA strands by trapping topoisomerase II which was involved in cutting and repairing tangled strands of DNA.12 Ellipticine (1) belongs to the group of compounds behaving as DNA topoisomerase II inhibitors capable of inducing an increase in the number of covalent enzyme–DNA complexes present on the cell chromatin at a given time thereby triggering processes that are responsible for cell death by apoptosis. Since topoisomerase II is involved in cell growth and division, blocking the activity of this enzyme by ellipticine (1) could be able to destroy cancer cells.68
Le Pecq et al. have investigated the interaction of several ellipticine derivatives including compounds 1–3 with DNA.69 Several DNA intercalating drugs can be cited which probably bind to both the DNA and the enzyme through intercalation of the chromophore at the DNA–enzyme interface through side chains.70, 71 In 1981, Ross et al. reported the ellipticine-induced strand cleavage in DNA from L1210 cells (concentrations ~1.25–5.0 mg/ml) with topoisomerase II inhibition.72 In 1995, a study report on activity of ellipticine (1) toward topoisomerase II was published by Froelich-Ammon et al. It was concluded that topoisomerase II is the chief cellular target of the ellipticine molecule whereby an ellipticine–enzyme–DNA ternary complex is initially formed.62 Auclair et al. in 1981, employed a biooxidation model system horseradish peroxidase (HRP) – hydrogen peroxide and reported that several ellipticine derivatives were capable of undergoing oxidation.73 For example, 9-hydroxyellipticine (2) is oxidized to the corresponding quinone imine 118 which is capable of oxidizing NADH to NAD+ and even irreversibly bind to bovine serum albumin (Scheme 25).
The simplicity of the molecular structure of ellipticine (1) motivated the research community to explore necessary modifications that are needed for pharmacological activitiy. Classic examples include hydroxylation at positions C-7 or C-9, quaternization at N-2 position through alkylation introducing an aliphatic hydrophobic chain, addition of a methyl group or aliphatic chains at positions N-6 or C-l. Being cytotoxic to cultured malignant cells, ellipticine derivatives have garnered wider interest for clinical purposes, especially owing to their restricted toxic side effects and due to the lack of hematological toxicity.74 Thus, ellipticine derivatives, including 9-hydroxyellipticine (2)68, 75 and 9-hydroxy-2-methylellipticinium (119) (Fig. 3), a quaternarized drug,76 have been found to be effective against murine leukemia L1210.
Several propositions have been made over the years regarding the fact that DNA has been the principal target for the ellipticine skeleton.77 One interesting perception was that the shape and size of the ellipticine skeleton resembled that of a purine–pyrimidine complementary base pair thereby providing favorable conditions for intercalation in doublestranded DNA.68 Another important opinion was that the aromatic character of this polycyclic system resulted in close-fitting interactions with suitable hydrophobic regions present in DNA. In 2005, in an experiment by Kuo et al., apoptosis in cancer cells was induced by ellipticine through caspase activation.78 However, in the G2M phase, the induction of a cell block was also found to be responsible. Cytotoxic activity evaluation was performed for several ellipticine derivatives toward cervical (A431), lung (A549), ovarian (2008), and colon (LoVo) cancer, as well as melanoma (A375) and leukemia (HL60).66
In spite of having high cytotoxicity, especially against tumor cells, only a limited number of clinical trials of ellipticine have been performed until the 1980s. This was primarily due to the poor solubility of ellipticine (1) in aqueous media and in several other organic solvents. During clinical trials, a few mild side effects were observed in the forms of intravascular hemolysis, decreasing heartbeat, and xerostomia.2, 79 In recent times, with the emergence of new technologies related to drug delivery, attention to this heterocycle has been renewed. Several instances can be cited where ellipticine and its derivatives could be covalently linked with peptides or polymers in order to generate the conjugates with considerably improved solubility.80,81,82 The interesting feature of such conjugates is related to their ability to act on several specific cancer cells. This is helpful to reduce several side effects that might otherwise be observed when specificity is absent. Delivering ellipticine (1) in vitro using copolymer micelles and using self-assembling peptides have been found a wise choice to transport such hydrophobic drugs.
Liu et al. in 2004, reported the physicochemical analyses of a few drug–polymer pairs.80 They also compared the difference in diverse solubility parameters of polymer and drug and calculated the partial and total solubility parameters for the drug as well as for the polymers using the group combination method. They also analyzed several pairs of drug–polymer systems using techniques like Fourier transform infrared and X-ray diffraction studies. Polycaprolactone and poly-β-benzyl-L-aspartate were found to be compatible whereas poly(d,l-lactide) was found incompatible with ellipticine (1).
Ellipticine (1) is reported to exhibit also photoendonuclease activity toward DNA.83 It is worth noting that, apart from the DNA binding, several other modes of action of this molecule have been discovered. This includes kinase inhibition, formation of adducts and interactions with p53 transcription factor.12
Ellipticine derivatives as small molecule fluorophores
For the past two decades, ellipticine (1)84,85,86 and other related carbazoles87,88,89,90,91,92 have been the focal point of interest with respect to photophysical studies in solvents of diverse nature. Photophysical studies related to solvent effects on the electronic absorption and fluorescence spectra of a variety of organic molecules are essential for the expansion of solution chemistry and undoubtedly constitute a significant subject area for photophysical research, since solvents play a major role, both in vitro and in vivo in the behavior of molecules in solution. Solvent molecules act by inducing stabilization of a chromophoric system often through the dipolar relaxation or, in certain cases, through some specific interactions especially hydrogen bonding.93
A wide range of wavelengths from 220 to 400 nm is absorbed by ellipticine (1) in solvents of diverse nature. Ellipticine (1) displays absorbance and emission maxima at 364 and 385 nm, respectively, in nonpolar solvents like hexane. Significantly high molar absorption coefficients (>104) are indicative of the fact that π–π* transition takes place in all the solvents.85, 94, 95
The change of solvent polarity and its effect on the shift of the absorption spectrum toward higher or lower wavelength is noteworthy in the field of photophysics. The fluorescence spectra of ellipticine (1) experience a greater bathochromic shift with the increase in solvent polarity than the absorption spectra due to the enhancement of dipole moment upon excitation.84, 86, 96 The excited state gets solvated with the polar solvents and thereby stabilized causing a bathochromic shift. In case of protic solvents, hydrogen bonding between the solvent and nitrogen centers of ellipticine induces redistribution of electrons thereby shifting the absorption maximum at longer wavelength.
In 2006, Fung et al. reported the photophysical behavior of ellipticine (1) in sixteen organic solvents of various nature to understand the fundamental solvation dynamics, hydrogen bond interactions, estimate the enhancement of electric dipole moment of ellipticine (1) in the excited states, and assess the microenvironmental features of biochemical systems.85 According to their study, both the UV absorption and fluorescence emission were found to be solvent-dependent. With the increase in the polarity of the solvent molecules, ellipticine (1) displayed a bathochromic shift for both absorption and fluorescence spectra. The Stokes shift was found to be large (10000–11000 cm–1) in polar solvents while in nonpolar solvents the shift was comparatively smaller (~8900 cm–1). The Stokes shifts were correlated with dielectric constants and polarities of the solvents using the Lippert–Mataga equation where there was observed a nonlinear trend in the corresponding Lippert–Mataga plot.91, 97 This observation led to the idea that specific solvent effects, especially hydrogen bonding between the molecules of solvent and ellipticine (1) were responsible for this phenomenon. Significantly high differences in dipole moment between ground and excited states were responsible for intramolecular charge transfer. In alcohols, a comparatively stronger hydrogen bonding was formed which was responsible for longer fluorescence lifetimes and large molar extinction coefficients.84, 85 However, in the case of MeOH, somewhat exceptional behavior was observed.
The fluorescence lifetime of ellipticine (1) was measured to be approximately 15 ns in nonpolar hexane medium. On moving from nonpolar hexane to polar DMSO and DMF, a considerable bathochromic shift was observed in the emission spectrum (~425 nm) along with an enhancement in emission intensity and lifetime (~27 ns), which was attributed to the hydrogen bonding involved in the charge transfer in solvents like DMSO and DMF. However, in the case of MeOH, a dual emission at 435 and 535 nm was observed. In 2006, Miskolczy et al. proposed that the long wavelength region (~535 nm) was due to the excited state proton transfer by the hydroxylic solvent, methanol. In the excited state, the rate of protonation has been measured (9.8 × 107 s–1).98 However, in 2011, Banerjee et al. proposed that the longer wavelength region in MeOH was due to solvent-assisted proton transfer from pyrrole moiety to the pyridine unit resulting in tautomerization.99 According to this proposition, a solvent reorganization took place in the excited state around the ellipticine skeleton to form a solvent cage assisting a rapid proton transfer thereby causing the formation of two bands. One is arising from the "normal" structure whereas the other one arose from the tautomer. This proposal was in agreement to that made by Cabo et al. in 1999 and proven by several steady-state and time-resolved experiments.100
Photophysical parameters of ellipticine (1) had been compared to those of its 6-methyl derivative. Both compounds emitted dual fluorescence in ethylene glycol as well as in methanol. Identical photophysical features of both ellipticine (1) and 6-methylellipticine in all investigated solvent systems clearly substantiated that the longdistance, solvent-assisted excited state proton transfer was not responsible for all observed effects. The fluorescence at the long wavelength region in case of MeOH was due to the photoinduced protonation by the protic solvent molecules rather than a 'quinoid-like' tautomeric form.98
We have previously discussed that low solubility of ellipticine (1) in aqueous medium is one of the major issues in the use of this heterocycle in pharmaceuticals. However, in aqueous medium, though it is feebly soluble, the molecule exists both in the protonated and deprotonated forms. At the physiological pH, both the cationic and neutral forms of ellipticine exist as its pKa value is around 7.4.62 As expected, ellipticinium salt of compound 1 is more soluble in H2O than the neutral form. The problem of solubility of ellipticine in H2O can be overcome through the attachment of the molecule 1 to a polymer, peptide, micelles, and reverse micelles.86
An increasing interest has been observed for the past four decades in the study of excited state photoprocesses within various organized microheterogeneous environments like micelles, reverse micelles, cyclodextrins, etc. The importance of these organized assemblies lies in the fact that they can mimic biological systems, and obviously a greater degree of organization is achieved compared to homogeneous environments.102,103,104,105
Thakur et al. in 2013, reported the photophysical study of ellipticine (1) inside reverse micelles, as the wellstructured network of H2O molecules present inside reverse micelles significantly resemble the behavior of H2O molecules observed in diverse biological systems.86 The photophysical responses of ellipticine (1) inside the reverse micelles and its existence in both neutral and cationic forms were indicative of its photophysical behavior within the biological membrane. Ellipticine molecules were trapped as a cationic intermediate within sodium bis-(2-ethylhexyl)sulfosuccinate (AOT) – system as the H2O content increased with the help of several steady-state and time-resolved experiments. However, with the increase in MeOH content, the cationic form of ellipticine (1) gradually converted to the neutral form. No solvent-assisted proton transfer was observed when the MeOH content was at maximum. This observation led to the conclusion that bulk MeOH was lacking in AOT–hexane–MeOH system as MeOH mostly preferred to bind at the AOT head group region thereby becoming unable to take part in solventassisted transfer of proton to ellipticine (1).
With the help of steady-state and time-resolved fluorescence spectroscopy related studies, the same group of researchers in 2014 reported the interaction of ellipticine (1) with human serum albumin (HSA) and the release of the encapsulated ellipticine (1) from 1,2-dimyristoylsn-glycero-3-phosphocholine (DMPC) and 1,2-dipalmitoylsn-glycero-3-phosphocholine (DPPC) liposomes upon addition of HSA.106 Ellipticine (1) exhibited an emission maximum at 535 nm at pH ~7, characteristic of the cationic form. However, at pH 12, an additional emission band was observed at 450 nm, characteristic of the neutral form, along with the band at 535 nm. Surprisingly, on adding HSA to an aqueous solution of ellipticine (1), a significant intensity enhancement was observed at 435 nm, but no change in emission intensity took place at 535 nm. This observation certainly indicated that the neutral form of ellipticine (1) was entrapped within the hydrophobic pocket of HSA. Similar to that of HSA, in liposomes, too, the emission maximum of ellipticine (1) was observed at 435 nm and the fluorescence quantum yield was found ~10 times higher compared to that in aqueous buffer solution. A smaller red shift was observed in the emission spectrum of ellipticine (1) in DMPC liposomes compared to DPPC liposomes, indicating that within DMPC exists a comparatively more polar microenvironment.
Sabín et al. in 2009 observed that upon addition of HSA to the liposome-entrapped ellipticine the emission intensity of ellipticine (1) at 435 nm decreased.107 It was proposed that HSA interacted with the hydrocarbon core of the liposomes and thereby affected the packing of the hydrocarbon tails of the lipids resulting in fluorescence quenching within liposomes. HSA was found to interact with DPPC to a higher extent than DMPC, as the latter is less hydrophobic. In spite of the decrease in fluorescence intensity at 435 nm, no significant enhancement was observed at 535 nm along with the absence of any isoemissive point clearly suggesting that ellipticine (1) did not migrate to aqueous phase upon adding HSA to liposomes.
The discovery of ellipticine, a promising anticancer drug from an Australian tree of Apocynaceae family has been blessing to the research community. This polycyclic, planar molecule and a few of its derivatives are capable of intercalating DNA and exhibit significantly high DNA binding affinity. Kinase inhibition, interaction with p53 transcription factor, and bioadduct formation are also among known activities of ellipticine. Since 1959, several research groups have synthesized this wonder molecule attempting to improve the yield and minimize the number of steps. Although, over the years, many other simple DNA intercalators have became known, the presence of protonable aromatic ring nitrogen atoms distinguished ellipticine from other DNA intercalators. Under physiological environments, both the neutral and charged (monocationic) forms of the molecule are found. The binding of ellipticine molecule to nucleic acids is enhanced by the positive charge of the cationic form, whereas the lipophilic character of the neutral form allows it to penetrate membrane barriers. The structural features of ellipticine and its derivatives offer multimodal actions like oxidative bioactivation, binding with DNA, and enzyme function modification. Synthetic exploration toward structural modification was performed to minimize toxic side effects that were otherwise observed in a few pharmacological studies. Apart from the DNA binding, several other modes of action of this molecule have been discovered. The fundamental solvation dynamics of ellipticine both in ground and excited states in several homogeneous and microheterogeneous environments has opened many significant insights that can be utilized in future photophysical and photobiological studies.
The author would like to acknowledge the financial assistance provided by the Department of Science and Technology, Government of West Bengal, India (Memo No. 1855(Sanc.)/ ST/P/S&T/15G-5/2019, 14/02/2020).
References
Gribble, G. W.; Saulnier, M. G. Heterocycles 1985, 23, 1277.
Garbett, N. C.; Graves, D. E. Curr. Med. Chem.: Anti-Cancer Agents 2004, 4, 149.
Larue, L.; Rivalle, C.; Muzard, G.; Paoletti, C.; Bisagni, E.; Paoletti, J. J. Med. Chem. 1988, 31, 1951.
Gribble, G. W. In Advances in Heterocyclic Natural Product Synthesis; Pearson, W. H., Ed.; JAI Press: London, 1990, Vol. 1, p. 43.
Kouadio, K.; Chenieux, J. C.; Rideau, M.; Viel, C. J. Nat. Prod. 1984, 47, 872.
Goodwin, S.; Smith, A. F.; Horning, E. C. J. Am. Chem. Soc. 1959, 81, 1903.
Marsais, F.; Pineau, P.; Nivolliers, F.; Mallet, M.; Turck, A.; Godard, A.; Queguiner, G. J. Org. Chem. 1992, 57, 565.
Ruckdeschel, J. C.; Modi, S. P.; El-Hamouly, W.; Portuese, E.; Archer, S. J. Med. Chem. 1992, 35, 4854.
Woodward, R. B.; Iacobucci, G. A.; Hochstein, I. A. J. Am. Chem. Soc. 1959, 81, 4434.
Mitra, A. K. Reson. 2021, 26, 1125.
Ibrahim-Ouali, M.; Dumur, F. ARKIVOC 2018, (i), 216.
Miller, C. M.; McCarthy, F. O. RSC Adv. 2012, 2, 8883.
Mitra, A. K. J. Iran. Chem. Soc. 2021. DOI: https://doi.org/10.1007/s13738-021-02444-0.
Knölker, H. J.; Reddy, K. R. Chem. Rev. 2002, 102, 4303.
Knolker, H. J. Curr. Org. Synth. 2004, 1, 309.
Yin, J.; Ma, Y.; Li, G.; Peng, M.; Lin, W. Coord. Chem. Rev. 2020, 412, 213257.
Altaf, A. A.; Shahzad, A.; Gul, Z.; Rasool, N.; Badshah, A.; Lal, B.; Khan, E. J. Drug Des. Med. Chem. 2015, 1, 1.
Golunski, S. E.; Jackson, D. Appl. Catal. 1986, 23, 1.
Abdel-Raheem, S. A. A.; El-Dean, A. M. K.; Abd ul-Malik, M. A.; Abd-Ella, A. A.; Al-Taifi, E. A.; Hassanien, R.; El-Sayed, M. E. A.; Mohamed, S. K.; Zawam, S. A.; Bakhite, E. A. Curr. Chem. Lett. 2021, 10, 337.
Kohn, K. W.; Waring, M. J.; Glaubiger, D.; Friedman, C. A. Cancer Res. 1975, 35, 71.
Canals, A.; Purciolas, M.; Aymamí, J.; Coll, M. Acta Crystallogr., Sect. D: Biol. Crystallogr. 2005, 61, 1009.
Dodin, G.; Schwaller, M. A.; Aubard, J.; Paoletti, C. Eur. J. Biochem. 1988, 176, 371.
Schwaller, M. A.; Aubard, J.; Auclair, C.; Paoletti, C.; Dodin, G. Eur. J. Biochem. 1989, 181, 129.
Monnot, M.; Mauffret, O.; Simon, V.; Lescot, E.; Psaume, B.; Saucier, J. M.; Charra, M.; Belehradek, J.; Fermandjian, S. J. Biol. Chem. 1991, 266, 1820.
Pedersen, J. M.; Bowman, W. R.; Elsegood, M. R.; Fletcher, A. J.; Lovell, P. J. J. Org. Chem. 2005, 70, 10615.
McKee, M. L.; Zheng, L.; O'Sullivan, E. C.; Kehoe, R.; Doyle Prestwich, B. M.; Mackrill, J. J.; McCarthy, F. O. Pathogens, 2020, 9, 58.
Dračínský, M.; Sejbal, J.; Rygerová, B.; Stiborová, M. Tetrahedron Lett. 2007, 48, 6893.
Zee, S.-H.; Su, H.-P. J. Chin. Chem. Soc. 1987, 34, 135.
Stillwell, R. N. Thesis; Harvard University, 1964.
Sainsbury, M. Synthesis 1977, 437.
Archer, S.; Ross, B. S.; Pica-Mattoccia, L.; Cioli, D. J. Med. Chem. 1987, 30, 1204.
Miller, R. B.; Dugar, S. Tetrahedron Lett. 1989, 30, 297.
Miyaura, N.; Yanagi, T.; Suzuki, A. Synth. Commun. 1981, 11, 513.
Liu, C. Y.; Knochel, P. J. Org. Chem. 2007, 72, 7106.
Negishi, E.; Valente, L. F.; Kobayashi, M. J. Am. Chem. Soc. 1980, 102, 3298.
Miller, R. B.; Stowell, J. G. J. Org. Chem. 1983, 48, 886.
Miller, R. B.; Moock, T. Tetrahedron Lett. 1980, 21, 3319.
Marsais, F.; Pineau, P.; Nivolliers, F.; Mallet, M.; Turck, A.; Godard, A.; Queguiner, G. J. Org. Chem. 1992, 57, 565.
May, C.; Moody, C. J. J. Chem. Soc., Perkin Trans. 1 1988, 247.
May, C.; Moody, C. J. J. Chem. Soc., Chem. Commun. 1984, 926.
Cranwell, P. A.; Saxton, J. E. J. Chem. Soc. 1962, 3482.
Fujiwara, A. N.; Acton, E. M.; Goodman, L. J. Heterocycl. Chem. 1968, 5, 853.
Konakahara, T.; Kiran, Y. B.; Okuno, Y.; Ikeda, R.; Sakai, N. Tetrahedron Lett. 2010, 51, 2335.
Bäckvall, J. E.; Plobeck, N. A. J. Org. Chem. 1990, 55, 4528.
Gribble, G. W.; Keavy, D. J.; Davis, D. A.; Saulnier, M. G.; Pelcman, B.; Barden, T. C.; Sibi, M. P.; Olson, E. R.; BelBruno, J. J. J. Org. Chem. 1992, 57, 5878.
Ramkumar, N.; Raghavendra, M. S.; Nagarajan, R. Synlett 2014, 25, 2791.
Ho, T.-L.; Hsieh, S.-Y. Helv. Chim. Acta 2006, 89, 111.
Miki, Y.; Tada, Y.; Yanase, N.; Hachiken, H.; Matsushita, K.-i. Tetrahedron Lett. 1996, 37, 7753.
Miki, Y.; Hachiken, H.; Yanase, N. J. Chem. Soc., Perkin Trans. 1 2001, 2213.
Vann, K. R.; Ergün, Y.; Zencir, S.; Oncuoglu, S.; Osheroff, N.; Topcu, Z. Bioorg. Med. Chem. Lett. 2016, 26, 1809.
Tewey, K. M.; Chen, G. L.; Nelson, E. M.; Liu, L. F. J. Biol. Chem. 1984, 259, 9182.
Ergün, Y.; Patir, S.; Okay, G. J. Heterocycl. Chem. 1998, 35, 1445.
Praly-Deprez, I.; Rivalle, C.; Huel, C.; Belehradek, J.; Paoletti, C.; Bisagni, E. J. Chem. Soc., Perkin Trans. 1 1991, 3165.
Ketcha, D. M.; Gribble, G. W. J. Org. Chem. 1985, 26, 5451.
Bennasar, M. L.; Roca, T.; Ferrando, F. J. Org. Chem. 2005, 70, 9077.
Ramkumar, N.; Nagarajan, R. Tetrahedron Lett. 2014, 55, 1104.
Ramkumar, N.; Nagarajan, R. J. Org. Chem. 2014, 79, 736.
Moody, D. L.; Dyba, M.; Kosakowska-Cholody, T.; Tarasova, N. I.; Michejda, C. J. Bioorg. Med. Chem. Lett. 2007, 17, 2380.
Palacios, F.; Alonso, C.; Aparicio, D.; Rubiales, G.; de Los Santos, J. M. Tetrahedron 2007, 63, 523.
Zhang, Q.; Shi, C.; Zhang, H.-R.; Wang, K. K. J. Org. Chem. 2000, 65, 7977.
Chaitanya, T. K.; Nagarajan, R. Org. Biomol. Chem. 2011, 9(12), 4662.
Froelich-Ammon, S. J.; Patchan, M. W.; Osheroff, N.; Thompson, R. B. J. Biol. Chem. 1995, 270, 14998.
Kizek, R.; Adam, V.; Hrabeta, J.; Eckschlager, T.; Smutny, S.; Burda, J. V.; Frei, E.; Stiborova, M. Pharmacol. Ther. 2012, 133, 26.
Sorace, R. A.; Sheid, B. Chem.-Biol. Interact. 1978, 23, 379.
Kohn, K. W.; Waring, M. J.; Glaubiger, D.; Friedman, C. A. Cancer Res. 1975, 35, 71.
Ferlin, M. G.; Marzano, C.; Gandin, V.; Dall'Acqua, S.; Dalla Via, L. ChemMedChem 2009, 4, 363.
Capranico, G.; Giaccone, G.; Zunino, F.; Garattini, S.; D'Incalci, M. Cancer Chemother. Biol. Response Modif. 1997, 17, 114.
Auclair, C. Arch. Biochem. Biophys. 1987, 259, 1.
Le Pecq, J. B.; Nguyen-Dat-Xuong; Gosse, C.; Paoletti, C. Proc. Natl. Acad. Sci. 1974, 71, 5078.
Ross, W. E.; Glaubiger, D. L.; Kohn, K. W. Biochim. Biophys. Acta, Nucleic Acids Protein Synth. 1978, 519, 23.
Ross, W. E.; Smith, M. C. Biochem. Pharmacol. 1982, 31, 1931.
Ross, W. E.; Bradley, M. O. Biochim. Biophys. Acta, Nucleic Acids Protein Synth. 1981, 654, 129.
Auclair, C.; Paoletti, C. J. Med. Chem. 1981, 24, 289.
Stiborová, M.; Poljaková, J.; Martínková, E.; Bořek- Dohalská, L.; Eckschlager, T.; Kizek, R.; Frei, E. Interdiscip. Toxicol. 2011, 4, 98.
Le Pecq, J. B.; Gosse, C.; Nguyen-Dat-Xuong; Cros, S.; Paoletti, C.; Raynal, B.; Morizet, J. Cancer Res. 1976, 36, 3067.
Auclair, C.; Voisin, E.; Banoun, H.; Paoletti, C.; Bernadou, J.; Meunier, B. J. Med. Chem. 1984, 27, 1161.
Bhuyan, B. K.; Fraser, T. J.; Li, L. H. Cancer Res. 1972, 32, 2538.
Kuo, P. L.; Hsu, Y. L.; Chang, C. H.; Lin, C. C. Cancer Lett. 2005, 223, 291.
Innis, M. A.; Lee, I. P. In Abstracts of Papers. Society of Toxicology. Eleventh Annual Meeting, Williamsburg, Virginia March 5–9, 1972; Academic Press, 1972, p. 30. https://www.toxicology.org/pubs/docs/Tox/1972Tox.pdf. DOI: https://doi.org/10.1016/0041-008X(72)90178-0.
Liu, J.; Xiao, Y.; Allen, C. J. Pharm. Sci. 2004, 93, 132.
Searle, F.; Gac-Breton, S.; Keane, R.; Dimitrijevic, S.; Brocchini, S.; Sausville, E. A.; Duncan, R. Bioconjugate Chem. 2001, 12, 711.
Liggins, R. T.; Burt, H. M. Adv. Drug Delivery Rev. 2002, 54, 191.
Perrouault, L.; Asseline, U.; Rivalle, C.; Thuong, N. T.; Bisagni, E.; Giovannangeli, C.; Doan, T. Le.; Helene, C. Nature 1990, 344, 358.
Thakur, R.; Das, A.; Chakraborty, A. Phys. Chem. Chem. Phys. 2012, 14, 15369.
Fung, S. Y.; Duhamel, J.; Chen, P. J. Phys. Chem. A 2006, 110, 11446.
Thakur, R.; Das, A.; Chakraborty, A. Chem. Phys. Lett. 2013, 563, 37.
Mitra, A. K.; Ghosh, S.; Sau, A.; Saha, C.; Basu, S. J. Lumin. 2015, 167, 233.
Mitra, A. K.; Ghosh, S.; Sarangi, M. K.; Chakraborty, S.; Saha, C.; Basu, S. J. Mol. Struct. 2014, 1074, 617.
Mitra, A. K. Reson. 2019, 24, 623.
Mitra, A. K.; Sau, A.; Bera, S. C.; Chakraborty, S.; Saha, C.; Basu, S. J. Fluoresc. 2015, 25, 1931.
Mitra, A. K.; Ghosh, S.; Chakraborty, S.; Basu, S.; Saha, C. J. Lumin. 2013, 143, 693.
Ghosh, S.; Mitra, A. K.; Saha, C.; Basu, S. J. Fluoresc. 2013, 23, 1179.
Lakowicz, J. R. Principles of Fluorescence Spectroscopy; Springer: New York, 2013.
Sbai, M.; Lyazidi, S. A.; Lerner, D. A.; Del Castillo, B.; Martin, M. A. J. Pharm. Biomed. Anal. 1996, 14, 959.
Sbai, M.; Lyazidi, S. A.; Lerner, D. A.; Del Castillo, B.; Martin, M. A. Anal. Chim. Acta 1995, 303, 47.
Chahine, J. M. E. H.; Bertigny, J. P.; Schwaller, M. A. J. Chem. Soc., Perkin Trans. 2 1989, 629.
Mataga, N.; Kaifu, Y.; Koizumi, M. Bull. Chem. Soc. Jpn. 1956, 29, 465.
Miskolczy, Z.; Biczók, L.; Jablonkai, I. Chem. Phys. Lett. 2006, 427, 76.
Banerjee, S.; Pabbathi, A.; Sekhar, M. C.; Samanta, A. J. Phys. Chem. A 2011, 115, 9217.
do Cabo, J. L.; Faria, H. B.; Portugal, S. G.; Silva, M. A.; Brinn, I. M. Photochem. Photobiol. 1999, 69, 664.
Froelich-Ammon, S. J.; Patchan, M. W.; Osheroff, N.; Thompson, R. B. J. Biol. Chem. 1995, 270, 14998.
Sarangi, M. K.; Mitra, A. K.; Sengupta, C.; Ghosh, S.; Chakraborty, S.; Saha, C.; Basu, S. J. Phys. Chem. C 2013, 117, 2166.
Ghosh, S.; Mitra, A. K.; Pal, U.; Basu, S.; Saha, C. J. Mol. Struct. 2017, 1130, 810.
Mitra, A. K.; Sau, A.; Pal, U.; Saha, C.; Basu, S. J. Fluoresc. 2017, 27, 1547.
Mitra, A. K.; Ghosh, S.; Sarangi, M. K.; Sau, A.; Saha, C.; Basu, S. J. Photochem. Photobiol., A 2014, 296, 66.
Thakur, R.; Das, A.; Chakraborty, A. J. Photochem. Photobiol., B 2014, 130, 122.
Sabín, J.; Prieto, G.; Ruso, J. M.; Messina, P. V.; Salgado, F. J.; Nogueira, M.; Costas, M.; Sarmiento, F. J. Phys. Chem. B 2009, 113, 1655.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2022, 58(4/5), 178–192
Rights and permissions
About this article
Cite this article
Mitra, A.K. Synthesis, Biological Activity and Photophysical Studies of Ellipticine and its Derivatives: State of the Art. Chem Heterocycl Comp 58, 178–192 (2022). https://doi.org/10.1007/s10593-022-03070-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-022-03070-1