Abstract
Understanding the spatial genetic structure of populations can provide insight into the ecological or evolutionary processes of the species, and enable wise conservation decisions. We examined the spatial genetic structure of a giant panda (Ailuropoda melanoleuca) population in a heterogeneous mountainous landscape using noninvasive genetic sampling and 12 microsatellite loci. Nonrandom genetic structure was detected through spatial autocorrelation analysis, demonstrating a significantly positive autocorrelation over closer distances. Additional spatial analyses showed significantly positive genetic correlation among spatially-proximate males, and no correlation among females and among male–female pairs. These findings suggest a female-biased dispersal pattern and cryptic family grouping among giant pandas on a large mountain-range scale. The spatial extent of genetic structure occurred within 12.5 km, measured by a least-cost path distance model integrating information of habitat quality and habitat preferences of this species. Using the bearing analysis of PASSAGE, we found that directional genetic autocorrelations were in agreement with habitat structure, and habitat heterogeneity may affect the direction of giant panda dispersal. The characterization of spatial genetic structure can provide potentially valuable information for the conservation and management of giant pandas and their habitat.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Movement of animals has an important impact on population dynamics (Macdonald and Johnson 2001) and especially gene flow (Dobson et al. 1997). Understanding animal movement is a focus of population ecology and evolutionary biology (Macdonald and Johnson 2001); however, these studies are often restricted because the movement patterns of animals can be difficult to observe directly. This is especially true across long distances or complicated topography (Turchin 1998; Manel et al. 2003) which, with natural and/or anthropogenic landscape heterogeneity, hampers animal movement (Manel et al. 2003). Recent researches into spatial genetic structure (i.e., nonrandom spatial distribution of genetic variation) have shown that this methodology can be used to elucidate movement patterns (Peakall et al. 2003; Fredsted et al. 2005), especially for threatened or elusive species (Støen et al. 2005; Goossens et al. 2006; Hammond et al. 2006).
The characterization of spatial genetic structure has been commonly used for analyses of gene flow between populations (e.g., F-statistics, Assignment Index—see Goudet et al. 2002), but less frequently applied within a single population. Spatial autocorrelation analysis (Sokal and Oden 1978a, b) can be performed at the individual level and has been gradually used to examine spatial genetic structure (Peakall et al. 2003; Double et al. 2005) and infer dispersal within a population, especially sex-biased dispersal (Peakall et al. 2003; Hazlitt et al. 2004). Spatial genetic autocorrelation measures the correlation of genetic and geographical distance between individuals separated by different distance classes and can reveal the shape and extent of the spatial relationship. Under restricted gene flow resulting from restricted movement of individuals, positive genetic autocorrelations are expected at closer distances (Peakall et al. 2003). Different distances or rates of dispersal by males and females may result in stronger spatial autocorrelation among individuals of the more philopatric sex. Additionally, directional autocorrelation analysis (Oden and Sokal 1986; Falsetti and Sokal 1993; Rosenberg 2000) can be used to explore the direction of spatial genetic structure, which can help to further our understanding of the processes shaping animal movement and spatial genetic structure (e.g., Arnaud 2003).
The giant panda (Ailuropoda melanoleuca), endemic to the eastern edge of the Qinghai-Tibet Plateau, is regarded as a flagship species of world wildlife conservation. Because it is rare, elusive and difficult to observe, studies on dispersal of this species often fail to portray a detailed and precise picture through direct observation or radiotelemetry. For example, field radiotelemetry studies have found very few cases of dispersal (Hu et al. 1985; Pan et al. 2001) and it has been hard to explore the real dispersal pattern at the population level. Recently, through measuring interpopulation differences of microsatellite allele frequencies and relatedness structure, Zhan et al. (2007) found a female-biased dispersal pattern of giant pandas at a fine scale—Wanglang Nature Reserve. This female bias is different from that of many other mammals (Lawson Handley and Perrin 2007), including other bears for which data are available (Proctor et al. 2004; Støen et al. 2005; Costello et al. 2008). This finding was based on a fine scale, and larger-scale studies are now needed. Importantly, giant pandas generally occupy heterogeneous habitat resulting from anthropogenic habitat loss and fragmentation (Loucks et al. 2001; Qi et al. 2009). As a typical habitat specialist, giant panda movement may be highly influenced by habitat structure. If so, we expect there to be directional differences of genetic correlation due to directional differences in habitat structure.
We investigated the spatial genetic structure of a giant panda population on a large mountain-range scale using spatial autocorrelation and directional autocorrelation analyses. We address four specific issues: (i) Is there nonrandom spatial genetic structure in giant pandas? (ii) Does female-biased dispersal occur on a larger scale? (iii) What is the spatial extent of genetic structure? (iv) Are there directional differences of genetic correlation consistent with differences in habitat structure?
Materials and methods
Study area and sample collection
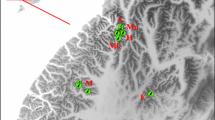
The Liangshan Mountains, the southernmost of six mountainous ranges containing wild giant pandas, are located in the southern Sichuan Province, China (28°14′–29°33′N, 102°35′–103°46′E). Giant panda habitat in the Liangshan Mountains covers an area of 2204 km2 across seven counties (State Forestry Administration of China 2006). The mountainous population has been isolated from the adjacent Daxiangling and Xiaoxiangling mountainous populations due to human activity (State Forestry Administration of China 2006). Within the Liangshan Mountains, giant pandas are confined to middle to high elevation areas between 2,300 and 3,300 m altitude under the pressure of anthropogenic habitat loss and degradation. A north–south county-level road (Ebian-Meigu road) has crossed core habitat for 50 years, further increasing habitat heterogeneity along with concomitant human settlement and agricultural activities (Fig. 1).
We searched for giant panda feces and hair on randomly-selected transect lines covering the entire giant panda habitat as in Qi et al. (2009). Most fecal samples were less than 2 weeks old, based on the status of the mucosal outer-layer of the feces. Upon collection in the field, outer layers were peeled off with sterile forceps and sterile plastic gloves and stored in 99.7% ethanol. Hair samples were air-dried and stored in sterile plastic bags. GPS positions were recorded for all samples. We sampled over two periods: March–September 2006 (81 fecal and 18 hair samples), and April–May 2007 (75 fecal and 12 hair samples). In total, we collected 156 fecal samples and 30 hair samples.
Molecular analysis
Total DNA was extracted from feces according to Zhang et al. (2006a) and from hair using the Chelex-100 method (Walsh et al. 1991). Blank controls were also performed in both extractions and downstream amplifications. We screened each sample using giant panda specific mitochondrial DNA primers (Zhang et al. 2002) to ensure extracts were from giant pandas.
Twelve microsatellite loci: Ame-μ5, μ10, μ13, μ15, μ22, μ26 (Lu et al. 2001), AY116213 (Shen et al. 2005), Ame-μ11, μ24, AY79, AY95, AY217 (redesigned loci; Wu et al. 2009) were used to amplify DNA extracts from fecal and hair samples. To obtain reliable genotypes, a stringent multi-tube approach was used (Taberlet et al. 1996). Firstly we amplified each extract three times simultaneously and then, if the genotype could not be determined, we performed four additional amplifications. PCR amplifications were performed in 10 μl containing 1 μl DNA, 5 μl HotstarTaq Master Mix (QIAGEN), 0.3 μM forward (5′ labeled with FAM, TAMRA or HEX) and reverse primers and 1 μg/μl BSA (Promega). PCR reactions were carried out in a Thermo MBS cycler, starting with 94°C for 15 min, followed by a touchdown PCR (a total of 35–39 cycles of 94°C/15 s, Ta/30 s, 72°C/45 s) and a final step of 60°C for 30 min. Ta was decreased by 2°C every second cycle from 60°C to a touchdown temperature (48–50°C), which was used for 25 subsequent cycles (Zhan et al. 2006). PCR products were separated using an ABI 3700 prism automated sequencer, and scored using GeneScan 3.7 and Genotyper 2.5 (Applied Biosystems).
A Y-linked sexing marker (ZX1, 210-bp) in combination with an X/Y-linked amplification control (ZFX/ZFY, 130-bp) was used to determine the sex of each sample (Zhan 2006). Sex identification was conducted three times for each DNA extract. PCR conditions for sex identification were similar to that of microsatellite amplification; the touchdown temperature was 50°C and products were electrophoresed in a 2% agarose gel. A sample was identified as male if at least two PCRs showed two bands of varying lengths, and as female if only one band (130-bp) was produced. DNA from blood of a male and a female giant panda from Wolong Nature Reserve, China, were used as positive controls, and a PCR reaction without DNA was used as a negative control.
Individual identification and genotyping checking
Individual identification was performed as in Zhan et al. (2006). To test the discrimination power of the set of microsatellites, we computed the probability of pairs of individuals bearing an identical multilocus genotype (P(ID)) using GIMLET 1.3.1 (Valière 2002). The more conservative P(ID) for full-sibs, P(sibs), was also estimated as an upper limit to the probability that pairs of individuals would share the same genotype.
Due to the low quantities and/or quality of DNA in feces and hair, genotyping errors such as allele dropout or false alleles often occur in noninvasive genetic studies (Taberlet et al. 1996, 1999). Here, the overall genotyping error rate was estimated following the method of Zhan et al. (2010). To minimize the genotyping error in the final individual identification results, besides the pre-selection of samples and the use of a multi-tube genotyping approach (Zhan et al. 2006; Zhang et al. 2007) we utilized the software MICRO-CHECKER (Van Oosterhout et al. 2004) to examine the presence of null alleles, large allele dropout or stuttering, and used DROPOUT (McKelvey and Schwartz 2005) to test for bimodality of genotyping errors and differences in capture history in order to verify whether genotyping errors were reduced to a non-significant level.
Genetic diversity and population genetic structure
We summarized genetic variation through the number of alleles per locus (A), expected (H E ) and observed heterozygosities (H O ), and inbreeding coefficients (F IS ) using FSTAT 2.9.3.2 (Goudet 2001). This software was also used to test linkage disequilibrium. Deviations from the Hardy–Weinberg equilibrium for each locus and the whole population were assessed using an exact probability test implemented in GENEPOP 3.4 (Raymond and Rousset 2003). Significance values for multiple comparisons were adjusted using the Bonferroni correction (Rice 1989).
We utilized the widely used Bayesian clustering method, STRUCTURE 2.1 (Pritchard et al. 2000), to detect genetic structure within this mountainous population. Admixture model and correlated allele frequencies between populations were chosen because this presumption is considered best in cases of subtle population structure (Falush et al. 2003). The range of possible clusters (K) tested was from 1 to 8, and 10 independent runs were carried out for each. The lengths of Markov Chain Monte Carlo (MCMC) iterations and burn-in were set at 1,000,000 and 100,000, respectively. The true K is selected using the maximal value of the log likelihood [Ln Pr(X/K)] of the posterior probability of the data for a given K (Pritchard et al. 2000).
Least-cost path distance
As shown in Fig. 1, habitat heterogeneity is a distinct feature of our study area, and might be influential on dispersal in a habitat specialist such as the giant panda. Under this circumstance, Euclidean distance (straight-line distance) may not reflect the true dispersal path of giant pandas, whereas least-cost path distance (Coulon et al. 2004; Cushman et al. 2006), accounting for possible effects of geographical barriers or landscape characteristics on dispersal behavior, may effectively model the real dispersal path.
We utilized two extensions of ArcView GIS 3.2a, XTOOLS and Center of Mass, to generate the centre of the movement range based on multiple GPS coordinates of each repeatedly sampled individual. Then we modeled the realistic movement paths of giant pandas based on species-specific habitat suitability. According to the rules of habitat selection in this species (Hu et al. 1985; Wei et al. 2000; Zhang et al. 2006b), habitat suitability was assessed using 21 eco-geographical variables following Qi et al. (2009) and computed at the level of 30 × 30 m grids using ecological niche factor analysis (ENFA) (Hirzel et al. 2002) in BIOMAPPER 4.0 (Hirzel et al. 2007). The cost value of habitat grid was then computed as 100 minus the relevant habitat suitability value, and a cost surface was created that assigned low cost values to landscape grids with high habitat suitability and high cost values to those with low habitat suitability. Finally, we computed the least-cost path distance using the PATHMATRIX 1.1 extension (Ray 2005) in Arcview GIS 3.2a (Fig. 1).
Spatial autocorrelation analysis
Spatial genetic structure of giant pandas was assessed using a multivariate spatial autocorrelation method which strengthens the spatial signal by reducing stochastic noise resulting from the traditional allele-by-allele and locus-by-locus analyses (Smouse and Peakall 1999). We calculated the squared genetic distance between two individuals following the method of Peakall and Beattie (1995), and used the least-cost path distance to represent pairwise geographical distance. The autocorrelation coefficient r is similar to Moran’s I and was calculated for a specified number of distance classes, which we based on the home range size of giant pandas. The 95% confidence intervals for the estimate of r were generated by bootstrapping (1,000 repeats) and nonrandom spatial genetic structure was tested via permutation (10,000 simulations). For small samples, bootstrap errors tend to be larger than permutation errors, and hence the bootstrap test is more conservative and will favor the null hypothesis more frequently than the permutation test (Peakall et al. 2003). In this study, significant autocorrelation was declared only when r exceeded the permutation 95% confidence intervals around the null hypothesis of zero, and the bootstrap 95% confidence intervals around r did not exceed zero. Significant positive r implies that individuals within a particular distance class are genetically more similar than expected by random. In order to examine sex-biased dispersal patterns, we conducted analyses not only for the entire sample, but also separately among males, among females, and among male–female pairs. We conducted all spatial autocorrelation analyses in GenALEx 6.0 (Peakall and Smouse 2006).
The distance classes of spatial autocorrelation analyses were determined by the size of home ranges of giant pandas. Two long-term radiotelemetry studies computed the home range sizes of giant pandas as 3.9–6.2 km2 in the Qionglai Mountains (Hu et al. 1985), and 10.62 km2 (average) in the Qinling Mountains (Pan et al. 2001). The difference in home range sizes in the two studies may result from seasonal movement of giant pandas in the Qinling Mountains (Pan et al. 2001), and no obvious seasonal movement in the Qionglai Mountains (Hu et al. 1985). Excluding seasonal home range variations we took the mean home range size of the giant panda as 5.5 km2. Following the method of Proctor et al. (2004), we assumed the home range of giant pandas to be approximately circular and hence determined 1.4 km as the radius of home ranges in the Liangshan Mountains. Considering the overall difference of 1.8 times between the least-cost path distance and the corresponding Euclidean distance in this study, 2.5 km (1.4 km × 1.8) was determined as the distance class size for spatial autocorrelation analysis including all giant pandas of identified sex. But, for separate autocorrelation analyses among males, females and male–female pairs, we selected 5 km as the distance class size due to the small number of pairwise comparisons within 2.5 km distance intervals. Previous studies have shown that spatial autocorrelation is likely only to occur in the first few distance classes (e.g., Peakall et al. 2003). Accordingly, in order to achieve greater statistical power without losing biologically important information we pooled pairwise comparisons from the last several distance classes so that there were at least 10 comparisons for the estimate of r in GenALEx 6.0.
We used two methods to study the spatial extent of genetic structure. First, the first X-intercept on the autocorrelogram was used to estimate the spatial extent of nonrandom genetic structure (Sokal and Wartenberg 1983; Peakall et al. 2003; Schweiger et al. 2004; Zamudio and Wieczorek 2007). Second, as in Peakall et al. (2003), multiple autocorrelation analyses for increments of a specified distance class size were used, because single spatial autocorrelation analysis is influenced by the interaction between chosen distance class size, sampling intensity, and true spatial extent of genetic structure. The extent of detectable spatial genetic structure was approximated by the distance class size at which r was no longer significantly positive (Peakall et al. 2003; Double et al. 2005). We performed multiple spatial autocorrelation analyses using 10 increments of 2.5 km to examine the spatial extent of genetic structure.
Directional autocorrelation analysis
We examined the directional difference of genetic variation by testing for the direction of maximum genetic correlation using the bearing procedure implemented in PASSAGE 1.0 (Rosenberg 2001). The bearing method analyzes the correlation coefficients (r) between geographical distance and genetic relatedness under fixed bearing angles (degrees north of due east), and geographical distances are weighted by the squared cosine of the angle between a fixed bearing and that of each pair of points. Because habitat heterogeneity may have an effect on spatial distribution of giant pandas, our analysis focused on the core distribution region of giant pandas in the Liangshan Mountains (Fig. 1) where we sampled 43 individuals. A weighted geographical distance matrix and an angular matrix (Rosenberg 2001) were calculated from the geographical coordinates of 43 individuals. Angular sectors were fixed at 10° north of due east (0°). Based on background gene frequencies from all 52 individuals, pairwise genetic relatedness values (Queller and Goonight (1989) estimator) of the 43 pandas were estimated with the bias corrected by pairs with Relatedness 5.0.8 (Queller and Goonight 1989). Because a directional correlation calculated in a particular direction will be identical to that in the opposite direction, we determined the bearing for each sector on a 0°–180° scale.
Results
Individual identification and genotyping checking
From fecal and hair samples 114 genotypes were obtained and 52 unique genotypes were identified (Fig. 1). Molecular sexing identified 25 male and 24 female giant pandas. Three other individuals failed to be successfully sexed and were removed from sex-specific analyses.
P(ID) analysis showed that the set of 12 loci would produce an identical genotype with a probability of 1.4 × 10−8, and with a probability of 3.49 × 10−4 for a full-sib. Even if the four most polymorphic loci could not be genotyped successfully, the probability was only 0.82% for full-sib identity. So, we used samples where more than eight loci could be reliably genotyped and discarded samples with genotypes for less than eight loci. Following the method of Zhan et al. (2010), the mean genotyping error rate per locus in our data was estimated at 0.17% and the combined genotyping error rate across 12 loci was estimated as 2.03%. Accordingly, we would expect 2–3 unreliable combined genotypes among 114 genotypes. However, because of multiple sampling of the same individual and individual identification rule of neglecting 1 allele mismatch, the 52 individuals identified should contain fewer genotyping errors than calculated by the method above. MICRO-CHECKER analysis indicated no evidences of null alleles, large allele dropout or stuttering in our final set of genotypes. DROPOUT analysis found a unimodal distribution of genetic differences between individuals, and the test for differences in capture history showed that no new individual was added based on the analysis of 11 loci determined by the requirements of P(ID) ≤ 1 × 10−6 and P(sibs) ≤ 1 × 10−3.
Genetic diversity and population genetic structure
Using 12 microsatellite loci, we detected a mean number of 4.0 alleles per locus among the 52 giant pandas. The mean H O and H E were 0.683 and 0.592, respectively. The mean F IS (−0.16) deviated significantly from zero (Table 1). Three loci (Ame-μ22, Ame-μ24 and Ame-μ26) significantly deviated from Hardy–Weinberg equilibrium, and there was no significant linkage disequilibrium after the Bonferroni correction was applied.
The STRUCTURE analysis showed that the average Ln Pr(X/K) value was maximum (−1225.32) at K = 1, and for K > 1, the proportion of the individual assigned to each cluster was roughly symmetric (i.e., 1/K), indicating there was no population structure according to Pritchard and Wen (2004). In addition, two other spatial Bayesian clustering methods, GENELAND (Guillot et al. 2005) and TESS (Francois et al. 2006), were also performed and the conclusions were the same as that of STRUCTURE analysis (data not shown). Hence, the giant pandas in the Liangshan Mountains are from only one population, for which we could further perform spatial autocorrelation and directional autocorrelation analyses.
Spatial genetic structure and dispersal
Spatial autocorrelation analysis for the 49 giant pandas of known sex indicated that significantly positive autocorrelations were found in the first (2.5 km, r = 0.104) and second distance classes (5 km, r = 0.097); r decreased gradually with distance (Fig. 2a), revealing a nonrandom spatial genetic structure for the giant panda population in the Liangshan Mountains such that spatially proximal individuals had higher genetic similarity than spatially distant individuals.
Spatial autocorrelograms of genetic correlation coefficient (r) as a function of least-cost path distance, with the permuted 95% confidence intervals (dashed lines) indicating random spatial genetic structure and the bootstrapped 95% confidence error bars around r. a All giant panda individuals with identified sex (n = 49); b males only (n = 25); c females only (n = 24); d male–female pairs only
Separate spatial autocorrelation analyses among males, females and male–female pairs revealed significantly positive autocorrelation among males, but not among females or male–female pairs. The coefficient r among males was significantly positive (r = 0.142) for the first distance class (5 km), and was positive (r = 0.121) for the second distance class (10 km) where the autocorrelation coefficient was much larger than that among females (r = 0.005) (Fig. 2b). For females or male–female pairs, however, no significantly positive autocorrelation was detected for any distance class (Fig. 2c, d). Particularly, among male–female pairs, the lower r values were found at even the closest distance class (r = 0.07) (Fig. 2d), reflecting a spatial segregation of related male–female pairs. Hence, genetic correlation among males contributed mainly to significantly positive global genetic structure over close distances. The sex-biased spatial distribution of genetic variation shows that dispersal in giant pandas is female-biased in our study population.
Extent and direction of spatial genetic structure
We estimated that the spatial extent of genetic structure occurred within 12.5 km through two analyses. Based on the first X-intercept on the correlogram, we obtained a spatial extent of 12.746 km using spatial autocorrelation for the distance class of 2.5 km (Fig. 2a). In addition, multiple spatial autocorrelation analyses showed that no significant positive autocorrelation occurred beyond the distance class of 12.5 km (Fig. 3).
Our bearing analysis found that the direction of maximal correlation (r = −0.07) was at 100–110° whereas the minimal correlation (r = 0.03) occurred at 0–10° (Fig. 4), demonstrating a nearly north–south spatial genetic correlation, in agreement with panda habitat structure across the Liangshan Mountains (Fig. 1).
Discussion
Spatial genetic structure and female-biased dispersal
Spatial autocorrelation analysis and noninvasive genotyping have allowed us to detect significant spatial genetic structure of the giant panda population in a large-scale area, the Liangshan Mountains. Restricted gene flow is often considered to be the main cause of spatial genetic structure (Peakall et al. 2003). Simulation studies have shown that, under restricted gene flow, spatial autocorrelation analysis reveals a pattern of genetic structure characterized by initial positive autocorrelations then declining with distance through zero to negative, sometimes followed by oscillation of positive and negative values (Epperson et al. 1999). We also found this pattern (Fig. 2a), suggesting that limited gene flow played an important role in shaping the spatial genetic structure of giant pandas in our study area.
Gene flow may be restricted by various factors including geographical barriers, population substructure and sex-biased dispersal. Here, the Bayesian clustering analyses showed that no significant genetic substructure occurred within this population. We are therefore confident that no obvious or cryptic geographical or anthropogenic barriers have limited gene flow of giant pandas and that sex-biased dispersal/philopatry was a key factor shaping the spatial genetic structure of our study population. Separate spatial autocorrelation results among males, females and male–female pairs were consistent with female-biased dispersal and male-biased philopatry patterns on a mountain-range scale (i.e., maximum pairwise Euclidean geographical distance of about 100 km). This finding is in agreement with previous researches (Pan et al. 2001; Zhan et al. 2007). Molecular analysis has shown that female-biased dispersal occurred at a fine scale (i.e., maximum pairwise Euclidean distance of only 12 km) (Zhan et al. 2007). Using field radiotelemetry, Pan et al. (2001) found that, in a giant panda family, a daughter established her home range far from her natal area, whereas a son of the same mother established a home range largely overlapping with that of his mother.
Population social structure could also result in the formation of spatial genetic structure (Scribner and Chesser 1993; Peakall et al. 2003). For instance, in a brown bear (Ursus arctos) population, home ranges of adult female kin overlapped more than those of nonkin and related females formed multigenerational matrilinear assemblages which produced a significant spatial genetic structure among females (Støen et al. 2005). The giant panda is generally regarded as a solitary species, but long-term field radiotelemetry has suggested some sort of cryptic social structure in wild populations. For example, a mother and her four offspring (one adult, two subadults and one cub) have been observed to occasionally aggregate and the home ranges of the mother and the offspring overlapped wholly or partially (Pan et al. 2001).
Short-distance dispersal for males
A trend was found that not all males were absolutely philopatric and some males dispersed short distances. Spatial autocorrelation analysis among males showed that, for the second distance class (10 km), a large and positive (non-significant) genetic correlation occurred, suggesting more related males than females within that distance interval. Ten kilometers markedly exceeds the radius (2.5 km of least-cost distance) of the size of a giant panda’s home range (5.5 km2 on average, Hu et al. 1985; Pan et al. 2001) under the least-cost path distance model, indicating short-distance dispersal beyond the natal area for at least some males. This pattern has also been found in brown bears with male-biased dispersal. McLellan and Hovey (2001) found that 18 males dispersed on average approximately 30 km and 12 females dispersed 10 km, and Zedrosser et al. (2007) reported that males had a dispersal probability of 94% yet females also had a 41% probability of dispersal, reflecting simultaneous dispersal of males and females with different probabilities and/or dispersal distances.
Habitat heterogeneity and giant panda dispersal
Spatial genetic structure can provide an indirect estimate of the average distance that animals disperse between birth and breeding sites (Clark and Richardson 2002). Using spatial autocorrelation analysis, Peakall et al. (2003) found that in the Australian bush rat (Rattus fuscipes), the spatial extent of detectable genetic structure exceeded 500 m, higher than the dispersal distance derived from field observations (200–400 m). Because it is difficult to track long-distance dispersal in giant pandas in the field, little is known about the distances they disperse. Our genetic analyses showed that the spatial extent of genetic structure occurred at 12.5 km, and indicates the average distance within which dispersal of giant pandas typically occurred. A spatial extent of 12.5 km was measured by a least-cost path distance model and cannot be directly compared with the output derived from a Euclidean distance method. However, this spatial extent revealed the effective dispersal distance in a heterogeneous landscape and provides a baseline for comparisons with similar work in other study areas.
The application of directional spatial autocorrelation extends one-dimensional analysis to two dimensions and advances our understanding of biological processes shaping spatial genetic structure (Rosenberg 2000). A nearly north–south genetic correlation was revealed in the giant panda population of the Liangshan Mountains, suggesting more gene flow on this axis. We believe that habitat heterogeneity in the Liangshan Mountains may be responsible for this trend. Although not an absolute barrier to dispersal, a north–south county-level road crosses core habitat and may inhibit the dispersal of giant pandas along an east–west axis. This was also supported by our observation that no individual was re-sampled on each side of the road. Thus, habitat heterogeneity may affect the direction of giant panda dispersal.
Implications for conservation
Understanding the spatial genetic structure of populations can provide insight into the ecological or evolutionary processes of the species, and enable wise conservation decisions. Despite habitat loss and fragmentation, giant pandas inhabiting the Liangshan Mountains form a single population without genetic substructure. This gives us hope regarding the long-term conservation of this mountainous population. However, the absence of genetic structure within this population does not mean that this population is free from the impacts of habitat heterogeneity as it is possible that dispersal is now being affected. We presume that if active conservation actions are not implemented, habitat heterogeneity will in the near future severely hamper gene flow within the mountains. Our findings also suggest that during habitat restoration, attention should be paid to not only habitat quality within patches but also habitat structure among patches.
References
Arnaud JF (2003) Metapopulation genetic structure and migration pathways in the land snail Helix aspersa: influence of landscape heterogeneity. Landscape Ecol 18:333–346
Clark SA, Richardson BJ (2002) Spatial analysis of genetic variation as a rapid assessment tool in the conservation management of narrow-range endemics. Invertebr Syst 16:583–587
Costello CM, Creel SR, Kalinowski ST, Vu NV, Quigley HB (2008) Sex-biased natal dispersal and inbreeding avoidance in American black bears as revealed by spatial genetic analyses. Mol Ecol 17:4713–4723
Coulon A, Cosson JF, Angibault JM, Cargnelutti B, Galan M, Morellet N, Petit E, Aulagnier S, Hewison AJM (2004) Landscape connectivity influences gene flow in a roe deer population inhabiting a fragmented landscape: an individual-based approach. Mol Ecol 13:2841–2850
Cushman SA, McKelvey KS, Hayden J, Schwartz MK (2006) Gene flow in complex landscapes: testing multiple hypotheses with causal modeling. Am Nat 168:486–499
Dobson FS, Chesser RK, Hoogland JL, Sugg DW, Foltz DW (1997) Do black-tailed prairie dogs minimize inbreeding? Evolution 51:970–978
Double MC, Peakall R, Beck NR, Cockburn A (2005) Dispersal, philopatry, and infidelity: dissecting local genetic structure in superb fairy-wrens (Malurus cyaneus). Evolution 59:625–635
Epperson BK, Huang Z, Li TQ (1999) Measures of spatial structure in samples of genotypes for multiallelic loci. Genet Res 73:251–261
Falsetti AB, Sokal RR (1993) Genetic structure of human populations in the British Isles. Ann Hum Biol 20:215–229
Falush D, Stephens M, Pritchard J (2003) Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics 164:1567–1587
Francois O, Ancelet S, Guillot G (2006) Bayesian clustering using hidden Markov random fields in spatial population genetics. Genetics 174:805–816
Fredsted T, Pertoldi C, Schierup MH, Kappeler PM (2005) Microsatellite analyses reveal fine-scale genetic structure in grey mouse lemurs (Microcebus murinus). Mol Ecol 14:2363–2372
Goossens B, Setchell JM, James SS, Funk SM, Chikhi L, Abulani A, Ancrenaz M, Lackman-Ancrenaz I, Bruford MW (2006) Philopatry and reproductive success in Bornean orang-utans (Pongo pygmaeus). Mol Ecol 15:2577–2588
Goudet J (2001) FSTAT: a program to estimate and test gene diversities and fixation indices, version 2.9.3. Lausanne University, Lausanne, Switzerland
Goudet J, Perrin N, Waser P (2002) Tests for sex-biased dispersal using bi-parentally inherited genetic markers. Mol Ecol 11:1103–1114
Guillot G, Mortier F, Estoup A (2005) GENELAND: a computer package for landscape genetics. Mol Ecol Notes 5:712–715
Hammond RL, Handley LJ, Winney BJ, Bruford MW, Perrin N (2006) Genetic evidence for female-biased dispersal and gene flow in a polygynous primate. Proc R Soc B 273:479–484
Hazlitt SL, Eldridge MDB, Goldizen AW (2004) Fine-scale spatial genetic correlation analyses reveal strong female philopatry within a brush-tailed rock-wallaby colony in southeast Queensland. Mol Ecol 13:3621–3632
Hirzel AH, Hausser J, Chessel D, Perrin N (2002) Ecological-niche factor analysis: how to compute habitat-suitability maps without absence data? Ecology 83:2027–2036
Hirzel AH, Hausser J, Perrin N (2007) Biomapper 4.0. Laboratory of Conservation Biology, Department of Ecology and Evolution, University of Lausanne, Switzerland. http://www2.unil.ch/biomapper
Hu JC, Schaller GB, Pan WS, Zhu J (1985) The giant panda of Wolong. Sichuan Publishing House of Science and Technology, Chengdu, China
Lawson Handley LJ, Perrin N (2007) Advances in our understanding of mammalian sex-biased dispersal. Mol Ecol 16:1559–1578
Loucks CJ, Lu Z, Dinerstein E, Wang H, Olson DM, Zhu CQ, Wang DJ (2001) Giant pandas in a changing landscape. Science 294:1465
Lu Z, Johnson WE, Menotti-Raymond M, Yuhki N, Martenson JS, Mainka S, Huang SQ, Zhang ZH, Li GH, Pan WS, Mao XR, O’Brien SJ (2001) Patterns of genetic diversity in remaining giant panda populations. Conserv Biol 15:1596–1607
Macdonald DW, Johnson DDP (2001) Dispersal in theory and practice: consequences for conservation biology. In: Clobert J, Danchin E, Dhondt AA, Nichols JD (eds) Dispersal. Oxford University Press, Oxford, UK, pp 358–372
Manel S, Schwartz MK, Luikart G, Taberlet P (2003) Landscape genetics: combining landscape ecology and population genetics. Trends Ecol Evol 18:189–197
McKelvey KS, Schwartz MK (2005) DROPOUT: a program to identify problem loci and samples for noninvasive genetic samples in a capture-mark-recapture framework. Mol Ecol Notes 5:716–718
McLellan BN, Hovey FW (2001) Natal dispersal of grizzly bears. Can J Zool 79:838–844
Oden NL, Sokal RR (1986) Directional autocorrelation: an extension of spatial correlograms to two dimensions. Syst Zool 35:608–617
Pan WS, Lu Z, Zhu XJ, Wang DJ, Wang H, Long Y, Fu DL, Zhou X (2001) A chance for lasting survival. Peking University Press, Beijing, China
Peakall R, Beattie AJ (1995) Does ant dispersal of seeds in Sclerolaena diacantha (Chenopodiaceae) generate local spatial genetic structure? Heredity 75:351–361
Peakall R, Smouse PE (2006) GenALEx 6: genetic analysis in excel. Population genetic software for teaching and research. Mol Ecol Notes 6:288–295
Peakall R, Ruibal M, Lindenmayer DB (2003) Spatial autocorrelation analysis offers new insights into gene flow in the Australian bush rat, Rattus fuscipes. Evolution 57:1182–1195
Pritchard JK, Wen W (2004) Documentation for STRUCTURE software: version 2. http://pritch.bsd.uchicago.edu
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Proctor MF, McLellan BN, Strobeck C, Barclay RMR (2004) Gender specific dispersal distances of grizzly bears estimated by genetic analysis. Can J Zool 82:1108–1118
Qi DW, Hu YB, Gu XD, Li M, Wei FW (2009) Ecological niche modeling of the sympatric giant and red pandas on a mountain-range scale. Biodivers Conserv 18:2127–2141
Queller DC, Goonight KF (1989) Estimating relatedness using genetic markers. Evolution 43:258–275
Ray N (2005) PATHMATRIX: a GIS tool to compute effective distances among samples. Mol Ecol Notes 5:177–180
Raymond M, Rousset F (2003) Genepop version 3.4. Updated from Raymond M, Rousset F 1995 Genepop (version 1.2): population genetics software for exact tests and ecumenicism. J Hered 86:248–249
Rice WR (1989) Analyzing tables of statistical tests. Evolution 43:223–225
Rosenberg MS (2000) The bearing correlogram: a new method of analyzing directional spatial autocorrelation. Geogr Anal 32:267–278
Rosenberg MS (2001) PASSAGE: pattern analysis, spatial statistics, and geographic exegesis, version 1.0. Department of Biology, Arizona State University, Tempe
Schweiger O, Frenzel M, Durka W (2004) Spatial genetic structure in a metapopulation of the land snail Cepaea nemoralis (Gastropoda: Helicidae). Mol Ecol 13:3645–3655
Scribner KT, Chesser RK (1993) Environmental and demographic correlates of spatial and seasonal genetic structure in the eastern cottontail (Sylvilagus floridanus). J Mammal 74:1026–1045
Shen FJ, Watts PW, Zhang ZH, Zhang AJ, Sanderson S, Kemp SJ, Yue BS (2005) Enrichment of giant panda microsatellite markers using dynal magnet beads. Acta Genet Sinica 32:457–462
Smouse PE, Peakall R (1999) Spatial autocorrelation analysis of individual multiallele and multilocus genetic structure. Heredity 82:561–573
Sokal RR, Oden NL (1978a) Spatial autocorrelation in biology. 1. Methodology. Biol J Linn Soc 10:199–228
Sokal RR, Oden NL (1978b) Spatial autocorrelation in biology. 2. Some implications and four applications of evolutionary interest. Biol J Linn Soc 10:229–249
Sokal RR, Wartenberg D (1983) A test of spatial autocorrelation analysis using an isolation-by-distance model. Genetics 105:219–237
State Forestry Administration of China (2006) The 3rd national survey report on giant panda in China. Science Press, Beijing, China
Støen OG, Bellemain E, Sæbø S, Swenson JE (2005) Kin-related spatial structure in brown bears Ursus arctos. Behav Ecol Sociobiol 59:191–197
Taberlet P, Griffin S, Goossens B, Questiau S, Manceau V, Escaravage N, Waits LP, Bouvet J (1996) Reliable genotyping of samples with very low DNA quantities using PCR. Nucleic Acids Res 24:3189–3194
Taberlet P, Waits L, Luikart G (1999) Noninvasive genetic sampling: look before you leap. Trends Ecol Evol 14:323–327
Turchin P (1998) Quantitative analysis of movement: measuring and modeling population redistribution in animals and plants. Sinauer Associates, Sunderland
Valière N (2002) GIMLET: a computer program for analysing genetic individual identification data. Mol Ecol Notes 2:377–379
Van Oosterhout C, Hutchinson WF, Wills DPM, Shipley P (2004) MICRO-CHECKER: software for identifying and correcting genotyping errors in microsatellite data. Mol Ecol Notes 4:535–538
Walsh PS, Metzger DA, Higuchi R (1991) Chelex-100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. Biotechniques 10:506–513
Wei FW, Feng ZJ, Wang ZW, Hu JC (2000) Habitat use and separation between the giant panda and the red panda. J Mammal 81:448–455
Wu H, Zhan XJ, Zhang ZJ, Zhu LF, Yan L, Li M, Wei FW (2009) Thirty-three microsatellite loci for noninvasive genetic studies of the giant panda (Ailuropoda melanoleuca). Conserv Genet 10:649–652
Zamudio KR, Wieczorek AM (2007) Fine-scale spatial genetic structure and dispersal among spotted salamander (Ambystoma maculatum) breeding populations. Mol Ecol 16:257–274
Zedrosser A, Stoen OG, Saebo S, Swenson JE (2007) Should I stay or should I go? Natal dispersal in the brown bear. Anim Behav 74:369–376
Zhan XJ (2006) Using noninvasive genetic sampling to estimate the population size and study dispersal of giant pandas. Dissertation, Institute of Zoology, Chinese Academy of Sciences
Zhan XJ, Li M, Zhang ZJ, Goossens B, Chen YP, Wang HJ, Bruford MW, Wei FW (2006) Molecular censusing doubles giant panda population estimate in a key nature reserve. Curr Biol 16:R451–R452
Zhan XJ, Zhang ZJ, Wu H, Goossens B, Li M, Jiang SW, Bruford MW, Wei FW (2007) Molecular analysis of dispersal in giant pandas. Mol Ecol 16:3792–3800
Zhan XJ, Zheng XD, Bruford MW, Wei FW, Tao Y (2010) A new method for quantifying genotyping errors for noninvasive genetic studies. Conserv Genet 11:1567–1571
Zhang YP, Wang XX, Ryder OA, Li HP, Zhang HM, Yong YG, Wang PY (2002) Genetic diversity and conservation of endangered animal species. Pure Appl Chem 74:575–584
Zhang BW, Li M, Ma LC, Wei FW (2006a) A widely applicable protocol for DNA isolation from fecal samples. Biochem Genet 44:503–512
Zhang ZJ, Wei FW, Li M, Hu JC (2006b) Winter microhabitat separation between giant and red pandas in Bashania faberi bamboo forest in Fengtongzhai nature reserve. J Wildlife Manage 70:231–235
Zhang BW, Li M, Zhang ZJ, Goossens B, Zhu LF, Zhang SN, Hu JC, Bruford MW, Wei FW (2007) Genetic viability and population history of the giant panda, putting an end to the “evolutionary dead end”? Mol Biol Evol 24:1801–1810
Acknowledgments
This research was funded by the National Basic Research Program of China (973 Program, 2007CB411600) and the National Natural Science Foundation of China (No. 30830020). We thank the staff of the Sichuan Forestry Department, Meigu-Dafengding Nature Reserve, Mabian-Dafengding Nature Reserve, Mamize Nature Reserve, Shenguozhuang Nature Reserve, Ma’anshan Nature Reserve, Heizugou Nature Reserve and Jinkouhe Forestry Bureau for assistance during fieldwork. We also thank M. W. Bruford (Cardiff University, UK) and R. C. Van Horn (San Diego Zoo, USA) for comments on this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hu, Y., Zhan, X., Qi, D. et al. Spatial genetic structure and dispersal of giant pandas on a mountain-range scale. Conserv Genet 11, 2145–2155 (2010). https://doi.org/10.1007/s10592-010-0100-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10592-010-0100-1