Abstract
In this article, we applied demographic and genetic approaches to assess how landscape features influence dispersal patterns and genetic structure of the common frog Rana temporaria in a landscape where anthropogenic perturbations are pervasive (urbanization and roads). We used a combination of GIS methods that integrate radiotracking and landscape configuration data, and simulation techniques in order to estimate the potential dispersal area around breeding patches. Additionally, genetic data provided indirect measures of dispersal and allowed to characterise the spatial genetic structure of ponds and the patterns of gene flow across the landscape. Although demographic simulations predicted six distinct groups of habitat patches within which movement can occur, genetic analyses suggested a different configuration. More precisely, BAPS5 spatial clustering method with ponds as the analysis unit detected five spatial clusters. Individual-based analyses were not able to detect significant genetic structure. We argue that (1) taking into account that each individual breeds in specific breeding patch allowed for better explanation of population functioning, (2) the discrepancy between direct (radiotracking) and indirect (genetic) estimates of subpopulations (breeding patches) is due to a recent landscape fragmentation (e.g. traffic increase). We discuss the future of this population in the face of increasing landscape fragmentation, focusing on the need for combining demographic and genetic approaches when evaluating the conservation status of population subjected to rapid landscape changes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Between one-third and one-half of the land surface has been transformed by human action: we live on a human-dominated planet (Vitousek et al. 1997). Habitat destruction and fragmentation can decrease the connectivity of landscapes, therefore altering dispersal processes of species that inhabit them (Clobert et al. 2001). Among vertebrates, pool-breeding amphibians can be relatively poor dispersers and philopatric, obliged to migrate between the aquatic breeding habitat and the terrestrial foraging habitat, placing them at risk from habitat loss and fragmentation (Blaustein et al. 1994; Hamer and McDonnell 2008). Breeding sites of pond-breeding amphibians are often clustered locally, allowing exchange between neighbouring ponds, raising the fundamental issue of whether the basic functioning unit is individual ponds or clusters of local ponds (Petranka et al. 2004). Following the seminal study of Gill (1978), on newt “metapopulation”, the “pond-as-patches” approach of metapopulation dynamics considers ponds as equivalent to subpopulations that exchange migrants and that are subject to local extinction and recolonisation from other pond subpopulations (review by Marsh and Trenham 2001). However, interpond terrestrial movements suggest that geographical units larger than single ponds are necessary for amphibian persistence (Marsh and Trenham 2001; Semlitsch 2003). Pond populations that are only a few hundred meters apart are not demographically independent, and therefore are best treated as subpopulations of the same monitoring unit (Petranka et al. 2004; Petranka 2007).
As a consequence the traditional view of amphibian population structure, related to metapopulation functioning can be questioned. It requires probably to be completed by a more functional landscape based analysis. Understanding the functioning of amphibian populations thus requires delimiting subpopulations and estimating landscape connectivity (e.g. Jehle et al. 2005; Waples and Gaggiotti 2006; Baguette and Van Dyck 2007; Stevens and Baguette 2008; Lee-Yaw et al. 2009). Landscape connectivity refers to functional (how dispersal is affected by landscape structure and elements) and structural connectivity (spatial configuration of habitat patches in the landscape, e.g. vicinity or presence of barriers) (Taylor et al. 1993; Baguette and Van Dyck 2007). Land conversion, degradation and fragmentation can decrease species richness and abundance (Beebee 1997; Pope et al. 2000; Joly et al. 2001), genetic diversity and increase genetic differentiation among populations (Reh and Seitz 1990; Hitchings and Beebee 1997; Seppä and Laurila 1999; Spear et al. 2005), and finally threaten amphibian population viability (Biek et al. 2002; Gibbs and Shriver 2005; Schmidt et al. 2005). Transportation infrastructure such as highways, roads and railways are identified as significant barriers to amphibian migrations (e.g. Fahrig et al. 1995; Mazerolle et al. 2005; Elzanowski et al. 2009).
Increasing number of studies integrate demographic and genetic approaches (Riley et al. 2006; Stevens et al. 2006; Gauffre et al. 2008; Purrenhage et al. 2009). This strategy allows the evaluation of the congruence between dispersal potential and gene flow, taking into account historical and current demographic processes (Zellmer and Knowles 2009) and highlighting the importance of habitat heterogeneity and landscape context for amphibian conservation strategies (Werner et al. 2009). The aims of this paper are: (1) to determine population genetic structure of the common frog in the investigated area and (2) to determine the influence of anthropogenic landscape features (urbanisation and traffic) on gene flow. We used a combination of GIS methods that integrate radiotracking of adults, landscape configuration data, and simulations to estimate the potential dispersal area around breeding ponds, clustering them according to landscape connectivity. Additionally, we used genetic data to investigate several spatial configurations (e.g. individuals as units, ponds as units), allowing the estimation of spatial genetic structure, and patterns of gene flow across the landscape.
Materials and methods
Sampling site
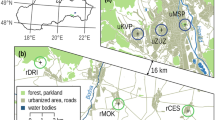
Our study focuses on a region in the northern French Alps, close to the city of Chambéry (300 m a.s.l.). The area covers approximately 135 km2 and is a geographically well defined glacial valley enclosed by the Epine massif (1500 m a.s.l.) on the west, the Bauges massif (2000 m a.s.l.) on the east, Le Bourget Lake (18 × 2 km) on the north and by the city of Chambéry (around 100,000 inhabitants) on the south. The landscape is composed of a large urbanized area (housing, commercial and industrial zones), grazed and ungrazed meadows, crops (wheat and maize), orchards (apples, pears and peaches) and forests (Fig. 1). A dense web of transportation infrastructure in the area includes 2 motorways (2 × 2 ways), 2 national roads (RN 201 and RN 504) and several local roads. Data on road traffic were obtained from the French State Service “Direction Départementale de l’Equipement” and the private motorway company (AREA). Motorway traffic density shows a constant increase over the past 20 years (Fig. 2) reaching up to 1600 vehicles/h in 2004. National and local roads also show a constant increase of traffic in the past 20 years.
Study area. 1–11 = codes of breeding patches of the common frog Rana temporaria (see details in Table 1). A43, A41 = motorways; RN201, RN504 = national roads; RD991, RD211 = local roads
Evolution of road traffic (in number of vehicles/h) from 1984 to 2005 on motorways and major roads of the studied area. A43, A41 = motorways; RN201, RN504 = national roads; RD991, RD211 = local roads. The dotted line is the traffic value (1000 vehicles/h) leading to impassable road to migrating common frog (i.e. complete mortality, see details in “Material and Methods”)
About 78 aquatic sites in the area considered as suitable habitat for common frog (ponds, marshes, gravel pits, surfaces with temporary water) were visited each year between 1998 and 2002 (referred to as ‘habitat patches’ hereafter). Presence of common frog (adults, eggs or larvae) was detected in 20 of these 78 habitat patches and those sites are referred to as ‘occupied habitat patches’. Reproduction during the 5 years of sampling was observed in 11 of those patches, further referred to as ‘breeding patches’. The breeding patches are permanent ponds or boggy marshes varying from 283 m a.s.l. (breeding patch 11) to 820 m a.s.l. (breeding patch 10) in altitude. Straight line distances between patches ranged from 0.9 to 9 km (Fig. 1).
We conducted genetic sampling on the breeding patches in the spring of 2002. We collected 20–25 eggs from each breeding patch, each from a different clutch to reduce parentage effect. Eggs were maintained in the laboratory until hatching and then tadpoles were stored in 90° ethanol. The breeding population sizes (estimated by counting clutches and assuming that each female lays one clutch per year; Miaud et al. 1999) varied from less than 20 to more than 2000 breeding females per patch (Table 1).
Demographic approach
Landscape permeability and friction map
Aerial photographs (Institut Géographique National, France) were used to identify land use, completed with field visits. A rasterised map of landscape structure and habitat types was obtained using Spatial analysis tool in ArcView (ESRI Inc., USA), with 10 m resolution. Grid missing data were replaced by the closest neighbor land use value with the Nibble function of spatial tool extension. Twenty-three habitats were described, with seven main types (urbanized areas, industrial areas, highways and roads, forested areas, cultivated fields and meadows, marshes and open water).
We estimated the landscape permeability to frog dispersal by relating spatial information on landscape to animal movements using “percolation theory” which consider individuals as particles that disperse according to patch quality and boundaries (Villalba et al. 1998; Adriaensen et al. 2003). We attributed a resistance cost to each habitat (friction coefficient) based on knowledge of habitat use by the common frog obtained by previous radiotracking studies (e.g. Loman 1978; Tramontano 1998; Kovar et al. 2009), and analysis of habitat preference by radiotracked adults in the studied area (Martin 2005). The friction coefficient of permeable habitats varied from 0 to 100 (lowest cost for wetlands and mixed-forest, and highest cost for crops and roads). Dense settlement/urban areas, industrial/commercial parks and highways were considered as impermeable habitats and received a maximum friction coefficient. Quantitative estimate of amphibian road mortality (Kuhn 1986; Hels and Buchwald 2001) showed that the probability of getting killed for an individual frog (taking into account velocity of this species and daily variation in traffic intensity) during a road crossing approached 1 when traffic reached 920 vehicles/h (Hels and Buchwald 2001). We thus considered a road as an impassable habitat type when traffic exceeded 1000 vehicles/h.
The friction map of the studied area, representing the landscape permeability to frog dispersal in each cell (10 m resolution), was obtained with the “Merge” function on the rasterised map (Spatial Analysis extension of ArcView).
Simulated dispersal area
The aim of the simulation was to define a dispersal potential area around the breeding patches and group them according to the landscape connectivity (friction map). It was based on the calculation of the energy that individuals would spend during their movements. We used the Costdistance function of ArcView, which allowed us to calculate the additive costs of migration and simulate the movement of individuals between breeding patches. In our simulations, the individuals dispersed along the path of least resistance and stopped when either they reached an impassable element (e.g. highways) or reached the Maximum Cost of Migration (Ray et al. 2002), defined as the minimum value of friction coefficient times the total migration distance allowed by the operator. This total migration distance was obtained by following 16 radiotracked common frogs from the breeding site (March) until hibernation (November) in the studied area (Martin 2005) and was estimated as the maximum cumulative distance between the breeding patches and the terrestrial locations (i.e. 1500 m, Janin et al. 2009). This value is in accordance with other common frog maximum dispersal estimates (Loman 1978; Tramontano 1998; Kovar et al. 2009).
Genetic approach
Twenty to 23 individuals from each breeding patch were genotyped for 7 microsatellite loci. We used three loci (RTempμJ, RTempμB, RTempμE) developed by Pidancier et al. (2002), three (RTempμ4, RTempμ1, RTempμ8) by Rowe and Beebee (2001), and one (RTempU4) by Berlin et al. (2000). Genomic DNA was extracted from the tails of tadpoles using DNeasy™ Tissue Kit (Qiagen) following manufactures’ instruction.
The PCR were conducted in a 12.5 μl total volume with 10 mM Tris–HCl pH 8.3, 50 mM KCl, 2 mM MgCl2, 0.5 μM of forward fluorescent labelled primer, 0.5 μM of reverse primer, bovine serum albumin (5 μg), 0.5 U of AmpliTaq Gold DNA polymerase (Perkin-Elmer) and 20–30 ng DNA. PCR reactions were performed on Perkin-Elmer thermocyclers (2400, 9600 and 9700) and the PCR conditions were optimized for each primers pair as reported in Pidancier et al. (2002), Rowe and Beebee (2001), and Berlin et al. (2000). PCR products were run in 6% denaturing polyacrylamide gels and sized with an internal lane standard (GeneScan ™ 350 Rox Size Standard, Applied Biosystems) using the GeneScan ™ (version 3.1) and Genotyper ™ (version 2.0) software programs (Applied Biosystems). In order to avoid allele mis-scoring three independent lectures of the microsatellite profiles were performed. No discrepancy was found between all independent lectures.
Individuals from breeding patch 9 “Les Fontaines” had a high percentage of missing data due to amplification problems (only 4 or less successfully amplified loci), so we removed them from the genetic analyses. At the end, we retained a sample consisting of 201 individuals from 10 breeding patches (between 19 and 23 individuals per patch). Locus RTempμ1 failed to amplify in all the individuals from breeding patch 7 “Les Molasses”.
Genetic diversity of breeding patches
We calculated number of alleles, observed and expected heterozygosity (unbiased estimate, Nei 1978) and inbreeding coefficients (F IS) for each breeding patch, and pairwise F ST between all pairs of breeding patches using Genetix (Belkhir 2001) and FSTAT (Goudet 1995). Each breeding patch was tested for departure from Hardy–Weinberg equilibrium expectations with exact tests (Guo and Thompson 1992), and linkage disequilibrium was tested across all pairs of loci with GENEPOP version 4.0 (Rousset and Leblois 2007). Corrections for multiple comparisons were applied using sequential Bonferroni correction when necessary (Rice 1989).
Genetic structure using breeding patches as units
The software BAPS5 (Corander et al. 2008) was used to infer spatial genetic structure of our dataset. BAPS5 (Corander et al. 2008) implements both non-spatial and spatial Bayesian clustering methods based on a non-reversible Markov chain Monte Carlo algorithm developed by Corander et al. (Corander et al. 2003, 2004, 2008; Corander and Tang 2007). The other available spatial Bayesian clustering methods (TESS/GENELAND) do not provide an option for spatial clustering of predefined groups of individuals, allowing only individual level analysis.
The spatial Bayesian clustering algorithm available in BAPS5 uses a Delaunay graph to specify hypothesised connections between individuals or sampling sites based on their locations. We used the mixture model for spatial clustering of predefined groups of individuals, where all individuals sampled within the same breeding patch were defined as one group. This model searches for the optimal spatial clustering using a prior that favors spatial smoothness in the clustering solution. We conducted 5 independent runs with the maximum number of putative spatial clusters (= groups of breeding patches) initially set to 12. Since the estimated number of spatial clusters was less than 12 in all 5 runs, this value was kept. Admixture analysis based on the result of the spatial clustering of groups from the mixture model was then used to estimate the number of individuals from each pond assigned to each of the detected spatial clusters. Maximum number of putative spatial clusters is the only parameter required for this analysis.
The effect of landscape connectivity on the genetic differentiation among breeding patches was studied using GESTE (Foll and Gaggiotti 2006), a Bayesian method that estimates F ST values for each local population and relates them to environmental factors using a generalized linear model. It evaluates likelihoods of the models that include all the factors and their combinations. We considered models with Euclidean distances between breeding patches and cost distances calculated from the friction map and traffic influence (in vehicles/h) as factors.
Genetic structure using individuals as units
STRUCTURE 2.0 (Pritchard et al. 2000), the most widely used clustering method, was used to infer the existence of a genetic structure without any a priori on individual belonging to specific breeding patches. We conducted 20 independent runs using the admixture model, for each value of K (= number of clusters) varying between 1 and 12 (i.e. 1 more then maximum number of sampled breeding patches), with burn in of 30,000 and total chain length of 100,000. Estimated log probabilities of data under each K were then compared between runs, and the partition with the highest probability was taken as the estimated optimal number of clusters (\( \hat{K} \)). Each individual was then assigned to the one of the estimated clusters (= group of breeding patches) according to the posterior probability.
A second analysis was performed with BAPS5, this time with the option for spatial clustering of individuals. In this case, geographical coordinates of individuals are included in the analysis which then estimates the spatial genetic structure (number and spatial location of clusters) and infers potential boundaries to gene flow. We conducted 5 independent runs with the maximum number of putative spatial clusters fixed to 12 (i.e. number of breeding patches observed +1). Since the result of a mixture analysis identified only one spatial cluster, an admixture analysis was not performed.
Analysis of molecular variance between clusters
In order to estimate significance of genetic differentiation among clusters, we performed hierarchical AMOVA among clusters previously defined by the demographic and genetic analysis. In the first case, clusters were groups of breeding patches whose dispersal areas were connected or overlapping by simulations. In the second case, clusters were based on the best solution of the spatial clustering of groups of individuals identified with BAP5.
To access the role of the road traffic on the genetic differentiation of frogs, we additionally defined clusters of breeding patches based on the location of roads with high traffic in this landscape. Using roads (RN201 and RN504) and highways (A41 and A43) as limits, we thus defined 3 clusters of breeding patches (cluster 1 = breeding patches 1, 2, 3, 4 and 5; cluster 2 = 6 and 7; cluster 3 = 8, 10 and 11) and tested their genetic differentiation.
Arlequin 3.11 (Excoffier et al. 2005) was used for the all AMOVA calculations. Significance of the variance components was tested using 10,000 permutations.
Inference of the number of first generation migrants
We applied the assignment test implemented in Geneclass 2 (Piry et al. 2004) to identify first generation migrants. This method allows estimating the probability of each individual to belong to each of the considered predefined populations. We used successively as population of reference, breeding patches and groups of breeding patches separated by roads with high traffic (group 1 = breeding patches 1, 2, 3, 4 and 5; group 2 = 6 and 7; group 3 = 8, 10 and 11). The Bayesian method of Rannala and Mountain (1997) was used as criteria for likelihood computations, and probabilities that each individual is a resident were estimated using the Monte-Carlo resampling algorithm of Rannala and Mountain (1997) with 10,000 simulated individuals and P-value of 0.01.
Results
Simulated dispersal area
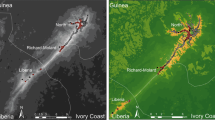
Simulations of dispersal areas were performed with a maximum dispersal distances of 1500 m (see methods) and were started from each the 11 breeding patches. Simulations of dispersal in the landscape surrounding the breeding patches generated six distinct areas (Fig. 3): area A included 17 vacant habitat patches, 10 occupied habitat patches (i.e. aquatic sites with frog presence during at least one breeding season) of which 3 were breeding patches (1, 2, and 3). Area B comprised 5 occupied habitat patches that included breeding patches 4 and 5. Area C included 1 vacant habitat patch, 7 occupied habitat patches including breeding patches 6 and 7. Area D included 2 vacant habitat patches, 7 occupied habitat patches including breeding patch 11. Area E included 2 vacant habitat patches, 4 occupied habitat patches that included patches 9 and 10. Area F included only breeding patch 8. The highway was not impassable for simulations starting from patches 4 and 5 because it is raised above ground along this sector. It was also permeable around breeding patch 11 because several bridges for roads and streams allowed frog dispersal (Martin 2005). Globally, 58 habitat patches were covered by these 6 simulated areas, including 34 occupied habitat patches.
Simulated dispersal area for the common frog R. temporaria. [A] to [F] = simulated dispersal areas. The shaded areas show the extent of the cumulative cost (white = lower cost, black = higher cost) for a frog to move in the landscape. The maximum migration distance of was fixed to 1500 m. Numbers 1–11 = breeding patches as in Fig. 1. These areas reflect potential connectivity between adjacent breeding ponds (e.g. 1, 2 and 3), or absence of dispersal possibility (e.g. 8 or 11)
Genetic diversity within and among breeding patches
Amount of polymorphism varied greatly between loci, from 6 to 33 alleles. Expected heterozygosity ranged from 0.63 to 0.78, while observed values were between 0.49 and 0.65, and all breeding patches except one (2, “Le Tremblay”) showed significant departure from HW equilibrium due to heterozygote deficit (Table 1). However, F IS values for all breeding patches were non-significant.
Out of 21 exact tests for linkage disequilibrium between pairs of loci across all populations, two of the tests were significant (P = 0.03674 and P = 0.02099) showing evidence of linkage between two pairs of loci (RtempμB–Rtempμ4 and Rtempμ8–Rtempμ4).
Table 2 presents F ST values between pairs of breeding patches a well as the modal population specific F ST values and 95% HDPIs intervals estimated by GESTE. These latter estimates evaluate the extent of genetic differentiation between each pond and the ancestral common population. Most of the pair-wise values were significant, indicating genetic differentiation between most breeding patches. For breeding patch 7 (Les Molasses), permutation test could not be performed due to missing data. GESTE results indicate that the genetic composition of La Tremblay is fairly distinct from that of the metapopulation as a whole. The second most differentiated population is Les Molasses, whose HPDI does not overlap with those of four other populations (1, 4, 6, 10) with low genetic differentiation.
Genetic structure using breeding patches as units
BAPS5 with the spatial option (with breeding patches as clustering units) detected 5 spatial clusters (Fig. 4). Three clusters were composed of only one breeding patch (2, 7 and 10), one cluster included two breeding patches (8 and 11), and the largest cluster included breeding patches 1, 3, 4, 5 and 6. The admixture analysis based on this solution confirmed very homogeneous genetic structure of breeding patch 2 “Le Tremblay”. Most of the individuals from the four other spatial clusters had high assignment probabilities, with low level of admixture. The largest cluster consisted only of breeding patches on the western side of road RN504. Overall, these results differ from those obtained with the simulations of dispersal; patches belonging to different areas using this latter method are grouped into the same genetic cluster.
Spatial structure of breeding patches detected with BAPS5 used with predefined groups of individuals i.e. individuals belonging to breeding patches. Numbers 1–11 indicate breeding patches described in Table 1 and letters A to E indicate detected spatial clusters. Pie charts indicate fraction of individuals from each breeding patch assigned to each of 5 spatial clusters, represented with different grey
Of all the possible models including Euclidian, cost distances or traffic influence between breeding patches, GESTE found the model with intercept only as the one with the highest posterior probability (77.43%). Euclidian, cost distances and traffic were not found influential on the genetic structure with breeding patches as units. Population based F ST values estimated by GESTE indicated similar levels of divergence for all breeding patches except for breeding patch 2 (“Le Tremblay”) that had much higher F ST (0.375) than all other breeding patches.
Genetic structure using individuals as units
STRUCTURE did not give a reliable estimation of the number of clusters (K = group of breeding patches). Values of ln(Pr(X/K)) increased as K increased, reaching a plateau at K = 9. We thus used this value of K to infer the global population structure. Out of 20 runs with this K, we chose the solution with the highest likelihood. All STRUCTURE clusters were composed of individuals from more than one breeding site, except one that consists only of all individuals from the breeding patch 2 “Le Tremblay”. There was no clear correspondence between the breeding patches and the clusters identified by STRUCTURE, indicating that there was no strong genetic differentiation of the individuals from different breeding patches.
BAPS5 performed at the individuals level was unable to detect any spatial genetic structure, converging to only one spatial cluster (K = 1).
Analysis of molecular variance
The results of hierarchical analysis of molecular variance between clusters of breeding patches defined by three criteria are given in Table 3. Two of the three comparisons for the genetic differentiation of clusters were significant: The five clusters defined by BAPS5 (with breeding patches as units) and the three clusters defined by the presence of roads with high traffic were significantly differentiated. Clusters defined by BAPS5 explained more of the total genetic variance (6.63%) than clusters defined by roads (1.84%). On the other hand, comparison among clusters built with the simulated dispersal areas around breeding patches (Fig. 3) did not lead to significant genetic differentiation between clusters.
Inference of the number of first generation migrants
The inference of the first generation migrants using the Geneclass 2 between (a) breeding patches and (b) groups of breeding patches separated by major roads are presented in Table 4. This result confirms exchange of genetic material between all of the breeding patches except patch number 2 (“Le Tremblay”) which did not receive nor send any migrants. Of all others, patch number 7 (“Les Molasses”) had the smallest amount of migrants (both sent or received).
Although the migration is expected to be reduced or completely prevented between isolated landscape fragments, Geneclass 2 results showed that this is not the case for three groups of breeding patches separated by major roads. Highest number of migrants was detected between groups marked as 2 and 3, giving indication that the influence of the road in this area is not strong.
Discussion
Individual dispersal and landscape structure
Dispersal simulations indicate that the current fragmentation pattern of the studied area can be described as consisting of six distinct areas within each of which adult frogs can move freely.
The clustering of habitat patches by the dispersal area simulation approach reflects the barrier effect of road traffic and urbanised areas. The studied area “cluse of Chambéry” is subject to very heavy road traffic, which continues to increase (Fig. 2). Road traffic kills involve many taxa, including amphibians (van Gelder 1973; Vos and Chardon 1998; Car and Fahrig 2001; Mazerolle 2004) and road mortality of amphibians is a worldwide conservation concern (e.g. Gibbs and Shriver 2005). Based on the results of Hels and Buchwald (2001), who quantified the probability of getting killed for an adult common frog as a function of traffic intensity, we infer that the highways became impassable as soon as they were put into service (1975–1980), and most of the main roads are impassable since about 1990.
The results from the simulation were based on the assumption that different land covers are expected to present variable resistance to movement in ground-dwelling animals (Wiens and Milne 1989; Charrier et al. 1997). This “matrix effect” (Ricketts 2001), now well established (review in Bowler and Benton 2005), has been described in several amphibian species using radiotracking to estimate habitat preferences (review in Miaud and Sanuy 2005). These habitat preferences were then used to infer movement costs and dispersal areas around breeding patches of newt (Ray et al. 2002) and toads (Joly et al. 2003; Stevens et al. 2004).
The simulations were based on adult dispersal ability, while the juvenile stage is often considered as responsible for most interpond dispersal in amphibians (Gill 1978; Berven and Grudzien 1990; Sjogren-Gulve 1994; Stevens et al. 2004). However, in this region, common frog dispersal distance is lower in juveniles than in adults (Miaud et al. 2005; Martin 2005). Nevertheless, it should be kept in mind that simulations describe present-day movement, and only genetic studies can help to estimate effective dispersal.
Genetic structure, gene flow and landscape influence
The combination of different analyses did not provide consistent results on the influence of landscape fragmentation on Rana temporaria population genetic structure. While spatial clustering of breeding patches using BAPS5 and AMOVA indicate genetic differentiation of clusters separated by major roads (especially RN504), this influence was not detected by any of the individual based analyses. BAPS5 analyses at the individual level assigned all sampled individuals to only one population, and STRUCTURE suggested that breeding patches are composed of highly admixed individuals. The difference between results of population-level and individual-level analyses may be the consequence of low information content in genetic data (small number of used markers and detected alleles), which makes individual-based analysis methods much less powerful than those carried out assuming predefined populations. However, we can also propose several potential biological explanations. One of them is that there has been extensive gene flow between local populations (breeding patches) in the past leading to extensive admixture. The time elapsed between the constructions of highways and road traffic level suppressing frog dispersal (about 30 years) is short given that the generation length of the common frog is 3–4 years (Miaud et al. 1999). The effect of fragmentation on genetic structure is more readily detected for species with shorter generation lengths. For example, roads constructed 30 years ago or forest fragmented 50 year ago strongly influenced genetic population differentiation of ground beetle (Keller and Lagardier 2003) and Rocky Mountain Apollo butterfly (Keyghobadi et al. 2005).
Both BAPS5 and STRUCTURE identified one breeding patch 2 (“Le Tremblay”) that was composed by genetically very homogeneous individuals, different from all others. This breeding patch is much more recent than all the others, being an artificial pond dating from 1990 that resulted from the collection of rainwater from nearby urbanized areas. It is thus possible that it was recently colonized by a small group of individuals all coming from the same breeding patch. Geneclass 2 analysis (Table 4a) did not detect any first generation migrants into, or from this patch, thus confirming its isolation. Another noticeable breeding patch is patch 7 “Les Molasses” (Fig. 4) which genetically differs from all the others. The founder effect is less applicable to this case because this breeding patch exhibited the largest number of breeders (Table 1). One hypothesis that remains to be tested is that subpopulations is (or was) genetically connected with other subpopulations in the south, outside the studied area.
Several studies with landscapes fragmented by roads and other forms of human activity (agriculture, urbanisation, etc.) have found an effect of geographic distance in the genetic structuring of amphibian populations (e.g. Hitchings and Beebee 1997; Stevens et al. 2006). However, a lack of isolation by distance was also observed for R. temporaria and Bufo bufo in natural landscapes (Seppä and Laurila 1999). In the present study, the genetic structure of the common frog population at the scale of the whole study area was not significantly related to landscape structure described by distance (Euclidian or least-cost), nor to the effect of the landscape fragmentation caused by road traffic (GESTE did not identify measurable effects for any of those factors) suggesting that more complex description of connectivity may be necessary to make inferences about amphibian (sub) population structuring.
Demographic and genetic approaches provided complementary insights that helped better describe population structure and functioning: the demographic simulations grouped the breeding patch 2 with neighbouring patches (Fig. 3) while it was clearly differentiated by the genetic analyses (Fig. 4). On the other hand, exchanges between clusters D and F (Fig. 3) were not possible according to the individual dispersal simulations but these clusters were grouped together by the genetic analyses. The difference between both approaches is explained by time-scale differences between ecological and genetic processes. Founder effect explains the singularity of patch 2 while ancestral polymorphism explains the lack of genetic differentiation between clusters D and F. This insight could not be obtained using only one of the two approaches, which represents another example of how combining them can help better understand the functioning of natural populations and the potential effects of landscape modifications (c.f. Riley et al. 2006; Stevens et al. 2006; Gauffre et al. 2008; Purrenhage et al. 2009; Lee-Yaw et al. 2009).
The future of this population
A moderate fragmentation of the landscapes might facilitate the movement of individuals of some species through the creation of roads and other linear corridors (Pither and Taylor 1998). For example, Nadorozny (1997) observed that radiotracked frogs remained in road-side ditches and followed roadways. Pool frogs and tree frogs are also able to move through the landscape matrix using canals and hedgerows (Ficetola and DeBernardi 2005). Landscape effects due to road traffic could also play directional selection on frog dispersal (e.g. Miaud et al. 2005). Isolation will affect the extinction of less mobile species, since it will disrupt gene flow and metapopulation functioning (With and King 1999). This is the case for an amphibian community of Northern Italy where only species with high dispersal capabilities persist in human-dominated landscape (Ficetola and DeBernardi 2005). On the other hand, species with high dispersal capabilities that do not disperse long distances may suffer the most, being exposed to mortality risk during dispersal (Law and Dickman 1998; Fahrig 2001): in an amphibian community of southern Connecticut, the species with the highest mobility suffer local extinction caused by habitat fragmentation (Gibbs 1998). In principle, these species could evolve characteristics such as tolerance for moving through heterogeneous mosaics (Eby 1995) but the speed at which fragmentation takes place will certainly preclude such a process. Local populations can also persist for several years in degraded landscapes before going extinct (Piha et al. 2007).
Our results show that the breeding patches are not necessarily equivalent to genetically distinct demographic units. The “pond-as-patches” paradigm (e.g. Marsh and Trenham 2001) does not properly describe the functioning of the common frog population in this area. Similar results were obtained for the marbled newt Triturus marmoratus (Jehle et al. 2005) and the Columbia spotted frog (Rana luteiventris, Funk et al. 2005). However, the metapopulation paradigm (Smith and Green 2005) is often used to described amphibian pond-breeding populations, and sometimes unnecessarily (review in Marsh and Trenham 2001; Smith and Green 2005). The metapopulation concept reflects the fact that most organisms have limited dispersal powers, hence there is a spatial scale at which most interactions occur “within populations”, whereas at larger spatial scales, these local populations are connected by migration and gene flow (Hanski and Gaggiotti 2004). In the case of amphibians, previous studies have justified the use of the metapopulation paradigm on the grounds of the presence of discrete habitat (breeding) patches, low dispersal capabilities and high site fidelity (e.g. Harrisson 1991; Sjogren-Gulve 1994; Alford and Richards 1999; Marsh and Trenham 2001; Smith and Green 2005). Demographic or genetic approaches have been used and studies using both simultaneously have found some disagreements between them. However, the demographic and genetic definitions of population are not necessarily equivalent (Waples and Gaggiotti 2006). Exchange of some few individuals between two patches each generation may suffice to create a panmictic population but the dynamics of each patch may still be fairly independent. In the studied area, the dynamics of each breeding patch (measured as number of spawn deposited each year) vary independently and only few adults migrated between breeding patches according to capture-mark-recapture studies (Martin 2005). The metapopulation concept can thus be applied to describe the common frog population structuring and functioning in the studied area. We may however be in presence of a non-equilibrium situation where ongoing fragmentation will eventually lead to complete isolation between different clusters of local populations.
Main processes leading to landscape fragmentation (e.g. urbanization and road traffic), will probably continue to accrue, leading breeding patches to become progressively more isolated and subject to a higher extinction risk. In the studied area, breeding patches 4, 5, 8 and 11 (Fig. 1) are threatened by isolation and small subpopulation size, and we can predict their rather rapid extinction. Patches 1, 3, 6, 7 and 10 may persist if terrestrial habitats continue to be accessible and favorable to juveniles and adults. Due to urbanization pressure and road traffic that will continue to increase in this landscape, the persistence of this common frog metapopulation will mainly depend on breeding patches situated on the edges on the valley. Thus, the managing strategy should focus on: (1) protecting existing breeding patches situated on these edges (breeding patches 3, 6, 7, 9, 10 and 11, both aquatic habitat and surrounding landscape); (2) increasing the number of breeding patches closed to existing but isolated patches (e.g. closed to patches 3, 7, 10); (3) restoring or favoring connection between breeding patches (e.g. between 10 and 11).
Further sampling efforts in coming years will allow comparing the current and past population structuring, and we predict the decrease of cluster number and a stronger genetic structuring between the clusters remaining in the two sides along the axis of human-dominated landscape.
References
Adriaensen F, Chardon JP, De Blust G, Swinnen E, Villalba S, Gulinck H, Matthysen E (2003) The application of ‘least-cost’ modelling as a functional landscape model. Landscape Urban Plan 64:233–247
Alford R, Richards S (1999) Global amphibian decline: a problem in applied ecology. Annu Rev Ecol Syst 30:133–165
Baguette M, Van Dyck H (2007) Landscape connectivity and animal behavior: functional grain as a key determinant for dispersal. Landscape Ecol 22:1117–1129
Beebee T (1997) Changes in dewpond numbers and amphibian diversity over 20 years on chalk Downland in Sussex, England. Biol Conserv 81:215–219
Belkhir K (2001) GENETIX, logiciel sous WindowsTM pour la génétique des populations. Laboratoire Génome et Populations, CNRS UPR 9060, Université de Montpellier II, Montpellier
Berlin S, Merila J, Ellegren H (2000) Isolation and characterization of polymorphic microsatellite loci in the common frog, Rana temporaria. Mol Ecol 9:1938–1939
Berven K, Grudzien T (1990) Dispersal in the wood frog (Rana sylvatica): implications for genetic population structure. Evolution 44:2047–2056
Biek R, Funk WC, Maxell BA, Mills LS (2002) What is missing in amphibian decline research: Insights from ecological sensitivity analysis. Conserv Biol 16:728–734
Blaustein A, Wake D, Sousa W (1994) Amphibian declines: judging stability, persistence, and susceptibility of population to local and global extinctions. Conserv Biol 8:60–71
Bowler D, Benton T (2005) Causes and consequences of animal dispersal strategies: relating individual behaviour to spatial dynamics. Biol Rev 80:205–225
Car L, Fahrig L (2001) Effect of road traffic on two amphibian species of differing vagility. Conserv Biol 15:1071–1078
Charrier S, Petit S, Burel F (1997) Movements of Abax parallelepipedus (Coleoptera, Carabidae) in woody habitats of a hedgerow network landscape: a radio-tracing study. Agric Ecosyst Environ 61:133–144
Clobert J, Wolff JO, Nichols JD, Danchin E, Dhondt A (2001) Introduction. In: Clobert J, Danchin E, Dhondt A, Nichols JD (eds) Dispersal. Oxford University Press, Oxford, pp XVII–XXI
Corander J, Tang J (2007) Bayesian analysis of population structure based on linked molecular information. Math Biosci 205:19–31
Corander J, Waldmann P, Sillanpaa MJ (2003) Bayesian analysis of genetic differentiation between populations. Genetics 163:367–374
Corander J, Waldmann P, Marttinen P, Sillanpaa MJ (2004) BAPS 2: enhanced possibilities for the analysis of genetic population structure. Bioinformatics 20:2363–2369
Corander J, Siren J, Arjas E (2008) Bayesian spatial modeling of genetic population structure. Comput Stat 23:111–129
Eby P (1995) The biology and management of flying foxes in New South Wales. Species Management Report Number 18
Elzanowski A, Ciesiołkiewicz J, Kaczor M, Radwańska J, Urban R (2009) Amphibian road mortality in Europe: a meta-analysis with new data from Poland. Eur J Wildl Res 55:33–43
Excoffier L, Laval G, Schneider S (2005) Arlequin (version 3.0): an integrated software package for population genetics data analysis. Evol Bioinform Online 1:47–50
Fahrig L (2001) How much habitat is enough? Biol Conserv 100:65–74
Fahrig L, Pedlar J, Pope S, Taylor P, Wegener J (1995) Effect of road traffic on amphibian density. Biol Conserv 73:177–182
Ficetola F, DeBernardi F (2005) Supplementation or in situ conservation? Evidence of local adaptation in the Italian agile frog Rana latastei and consequences for the management of populations. Anim Conserv 8:1–8
Foll M, Gaggiotti O (2006) Identifying the environmental factors that determine the genetic structure of populations. Genetics 174:875–891
Funk WC, Bloin MS, Stephen P et al (2005) Population structure of columbia spotted frogs (Rana luteiventris) is strongly affected by the landscape. Mol Ecol 14:483–496
Gauffre B, Estoup A, Bretagnolle V, Cosson JF (2008) Spatial genetic structure of a small rodent in a heterogeneous landscape. Mol Ecol 17:4619–4629
Gibbs JP (1998) Distribution of woodland amphibians along a forest fragmentation gradient. Landscape Ecol 13:263–268
Gibbs J, Shriver W (2005) Can road mortality limit populations of pool-breeding amphibians? Wetlands Ecol Manage 13:281–289
Gill D (1978) The metapopulation ecology of the red-spotted newt, Notophthalmus viridescens (Rafinesque). Ecol Monogr 48:145–166
Goudet J (1995) Fstat version 1.2: a computer program to calculate Fstatistics. J Hered 86:485–486
Guo SW, Thompson E (1992) Performing the exact test of Hardy–Weinberg proportion for multiple alleles. Biometrics 48:361–372
Hamer AJ, McDonnell MJ (2008) Amphibian ecology and conservation in the urbanising world: a review. Biol Conserv 141:2432–2449
Hanski I, Gaggiotti O (2004) Ecology, genetics, and evolution of metapopulation. Elsevier Academic Press, Amsterdam
Harrisson S (1991) Local extinction in a metapopulation context: an empirical evaluation. Biol J Linn Soc 42:73–88
Hels T, Buchwald E (2001) The effect of road kills on amphibian populations. Biol Conserv 99:331–340
Hitchings S, Beebee T (1997) Genetic substructuring as a result of barriers to gene flow in urban Rana temporaria (common frog) populations: implications for biodiversity conservation. Heredity 79:117–127
Janin A, Léna JP, Ray N, Delacourt C, Allemand P, Joly P (2009) Assessing landscape connectivity with calibrated cost-distance modelling: predicting common toad distribution in a context of spreading agriculture. J Appl Ecol 46:833–841
Jehle R, Burke T, Arntzen JW (2005) Delineating fine-scale genetic units in amphibians: probing the primacy of ponds. Conserv Genet 6:227–234
Joly P, Miaud C, Lehmann A, Groleto O (2001) Habitat matrix effect on pond occupancy in newts. Conserv Biol 15:239–248
Joly P, Morand C, Cohas A (2003) Habitat fragmentation and amphibian conservation: building a tool for assessing landscape matrix. C R Biol 326:S132–S139
Keller I, Lagardier CR (2003) Recent habitat fragmentation caused by major roads leads to reduction of gene flow and loss of genetic variability in ground beetles. Proc R Soc Lond B 270:417–423
Keyghobadi N, Roland J, Matter SF, Strobek C (2005) Among-and within-patch components of genetic diversity respond at different rates to habitat fragmentation: an empirical demonstration. Proc R Soc B 272:553–560
Kovar R, Brabec M, Vita R, Bocek R (2009) Spring migration distances of some Central European amphibian species. Amphib-Reptil 30:367–378
Kuhn J (1986) Strassentod der Erdkröte (Bufo bufo): Verlustquoten und Verkehrsaufkommen, Verhalten auf der Strasse. Beih.Veröff.Naturschutz & Landschaftspflege in Baden. Württemberg 41:175–186
Law B, Dickman C (1998) The use of habitat mosaics by terrestrial vertebrate fauna: implications for conservation and management. Biodivers Conserv 7:323–333
Lee-Yaw JA, Davidson A, McRae BH, Green DM (2009) Do landscape processes predict phylogeographic patterns in the wood frog? Mol Ecol 18:1863–1874
Loman J (1978) Macro- and micro- habitat distribution in Rana arvalis and Rana temporaria during summer. J Herpetol 12:29–33
Marsh P, Trenham P (2001) Metapopulation dynamics and amphibien conservation. Conserv Biol 15:40–49
Martin R (2005) Biodiversité génétique et fonctionnelle chez Rana temporaria L. (Amphibia: Anura). Approche intégrative le long d’un gradient altitudinal. Thèse
Mazerolle M (2004) Amphibian road mortality in response to nightly variations in traffic intensity. Herpetologica 60:45–53
Mazerolle MJ, Huot M, Gravel M (2005) Behavior of amphibians on the road in response to car traffic. Herpetologica 61:380–388
Miaud C, Sanuy D (2005) Terrestrial habitat preferences of the natterjack toad during and after the breeding season in a landscape of intensive agricultural activity. Amphib-Reptil 26:1–8
Miaud C, Guyétant R, Elmberg J (1999) Variation in life-history traits in the common frog Rana temporaria (Amphibia: Anura): a literature reviews and new data from the French Alps. J Zool Lond 249:61–73
Miaud C, Serandour J, Martin R, Pidancier N (2005) Preliminary results on the genetic control of dispersal in the common frog Rana temporaria froglets. In: Ananjeva N, Tsinenko O (eds) Society Europea Herpetologica, Herpetologia petropolitana, pp 193–197
Nadorozny ND (1997) A temporal and spatial comparison of the movements of three frogs (Genus Rana) among farm and forested landscapes in the Annappolis Valley, Nova Scotia. MSc thesis, Acadia University, Wolfeville
Nei M (1978) Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 89:583–590
Petranka J (2007) Evolution of complex life cycles of amphibians: bridging the gap between metapopulation dynamics and life history evolution. Evol Ecol 21:751–764
Petranka J, Smith C, Scott A (2004) Identifying the minimal demographic unit for monitoring pond-breeding amphibians. Ecol Appl 14:1065–1078
Pidancier N, Gauthier P, Miquel C, Pompanon F (2002) Polymorphic microsatellite DNA loci identified in the common frog (Rana temporaria, Amphibia, Ranidae). Mol Ecol Notes 3:304–305
Piha H, Luoto M, Merilä J (2007) Amphibian occurrence is influenced by current and historic landscape characteristics. Ecol Appl 17:2298–2309
Piry S, Alapetite A, Cornuet J-M, Paetkau D, Baudouin L, Estoup A (2004) GeneClass2: a software for genetic assignment and first-generation migrant detection. J Hered 95:536–539
Pither J, Taylor P (1998) An experimental assessment of landscape connectivity. Oikos 83:166–174
Pope S, Fahrig L, Merriam NG (2000) Landscape complementation and metapopulation effects on leopard frog populations. Ecology 81:2498–2508
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Purrenhage JL, Niewiarowski PH, Moore FBG (2009) Population structure of spotted salamanders (Ambystoma maculatum) in a fragmented landscape. Mol Ecol 18:235–247
Rannala B, Mountain JL (1997) Detecting immigration by using multilocus genotypes. Proc Natl Acad Sci USA 94:9197–9201
Ray N, Lehmann A, Joly P (2002) Modeling spatial distribution of amphibian populations: a GIS approach based on habitat matrix permeability. Biodivers Conserv 11:2143–2165
Reh W, Seitz A (1990) The influence of land use on the genetic structure of populations of the common frog Rana temporaria. Biol Conserv 54:239–249
Rice WR (1989) Analysing tables of statistical tests. Evolution 43:223–225
Ricketts T (2001) The matrix matters: effective isolation in fragmented landscapes. Am Nat 158:87–99
Riley SPD, Pollinger JP, Sauvajot RM et al (2006) A southern California freeway is a physical and social barrier to gene flow in carnivores. Mol Ecol 15:1733–1741
Rousset F, Leblois R (2007) Likelihood and approximate likelihood analyses of genetic structure in a linear habitat: Performance and robustness to model mis-specification. Mol Biol Evol 24:2730–2745
Rowe G, Beebee T (2001) Polymerase chain reaction primers for microsatellite loci in the common frog Rana temporaria. Mol Ecol Notes 1:6–7
Schmidt BR, Feldmann R, Schaub M (2005) Demographic processes underlying population growth and decline in Salamandra salamandra. Conserv Biol 19:1149–1156
Semlitsch R (2003) Conservation of pond-breeding amphibians. In: Semlitsch RD (ed) Amphibian conservation, 1st edn. Smithsonian Books, Washington, DC, pp 8–23
Seppä P, Laurila A (1999) Genetic structure if island populations of the anurans Rana temporaria and Bufo bufo. Heredity 82:309–317
Sjogren-Gulve P (1994) Distribution and extinction patterns within a northern metapopulation of the pool frog, Rana lessonae. Ecology 75:1357–1367
Smith MA, Green DM (2005) Dispersal and the metapopulation paradigme in amphibian ecology and conservation: are all amphibian populations metapopulations? Ecography 28:110–128
Spear SF, Peterson CR, Matocq MD, Storfer A (2005) Landscape genetics of the blotched tiger salamander (Ambystoma tigrinum melanostictum). Mol Ecol 14:2553–2564
Stevens VM, Baguette M (2008) Importance of habitat quality and landscape connectivity for the persistence of endangered natterjack toads. Conserv Biol 22:1194–1204
Stevens V, Polus E, Wesselingh R, Schtickzelle N, Baguette M (2004) Quantifying functional connectivity: experimental evidence for patch-specific resistance in the Natterjack toad (Bufo calamita). Landscape Ecol 19:829–842
Stevens V, Verkenne C, Vandewoestijne S, Wesselingh RA, Baguette M (2006) Gene flow and functional connectivity in the Natterjack toad. Mol Ecol 15:2333–2344
Taylor PD, Fahrig L, Henein K, Gray M (1993) Connectivity is a vital element of landscape structure. Oikos 68:571–573
Tramontano R (1998) The post-breeding migration of the European common frog Rana temporaria. Lund University
van Gelder J (1973) A quantitative approach to the mortality resulting from traffic in a population of Bufo bufo L. Oecologia 13:93–95
Villalba S, Gulinck H, Verbeylen G, Matthysen E (1998) Relationship between patch connectivity and the occurrence of the European red squirrel, Sciurus vulgaris, in forest fragments within heterogenous landscapes. In: Dover JW, Bunce RGH (eds) Key concepts in landscape ecology. IALE Publications, Preston, pp 205–220
Vitousek P, Mooney H, Lubchenco J, Melillo J (1997) Human domination of Earth’s ecosystems. Science 277:494–499
Vos C, Chardon J (1998) Effect of habitat fragmentation and road density on the distribution pattern of the moor frog Rana arvalis. J Appl Ecol 35:44–56
Waples RS, Gaggiotti O (2006) What is a population? An empirical evaluation of some genetic methods for identifying the number of gene pools and their degree of connectivity. Mol Ecol 15:1419–1439
Werner EE, Relyea RA, Yurewicz KL, Skelly DK, Davis CJ (2009) Comparative landscape dynamics of two anuran species: climate-driven interaction of local and regional processes. Ecol Monogr 79:503–521
Wiens J, Milne B (1989) Scaling of landscape in landscape ecology, or landscape ecology from a beetle’s perspective. Landscape Ecol 3:87–96
With KA, King AW (1999) Extinction thresholds for species in fractal landscapes. Conserv Biol 13:314–326
Zellmer AJ, Knowles LL (2009) Disentangling the effects of historic vs. contemporary landscape structure on population genetic divergence. Mol Ecol 18:3593–3602
Acknowledgments
We thank Jean-Noël Avrillier for technical assistance with GIS and figure drawing and Régis Martin for help with field study (radiotracking). We also thank Francesco Ficetola and Alice Valentini for helpful comments on a previous draft of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Safner, T., Miaud, C., Gaggiotti, O. et al. Combining demography and genetic analysis to assess the population structure of an amphibian in a human-dominated landscape. Conserv Genet 12, 161–173 (2011). https://doi.org/10.1007/s10592-010-0129-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10592-010-0129-1