Abstract
Galaxiella pusilla is a small, non-migratory freshwater fish, endemic to south-eastern Australia and considered nationally threatened. To assist in the conservation of the species, microsatellite markers were developed and used to characterize genetic variation in 20 geographically distinct populations across its range. Substantial genetic differentiation was found between an eastern (Victoria east of the Otway Ranges and Tasmania) and western (South Australia and Victoria west of, and including, the Otway Ranges) region. This major separation was also observed in data from a mitochondrial gene and supports a previously proposed split. Populations from the eastern region had overall lower genetic diversity for both the microsatellite and mtDNA markers. There was substantial genetic differentiation between populations within the two regions, suggesting that gene flow is limited by the isolation of freshwater streams. Genetic structure, consistent with an isolation-by-distance model, was also evident in both regions. Patterns of genetic variation in this threatened species are compared to those obtained for other taxa across the same region. The need to consider separate conservation strategies for the two sets of populations is emphasized.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Genetic studies have become an increasing part of conservation planning in threatened fish species. Genetic tools can provide information on the evolutionary history, current levels of genetic diversity and gene flow, and key factors contributing to fitness of fish populations (e.g. Vrijenhoek 1998; Moran 2002; Chistiakov et al. 2006). This can help in the identification of critical populations for protection, development of programs to facilitate and maintain gene flow, and the design of captive breeding programs (e.g. Gleeson et al. 1999; Knaepkens et al. 2004; Danancher et al. 2008). Genetic studies can also help identify and resolve taxonomic issues by defining species boundaries, particularly in cryptic species complexes where there is substantial overlap in phenotypes between taxa or where there is substantial divergence in phenotypes within a single taxon, as in rainbowfish (Zhu et al. 1998) and Australian smelt (Hammer et al. 2007).
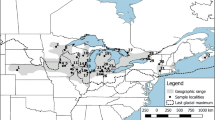
The dwarf galaxias (Galaxiella pusilla) is one of three recognised species of the galaxiid genus Galaxiella and the only member found in eastern Australia (McDowall and Frankenberg 1981). It is a small freshwater dependant fish, with females to ~40 mm in total length (TL) and males to ~35 mm TL (Backhouse and Vanner 1978; McDowall and Frankenberg 1981). Sexual dimorphism is present in G. pusilla—with males tending to be smaller and more brightly coloured including the development of a bright orange-red stripe along their sides (McDowall 1978). Although some uncertainty exists (McDowall and Waters 2004), sexual dimorphism in G. pusilla is potentially unique among the Galaxiidae. G. pusilla is generally found in shallow, still or slow flowing permanent or ephemeral habitats with abundant aquatic vegetation (emergent and submerged forms), including lakes, wetlands, swamps, billabongs, small creeks and earthen drains (Backhouse and Vanner 1978; McDowall and Frankenberg 1981; Beck 1985). Populations are only known from south-eastern Australia, including southeast South Australia, southern coastal Victoria and northern Tasmania (as well as Flinders Island) (Backhouse and Vanner 1978; McDowall and Frankenberg 1981; Beck 1985). Historic survey records held on State fauna databases for south-eastern Australia indicate that dwarf galaxias have been found within close to 50 separate waterway systems. Based on the age of many records (~20–40 years) and subsequent surveys in those areas, however, the number of these systems still supporting dwarf galaxias is likely to be closer to 40.
Galaxiella pusilla is considered a threatened species and is listed at national (EPBC Act 1999) and state (Victoria and Tasmania) levels. It is also listed as vulnerable under the 2008 IUCN Red List of Threatened Species (Wager 1996). Habitat loss resulting in fragmentation of populations, water pollution, and interaction with alien fishes (e.g. eastern gambusia, Gambusia holbrooki) are believed to be key threats (Koster 2003). The lack of diadromy and tendency to occur in freshwater habitats that are hydrologically isolated for extended periods of time (e.g. billabongs, wetlands, swamps, slow flowing streams) may contribute to the fragmentation and vulnerability of G. pusilla populations. Contemporary fragmentation of populations due to human activity (e.g. land use change, water extraction, creation of physical barriers such as weirs or dams) is expected to have led to substantial reductions in gene flow within and between some river systems. For example, a number of expansive swamplands that once existed across their range (e.g. Koo Wee Rup and Carrum swamps near Melbourne) have now been drained for farming or housing. It is probable that these swamps provided frequent opportunities for gene flow between rivers and creeks that were formerly connected via the swamps. Large swamps and lakes may have also been important refuges during times of drought—enabling the persistence of large populations and a subsequent source of colonists when rainfall and river flows increased. It is possible therefore that the recent fragmentation and isolation of G. pusilla populations has resulted in a number of localised extinctions, or alternatively where isolated populations have managed to persist, increased genetic differentiation over time within or between formerly connected river systems.
There are a number of gaps in knowledge about the biology of this species that are hindering conservation efforts. As a priority for G. pusilla conservation, Koster (2003) identified the need to understand its population genetics, including the establishment of a spatial genetic framework. An understanding of the geographical scale at which populations are differentiated and the level of gene flow among them is critical to the success of any management plan (Salgueiroa et al. 2003); this can assist in the determination of conservation management boundaries, identification of levels of historical gene flow that may guide the selection of suitable stock for translocation/reintroduction programs, and contemporary barriers to gene flow that may place certain populations at a high risk of inbreeding. This study uses both mitochondrial (cytochrome c oxidase subunit I) and nuclear (microsatellites) genetic markers to describe broad patterns of genetic structure of 20 G. pusilla populations spanning their known geographic distribution. It builds on information provided by Unmack and Hammer (unpublished) who previously used allozymes and mtDNA cytochrome b sequences to assess the genetic structure of various G. pusilla populations and suggested a distinct east–west geographic separation of populations. The purpose of the present research was to test if such a split could be validated with newly developed microsatellite markers and a different mtDNA gene. We were also interested in testing for geographic patterns in genetic diversity and characterizing levels of isolation within regions, although sampling was necessarily limited because of the threatened nature of this species.
Materials and methods
Sample collection
Due to the threatened status of G. pusilla, the numbers of fish collected was limited to 10 individuals from each of 20 populations across south-eastern Australia (Fig. 1). Locations were chosen to cover a diversity of habitats and geographic areas. Most populations were sourced from discrete river systems, with the exceptions being that Shaw River is a tributary of Eumeralla River, Monkey Creek is a tributary of Merriman Creek and Crawford River is a tributary of Glenelg River. The Crawford and Glenelg populations are geographically separated to a much greater degree (~90 km great-circle distance) than the other sites that share the same river system (~13 and 21 km great-circle distance). Fish were captured using a combination of dip nets and non-baited fine mesh bait traps and were mainly caught in shallow (<1 m) vegetated margins of each site. Bait traps were set overnight and placed on the substrate with part of the trap above the water. In most cases dip netting alone was sufficient to capture 10 individuals. Sampling was conducted progressively across the range of the species from December 2007 to December 2008. Specimens were euthanized with clove oil prior to preserving the whole fish in 100% ethanol and storing it in the laboratory at −20°C.
DNA extraction
Small sections (~2 mm2) of tissue from the caudal fin were used to extract DNA from G. pusilla specimens. DNA extractions for Polymerase Chain Reactions (PCRs) were based on the Chelex® protocol (Walsh et al. 1991). Fin tissue from individual fish was placed into separate 0.5 ml microcentrifuge tubes and 5 μl of proteinase K was added to each tube. The tissue was then crushed using a clean 0.5 ml pestle and 300 μl of 5% Chelex® solution was added. The pestle was removed and the tubes were incubated at 55°C for 60 min followed by 100°C for 8 min. Extractions were stored at −20°C until required. Prior to PCRs, extractions were spun at 13 000 rpm for 2 min. The supernatant from just above the Chelex® resin was used in the PCR amplifications.
Microsatellite isolation
Microsatellites were isolated with the FIASCO protocol of Zane et al. (2002) using modifications described in Rugman-Jones et al. (2005). The microsatellite library was developed using DNA extractions from four individuals from geographically distinct populations, i.e. one from each of Bridgewater Lake, Shaw River, Tuerong Creek and Monkey Creek. Two hundred and twenty-five clones were sequenced by Macrogen laboratories (Seoul, Korea) from which 43 clones were identified to contain six or more di-nucleotide repeat units. Primer pairs were designed in the flanking regions of these positive clones with the software PRIMER Version 3 (Rozen and Skaletsky 2000) and OLIGO Version 4.1 (National Biosciences). In some cases primers were designed partly inside the repeat motif, with a maximum of four di-nucleotide repeats incorporated into the primers. By screening PCR products using both 2% agarose and 5% polyacrylamide denaturing gels and annealing temperatures of 55°C, 11 of these microsatellite loci were successfully amplified and shown to be polymorphic (Table 1) and selected to study genetic differentiation and diversity within G. pusilla populations.
Microsatellite PCR
Reactions were adjusted to a final volume of 10 μl with ddH2O and contained 1x polymerase reaction buffer, 2 mM MgCl2, 0.2 mM dNTPs, 0.5 mg/ml bovine serum albumin (BSA) (Biolabs, New England, USA), 0.03 U of Immolase DNA polymerase (Bioline, Alexandria, Australia), 0.3 μM forward primer end-labelled with [γ33P] ATP, 0.2 μM unlabelled forward primer, 0.5 μM unlabelled reverse primer and 2 μl of template DNA (from Chelex extractions). 0.2 μM unlabelled forward primer was added so that the efficiency of the PCR was not compromised and to reduce the use [γ33P] ATP.
PCRs were carried out with either an Eppendorf Mastercycler (Eppendorf, Hamburg, Germany) or GeneAmp PCR System 9700 thermal cycler (PE Biosystems, California, USA). Thermocycling was the same for all primer pairs, with an initial denaturation at 95°C for 10 min, followed by 35 cycles of 95°C for 20 s, 55°C for 30 s and 72°C for 30 s. A final extension step of 72°C for 3 min preceded an indefinite hold period at 4°C. PCR products were then run through 5% polyacrylamide denaturing gels at 65 W for ~2 h and exposed to autoradiography film (OGX, CEA, Sweden) after 1–5 days. Alleles were initially sized by comparison with λgt11 ladders (Promega fmol® DNA Cycle Sequencing System, Madison, USA). PCR products run on subsequent gels were sized using previously sized PCR products.
Microsatellite data analysis
Mean observed heterozygosity, mean expected heterozygosity and mean number of alleles per locus were calculated with Microsatellite Tools for Excel (Park 2001). Allelic richness averaged over loci, Weir and Cockerham’s (1984) F statistics (F IS and F ST) and tests for linkage disequilibrium based on a log-likelihood ratio test, were calculated with FSTAT 2.9.3 (Goudet 2001). Two-sided P values after 9999 permutations were also calculated in FSTAT to test for differences in heterozygosity and allelic richness between the east and west regions. Deviations from Hardy–Weinberg equilibrium were determined by exact tests and significance determined through permutation in GDA Version 1.1 (Lewis and Zaykin 2001). An estimate of the null allele frequency for each locus across all populations was performed in GENELAND (Guillot et al. 2005, 2008, 2009), and evidence for null alleles and large allele dropout for each locus within each population was assessed using MICRO-CHECKER Version 2.2.3 (Van Oosterhout et al. 2004), with an analysis setting of 1,000 and a Bonferroni confidence interval.
To compare differences between populations, a hierarchical analysis of molecular variance (AMOVA) was performed with ARLEQUIN Version 3.1 (Excoffier et al. 1992, 2006). Microsatellite data were partitioned to enable a comparison of variation among regions (‘west’ = western Victoria/South Australia and ‘east’ = eastern Victoria and Tasmania), among populations within regions and among individuals within populations. Regions were defined according to the previous finding by Unmack and Hammer (unpublished) of two distinct geographic groups of dwarf galaxias populations. Pairwise F ST values were calculated by ARLEQUIN and used as the distance measure. AMOVA computations were performed with 10,000 permutations to test for significance.
To test for isolation by distance (Wright 1943), Slatkin’s (1995) linearized F ST transformation (F ST/1 − F ST) was regressed onto the natural log of geographic distance (Rousset 1997). The significance of this relationship was determined with a Mantel test (10 000 permutations) in POPTOOLS (Mantel 1967; Hood 2002). GENALEX Version 6 (Peakall and Smouse 2006) was used to generate a pairwise geographic distance matrix between individuals from longitudes and latitudes. Geographic distance calculations followed a modified Haversine Formula (Sinnott 1984) to produce great-circle distances between two points on a sphere. As most populations were collected from disconnected waterways, a geographic distance measure directed primarily at opportunities for ‘overland’ gene flow (e.g. major floods, geologic changes in drainage boundaries) was considered appropriate.
Two contrasting Bayesian clustering methods were applied to examine population genetic structure without allocating individuals to populations prior to analysis: STRUCTURE Version 2.2 (Pritchard et al. 2000) and SAMOVA Version 1.0 (Dupanloup et al. 2002). Based solely on genetic data, STRUCTURE identifies the number of distinct clusters, assigns individuals to clusters and identifies migrants and admixed individuals. To determine the number of populations (K) within the complete data set, three independent simulations for K = 1–22 with 100,000 burn in iterations and 500,000 data iterations were run. Three independent simulations for K = 1–12 with the same parameters were also run for each of the east and west regions independently, although the ability to detect population structure within regions is likely to be low given the small sample sizes,. Analysis was performed using the admixture model of population structure (i.e. each individual draws some fraction of their genome from each of the K populations) and allele frequencies correlated among populations (i.e. allele frequencies in the different populations are likely to be similar due to factors such as migration or shared ancestry) (Pritchard et al. 2000).
Using genetic data and geographic coordinates, SAMOVA (spatial analysis of molecular variance) defines groups of populations that are geographically homogenous and maximally differentiated from each other (Dupanloup et al. 2002). Similar to STRUCTURE, SAMOVA is a Bayesian clustering method that finds group structure from genetic data. However, rather than defining groups of individuals, SAMOVA defines groups of populations with the constraint that they must be geographically adjacent and genetically homogenous (Dupanloup et al. 2002). The method is based on the proportion of total genetic variance due to differences between groups (F CT), where the largest mean F CT is expected to be associated with the correct number of simulated groups (Dupanloup et al. 2002). As opposed to STRUCTURE, SAMOVA makes no assumptions about Hardy–Weinberg equilibrium within populations, or about linkage equilibrium between loci (Dupanloup et al. 2002). To determine the number of groups within the complete data set, 100 simulated annealing processes for each K = 2–19 were run on a sum of square size differences distance matrix. Simulations were also run for the east and west regions independently with K = 2–9.
To visualise the genetic structure of individuals over multiple loci, multivariate factorial correspondence analysis was undertaken with GENETIX Version 4.05 (Belkhir et al. 2004). The two factors that explain the majority of the variation in multilocus genotypes were plotted.
All tests involving multiple comparisons were corrected at the table-wide α′ = 0.05 level with a Dunn-Šidák test (Sokal and Rohlf 1995).
mtDNA PCR
Reactions were adjusted to a final volume of 30 μl with ddH2O and contained 1x polymerase reaction buffer, 2 mM MgCl2, 0.2 mM dNTPs, 0.03 U Immolase DNA polymerase (Bioline, Alexandria, Australia), 0.5 μM of each primer and 5 μl of template DNA. Primers used to amplify a 655 bp region of the Cytochrome c oxidase subunit I gene (COI) were FishF1 and FishR1 as described in Ward et al. (2005). PCR conditions were modified slightly from Ward et al. (2005) and comprised an initial 7 min denaturing step at 94°C, then 40 cycles of denaturation at 94°C (20 s), annealing at 54°C (30 s), and extension at 72°C (60 s), with a final extension step of 72°C for 1 min preceding an indefinite hold period at 4°C.
PCR amplification of double-stranded product was carried out with either an Eppendorf Mastercycler (Eppendorf, Hamburg, Germany) or Geneamp PCR System 9700 thermal cycler (PE Biosystems, California, USA). PCR products were sent to Macrogen laboratories (Seoul, Korea) for purification and sequencing on an ABI3730 XL DNA sequencer (Applied Biosystems, Foster City, California). All products were sequenced in both directions and primers from the initial PCR reaction (FishF1 and RishR1) were used for sequencing.
mtDNA data analysis
Forward and reverse sequences were aligned and manually edited in Sequencher Version 4.6 (Genecodes, Ann Arbor, Michigan). Consensus sequences were then imported into MEGA Version 4 (Tamura et al. 2007) for multiple alignments with ClustalW. ClustalW analysis was performed with the default parameters, namely an opening gap penalty = 15 and gap extension penalty = 6.66 for both pairwise and multiple alignments.
To determine the extent of genetic structure and identify relationships between haplotypes, phylogenetic trees were constructed using both Bayesian inference and maximum parsimony methods. These methods differ in their approach in that the Bayesian inference method searches for trees that maximises the probability of the tree given the data and the model of evolution, while parsimony selects trees that require the fewest number of changes to explain the data in the alignment. COI sequences of 582 bp in length were used to construct trees. A congener, the black-stripe minnow Galaxiella nigostriata, and the common galaxias Galaxias maculatus (Galaxiidae), were included as out-groups—GenBank accession numbers for these taxa were NC008448 and NC004594, respectively. Genetic differentiation between haplotypes was also examined by calculating pairwise genetic distances under a Kimura-2-parameter model within MEGA (Tamura et al. 2007).
MODELTEST Version 3.7 (Posada and Crandall 1998) provided the optimum nucleotide substitution model for the data prior to constructing the Bayesian inference phylogenetic tree within MRBAYES Version 3.1.2 (Huelsenbeck and Ronquist 2001; Ronquist and Huelsenbeck 2003). MRBAYES uses the Metropolis-coupled Monte Carlo Markov Chain (MCMC) method to sample possible trees. Four chains were run for 5,000,000 generations, and sampled trees every 100 generations. The default temperature parameter of 0.2 was applied and the swap statistics matrix examined following analysis to ensure heated chains were all successfully contributing states to the cold chain (i.e. were contributing possible trees). Convergence of runs is indicated by an average standard deviation of split frequencies between duplicate runs of less than 0.01 (a final value of 0.002803 was obtained here). Stationarity of the chains was assessed by plotting log likelihood values against generation time and observing the point where a stable mean equilibrium value was reached. Burn in values were conservatively set to discard the first 5,000 trees (to remove trees outside the stable mean equilibrium) prior to calculation of a consensus topology and estimates of posterior probabilities for the 50% majority rule consensus tree.
The construction of a maximum parsimony phylogenetic tree was performed using PAUP Version 4.0b10 (Swofford 1998). A heuristic search with 1,000 replicates was run with tree-bisection–reconnection (TBR) branch swapping to estimate the most parsimonious trees. Equally parsimonious trees produced by the search were then summarised in PAUP by constructing a strict consensus tree (any clade that does not occur in all trees is displayed as a polytomy). Bootstrapping analysis with 1,000 replicates was also performed to estimate support for nodes within the tree.
In addition to the microsatellite data, differences between populations using the 582 bp COI sequences were assessed with a hierarchical analysis of molecular variance (AMOVA) in GENALEX. Once again, COI data were partitioned to enable a comparison of variation among east and west regions, among populations within regions and among individuals within populations. Pairwise Φ PT values (an analogue of F ST) were calculated by GENALEX as the genetic distance measure and were based on the number of differences at 62 polymorphic sites on the 582 bp fragments. AMOVA computations were performed with 9,999 permutations to test for significance.
Demographic history was explored using the software DnaSP version 4.50.3 (Rozas et al. 2003). Using COI haplotypes, signatures of recent bottleneck events were examined by assessing deviations from neutrality using Tajima’s D statistic (Tajima 1989). Evidence for population range expansion was assessed using mismatch analysis (Rogers et al. 1996; Rogers and Harpending 1992) of COI haplotypes with the raggedness index determined via coalescent simulations using 1,000 replicates (Harpending et al. 1993). Evidence for population growth was also assessed using coalescent simulations with 1,000 replicates to calculate Fu and Li’s F* and D* (Fu and Li 1993), and Fu’s F s (Fu 1997) statistics.
All unique mtDNA sequences (haplotypes) used in the analyses were submitted to Genbank under accession numbers GU580944 to GU580964.
Results
Microsatellites
A total of 178 alleles were detected with 11 microsatellite loci from 20 populations (198 individuals), with summary statistics presented in Tables 1 and 2, and detailed statistics for each locus and population separately presented in Supplementary Table 1. ‘West’ region populations were more diverse with 131 alleles in populations sampled from western Victoria and South Australia compared with 74 in ‘east’ region populations from eastern Victoria and Tasmania. Only 25 alleles (14%) were shared between the two regions—although given the diversity of alleles at some loci, there is the potential that further sampling will reveal a greater level of allele sharing. The size of microsatellite loci ranged from 89 to 183 bp, with low estimates of null allele frequencies for each locus across all populations. When each population was analysed separately, there was no evidence for large allele dropouts and there was no evidence of null alleles for at least 18 of the 20 populations for every locus.
Mean number of alleles and allelic richness ranged from a = 1.64 and r = 1.61 (Gosling Creek) to a = 6.36 and r = 5.37 (Darlot Creek), with lower values tending to occur in the eastern region. Observed heterozygosity (H o) ranged from 0.20 (Cobblers Creek) to 0.65 (Darlot Creek) and, as in the case of allele number and richness, values tended to be relatively lower in the eastern region. Across all populations in the western region, mean number of alleles, allelic richness and H o were 4.23 (SE 0.41), 3.75 (SE 0.33) and 0.51 (SE 0.04), respectively. Across all populations in the eastern region, mean number of alleles, allelic richness and H o were 2.18 (SE 0.08), 2.07 (SE 0.07) and 0.29 (SE 0.02), respectively. Significant differences in mean allelic richness and H o between regions were observed (P values < 0.001).
Over all loci, F IS was not significantly greater than zero in any of the 20 populations after (or before) corrections for multiple comparisons. Except for the Tuerong Creek population, there were no significant deviations from Hardy–Weinberg equilibrium and tests for genotypic linkage disequilibrium found no significant associations between pairs of loci within populations or across all populations after correcting for multiple comparisons.
In an AMOVA, where populations were divided among the east and west regions, there was significant variation at all three hierarchical levels. The highest level of variation was observed within populations (40.89%, F ST = 0.5911, P < 0.0001) and the lowest level of variation among populations within regions (21.50%, F SC = 0.3447, P < 0.0001). Differences between the east and west regions explained 37.61% (F CT = 0.3761, P < 0.0001) of the variation in the microsatellite data.
The global estimate of F ST over all populations and loci was significantly different from zero (F ST = 0.503, 99% confidence intervals 0.409–0.600), and suggests that there are substantial genetic differences between populations. Pairwise F ST estimates differ significantly in 178 of the 190 population comparisons after correcting for multiple comparisons (see Table 4 in Appendix). Over all populations, pairwise F ST values ranged from 0.011 (Darlot Creek Tributary and Eumeralla River) to 0.779 (Gosling Creek and Cobblers Creek) and a mean of 0.477 (SE = 0.012). Within the east region only, pairwise F ST values ranged from 0.181 (Merriman Creek and Monkey Creek) to 0.599 (Moe Main Drain and Icena Creek Tributary) with a mean of 0.448 (SE = 0.015). In the west region, pairwise F ST values ranged from 0.011 (Darlot Creek Tributary and Eumeralla River) to 0.622 (Gosling Creek and Hammerhead Lagoon) with a mean of 0.251 (SE = 0.020). Pairwise F ST values, both within and between regions, were lowest between adjacent river systems and highest between sites that were separated by large geographic distances. For higher F ST values it is likely that, due to relatively long periods of population isolation and anticipated affects of size homoplasy, these values underestimate genetic differentiation (e.g. Balloux and Lugon-Moulin 2002).
A significant positive relationship between genetic distance (Slatkin’s linearized F ST) and geographic distance (natural log transformed) of all populations was identified using a Mantel test (r = 0.575, P < 0.001). This relationship was also significant when populations within east and west regions were tested independently (r = 0.515, P = 0.005 and r = 0.591, P = 0.013, respectively). Regression analysis identified a significant linear relationship between genetic distance and geographic distance, for all populations (R 2 = 0.331, F = 92.944, P < 0.001) and for the east (R 2 = 0.293, F = 17.834, P < 0.001) and west (R 2 = 0.356, F = 23.784, P < 0.001) regions considered separately.
Analysis of population structure on the entire dataset using STRUCTURE indicated that the most likely value for K is 11 based on the highest mean estimated log probability of data −4551.4 (SD = 9.4) and based on the value close to the initial ‘plateau’ of the curve when K is plotted versus mean posterior probability (Pritchard et al. 2000). From this analysis, the west region was divided into eight clusters and the east region into three clusters for all three runs. For the west region, outputs consistently grouped individuals from Shaw and Moyne Rivers into one cluster, and Darlot Creek Tributary and Eumeralla River into another cluster. For the east region, outputs from the three runs consistently grouped Hallam Main Drain and Tuerong Creek into one cluster, Yallock Creek, Cardinia Creek Tributary and Darby River into another cluster, and Moe Main Drain, Merriman Creek, Cobblers Creek and Monkey Creek into the remaining cluster. Icena Creek Tributary either grouped with the Hallam Main Drain and Tuerong Creek cluster or the Yallock Creek, Cardinia Creek Tributary and Darby River cluster. When regions were analysed independently, the most likely K for the west region was 9 (mean estimated log probability of data −2745.0, SD = 5.4) and the most likely K for the east region was 7 (mean estimated log probability of data −1354.1, SD = 5.7). In the west region, the Shaw and Moyne Rivers consistently grouped into one cluster, while Darlot Creek Tributary and Eumeralla River shared a similar profile (Fig. 2a). From the three runs for the east region, Tuerong Creek and Hallam Main Drain grouped into one cluster, as did Moe Main Drain and Cobblers Creek, and Merriman and Monkey Creek grouped into a third cluster (Fig. 2b).
Summary plots of the estimated membership coefficient (x axis) for each individual from Galaxiella pusilla populations in the a west region (K = 9 and n = 100) and b east region (K = 7 and n = 98) based on 11 microsatellite loci. Each individual is represented by a single vertical line broken into shaded segments, where segments are proportional to the membership coefficient for each of the K clusters. Individuals are arranged into populations from which they were sampled and symbols indicate clustered populations
Analysis of population structure on the entire dataset using SAMOVA indicated F CT values progressively increased from K = 2 (0.373) to 19 (0.477), with no clear indication of the most appropriate K. At K = 2, the populations were separated into the east and west regions. At K = 3 the first split within regions was Gosling Creek from the rest of the west region, while in the east region Gippsland sites (Moe Main Drain, Merriman Creek, Cobblers Creek and Monkey Creek) were split from the others. At K = 19 the last remaining cluster was Darlot Creek Tributary and Eumeralla River in the west region, while the last remaining cluster in the east region was Yallock and Cardinia Creeks (K = 17). The SAMOVA results for each region independently are given in Table 3. In the west region, the first population to split from the others was Gosling Creek (F CT = 0.20). After an initial decline in F CT, as K increases from 4 to 9 there is a corresponding increase in F CT. At K = 8, Shaw and Moyne Rivers are still grouped, as are Darlot Creek Tributary and Eumeralla River (also K = 9). In the east region, the first population split is between the Gippsland sites from the others (F CT = 0.18). The greatest F CT value was 0.34 at K = 8, where Yallock and Cardinia Creeks are still grouped, as are Cobblers and Monkey Creeks.
The degree of relationship between populations is visually represented with two-dimensional factorial correspondence analysis of the microsatellite data (Fig. 3). The factor that explained most of the variance for all populations combined (factor 1, 23.06%) strongly separated the east and west regions and the second factor (factor 2, 8.72%) showed some separation within regions (Fig. 3a). Relationships between populations within regions are more clearly shown in Fig. 3b, c. For populations in the east region, factor 1 and factor 2 explained 22.26 and 17.99% of the variation, respectively. These factors most notably separate the Tasmanian population (Icena Creek Tributary), a broad group comprising the Gippsland populations (Moe Main Drain, Merriman Creek, Monkey Creek, Cobblers Creek) and another broad group comprising the Melbourne and Wilsons Promontory populations (Hallam Main Drain, Tuerong Creek, Cardinia Creek, Yallock Creek, Darby River). For populations in the west region, factor 1 and factor 2 explained 21.25 and 16.27% of the variation, respectively. These factors identify one of the South Australian populations (Hammerhead Lagoon) as a group and the Otways population (Gosling Creek) as another group, independent from the other west Victoria and South Australian populations.
Two-dimensional plot showing the relationships among populations of Galaxiella pusilla based on a multivariate factorial correspondence analysis of 11 loci from a all 20 populations, b ‘east’ region only and c ‘west’ region only. The first two factors are shown, with the percentage of variance explained in parenthesis
mtDNA sequences
The west region contained 15 COI haplotypes from 100 individuals, while the east region contained six COI haplotypes from 98 individuals (Table 2). The number of individuals within a haplotype ranged from n = 1 to n = 38, with the latter composed of G. pusilla from the Tuerong, Hallam, Cardinia and Yallock systems (i.e. the greater Melbourne area).
Phylogenetic trees constructed with both maximum parsimony (MP) and Bayesian inference (BI) infer strong haplotype divergence (BI posterior probability and MP bootstrap estimate both = 100) between the east and west regions (Figs. 4, 5). In contrast, there is a lack of agreement between the MP and BI groupings within regions. Kimura 2-parameter distances within populations from the east and west regions varied from 0.002 to 0.014 (mean = 0.008, SE = 0.001) and 0.002 to 0.028 (mean = 0.012, SE 0.001), respectively (see Table 5 in Appendix). Much greater distances were observed between regions and those differences varied from 0.077 to 0.094 (mean = 0.084, SE 0.001). Inter-regional distances were lowest between the Glenelg haplotypes (‘A’ and ‘B’) and Merriman/Monkey B haplotype, the Hammerhead haplotype and Merriman/Monkey B haplotype and the Gosling and Moe/Monkey A/Cobblers haplotypes. Inter-regional distances were greatest between the Hatherleigh haplotypes (‘A’ and ‘B’) and Tuerong/Hallam/Cardinia/Yallock, Moe/Monkey A/Cobblers, Monkey C and Icena haplotypes. Kimura 2-parameter distances between G. pusilla populations and the genus outgroup G. nigostriata varied from 0.217 to 0.250 (mean = 0.229, SE = 0.002) and between the family outgroup Galaxias maculatus distances varied from 0.242 to 0.261 (mean = 0.251, SE = 0.001).
Bayesian inference phylogenetic tree for Galaxiella pusilla haplotypes generated from a 502-bp fragment of the mtDNA gene cytochrome c oxidase subunit I. The black-stripe minnow, Galaxiella nigostriata, and common galaxias, Galaxias maculatus, were used as outgroups. Branches with <50% Bayesian posterior probability support are collapsed. ‘A’, ‘B’ and ‘C’ are used to distinguish different haplotypes within populations
Maximum parsimony phylogenetic tree for Galaxiella pusilla haplotypes generated from a 502-bp fragment of the mtDNA gene cytochrome c oxidase subunit I. The black-stripe minnow, Galaxiella nigostriata, and common galaxias, Galaxias maculatus, were used as outgroups. Strict consensus tree based on the 15 most parsimonious trees. Bootstrap estimates are derived from 1,000 replicates and values less than <50% are not shown. ‘A’, ‘B’ and ‘C’ are used to distinguish different haplotypes within populations
Using the mtDNA data, an AMOVA with the same three hierarchical levels as the microsatellite AMOVA showed that the majority of observed variation was among the east and west regions (89.86%), and only small levels of variation were observed among populations within regions (9.66%) and within populations (0.49%). Values for Φ RT (correlation within a region relative to the total), Φ PR (correlation between individuals within a population relative to the individuals from the same region) and Φ PT (correlation between individuals within a population relative to the total) were 0.899 (P < 0.001), 0.952 (P < 0.001) and 0.995 (P < 0.001), respectively.
For the east region, demographic history was explored using COI haplotypes in an effort to explain the comparatively low genetic diversity observed. Tajima’s D (−0.0650) was non-significant (P > 0.10) suggesting no evidence for a recent bottleneck event. The mismatch analysis (initial θ = 0, final θ = 1,000, expansion parameter τ = 2μt = 2.167) produced a slightly ragged unimodal distribution with a nonsignificant raggedness index (r = 0.2778, P = 0.3605), suggesting a stationary population. Fu and Li’s F* (−0.071) and D* (−0.07841), and Fu’s F s (0.34697) were also non-significant (P > 0.10) and in combination do not indicate population growth.
Discussion
Eleven polymorphic microsatellite loci were isolated from Galaxiella pusilla specimens and used to assess the genetic structure of 20 populations across the species geographic range in south-eastern Australia. Microsatellite data from these populations consistently showed substantial genetic differences between east (Victoria east of the Otway Ranges and Tasmania) and west (South Australia and Victoria west and including, the Otway Ranges) regions. The broad separation of G. pusilla populations was also apparent in the mtDNA data for fragments of the Cytochrome c oxidase subunit I gene. These findings agree with Unmack and Hammer (unpublished) who used allozymes and Cytochrome b to investigate the genetic structure of G. pusilla populations.
Based on a study of 207 fish species (mostly Australian marine fish), Ward et al. (2005) observed mean within species and within genus Kimura 2-parameter (K2P) distances for COI of 0.39% (SE 0.031) and 9.93% (SE 0.096), respectively. The maximum within-species K2P distance observed was 14.08% (Ward et al. 2005). Based on the mean COI divergence of 8.43% (SE 0.05) between populations in the east and west regions, it is possible that Galaxiella pusilla (Mack 1936) as it is currently recognised, may comprise two separate species. Additional biological and ecological comparisons (e.g. differences in morphometrics, habitat preferences, breeding patterns, sexual isolation or fitness of offspring) are required to further clarify the taxonomic status. Observations during the study surveys did not indicate clear inter-regional habitat differences (e.g. altitude, aquatic vegetation, hydrology, water quality, benthic substratum) to suggest that substantial habitat adaption at the region scale is likely to have occurred. Current climate conditions (based on data from 1961 to 1990), such as mean annual rainfall and mean January maximum temperatures (Unmack 2001), are also broadly similar across the current species range. However, historical climate patterns and/or the unique hydrology of certain river systems (e.g. long-term wetting and drying cycles of expansive swamps or lakes) may drive local adaption. If such adaptations have occurred, they may include changes to the timing or regularity of breeding seasons or the ability to survive periods of low water level (e.g. tolerance to elevated water temperatures and low dissolved oxygen or a capacity to aestivate in benthic habitat when there is no surface water). The ability of G. pusilla to aestivate as a survival strategy during periods of low water level has been suggested by some authors (e.g. McDowall and Frankenberg 1981; Beck 1985; Chilcott and Humphries 1996). Understanding whether these types of adaptations have occurred could be particularly important for conservation strategies in light of recent climate change scenarios that predict reductions in the volume and frequency of rainfall across southeastern Australia over the next few decades (CSIRO 2007).
In a review of the biogeography of Australian native freshwater fish, Unmack (2001) identified an east–west faunal divide (based on the extent of endemism) near Wilson’s Promontory—separating South Western Victoria and Northern Tasmania regions (collectively referred to as the Bass Province) from South Eastern Victoria. Hammer (2001) observed a similar genetic divide (using allozymes) within southern pygmy perch (Nannoperca australis), an Australian native freshwater fish that frequently co-exists with G. pusilla. Hammer (2001) proposed a species boundary between eastern (Gippsland, Flinders Island and north eastern Tasmania) and western (south east South Australia, western Victoria and northern Tasmania) populations. The genetic grouping of G. pusilla populations into west and east regions is broadly consistent with these faunal divides. Where the genetic structure of G. pusilla appears to differ is the similarity of Tasmanian and central Victorian populations (i.e. greater Melbourne) to South Eastern Victoria populations, rather than South Western Victoria populations.
Freshwater-dependent species may show strong phylogeographic structure, with connectivity of contemporary and ancient river systems providing a basis for the interpretation of patterns (e.g. Wong et al. 2004; Thacker et al. 2007; Burridge et al. 2008; Schultz et al. 2008). It is possible that the geographic split of G. pusilla into two broad east and west lineages relates to an ancient network of freshwater systems that facilitated gene flow across its range during a period of substantially lowered sea level (e.g. Schultz et al. 2008). The genetic isolation of the east and west lineages could then have followed as the sea level rose and progressively disconnected paleodrainages. For example, the genetic grouping of G. pusilla populations from Tasmania and eastern Victoria indicated by the COI data is best explained by former landbridges and ancient river systems that occurred throughout Bass Strait at various glacial maxima and sea level regressions (Jennings 1958; Horwitz 1988; Chilcott and Humphries 1996; Unmack 2001; Schultz et al. 2008).
As well as paleodrainage connectivity and subsequent disconnection as an explanation for the separation of east and west populations, alternative explanations include significant geomorphic events (e.g. tectonic uplift, lava flows) or extensive wet periods that alter drainage basin boundaries (e.g. Unmack 2001). For example, extensive tectonic uplift occurred during the late Pliocene (Pliocene = 5.3–1.8 Ma) and early Pleistocene (Pleistocene = 1.8–0.1 Ma) in southern Victoria, including elevation along the Otway Ranges and South Gippsland Uplands (Hills 1975; Douglas and Ferguson 1976). In addition a major peak in volcanic eruptions occurred throughout the western Victoria plains around 2 Ma (with most activity occurring between 4.5 and 2.0 Ma) (Birch 2003). In terms of historical wet periods, combined paleontological and climatic data for south-eastern Australia indicate during 4–5 Ma the climate was significantly warmer and wetter (mean annual temperatures and rainfall of ~20°C and >1,000 mm) than at present (mean annual temperatures and rainfall of 13°C and 680 mm) (Birch 2003). To interpret patterns of freshwater crayfish distribution between western and central Victoria, Schultz et al. (2008) discussed the connection of river systems via the historical expansion and contraction of Lake Corangamite (between ~1.6 and 0.1 Ma) that could equally apply to G. pusilla populations. Alternatively, perhaps the broad distribution of populations across the east–west regional divide was initially made possible by the occurrence of diadromy in an ancestral stock, i.e. that has since lost a marine life history and subsequently isolated populations into various freshwater systems. The loss of diadromy in New Zealand Galaxiidae fish has been reported by Waters and Wallis (2001).
Using a molecular-clock approach and (in the absence of established COI mutation rates for G. pusilla) the ‘universal’ rate of 2% sequence divergence per million years for mtDNA (Brown et al. 1982), the average divergence of the COI sequences between the east and west regions of 8.4% equates to a separation of around 4 Ma. Although this date estimate seems roughly consistent with some of the historical geologic and climatic events described above, the use of molecular clocks is controversial– especially where mutation rates have not been developed for the specific taxon and gene in question (Martin et al. 1992) and due to a lack of sufficient calibration points with 95% confidence intervals on date estimates (Hillis et al. 1996). Other studies of COI mutation rates in fish may be much slower than 2% per million years, e.g. 0.9% in Goodeidae (Webb et al. 2004).
Analysis of genetic structure within the two regions using microsatellites suggests that, as well as river network connections (e.g. Eumeralla River system in the west region and Merriman Creek system in the east region), overland connections between geographically close populations during wet periods (e.g. large floods, swampland connections) may be important for the facilitation of gene flow. For example, in the west region historical overland connections seem to have occurred between the Shaw and Moyne River systems, and between the Darlot Creek and Eumeralla River systems. Gene flow may also have been made possible via paleodrainage connections during times of lower sea level between the last glacial maxima approximately 18,000 years ago and the post-glacial transgression a few thousand years later (Harris et al. 2005), e.g. Tuerong Creek and Hallam Main Drain via the Port Phillip paleodrainages, or Moe Contour Drain, Merriman Creek, Monkey Creek and Cobblers Creek via the a large paleo-catchment incorporating much of Gippsland.
Sea level transgression across Bass Strait and the isolation of Tasmania from mainland Australia from about 14,000 years ago (Lambeck and Chappell 2001) as well as the subsequent isolation of river systems on Wilsons Promontory (Darby River) could explain the distinctiveness of microsatellite genotypes in these populations. In the case of Cardinia and Yallock Creeks, the former Koo Wee Rup swamplands that were drained in the late 1800s to early 1900s for farming would have provided frequent connection between Cardinia and Yallock Creek populations. On the other hand, individuals from the Gosling Creek population seem to have been isolated from the other west populations to the greatest extent. The expansion and retraction of the Lake Corangamite system that once connected the headwaters of the Barwon River system (of which Gosling Creek is a tributary) and the Mt Emu Creek system in the Hopkins River basin (Currey 1964; Schultz et al. 2008) is a probable explanation for the connection and subsequent isolation of the Gosling Creek population.
Populations from the east region tended to have lower genetic diversity than the west region, as indicated by both the microsatellite data and COI haplotypes. Based on microsatellite analyses, DeWoody and Avise (2000) showed that mean values for heterozygosity and mean number of alleles per locus in freshwater fish were 0.46 (SD 0.34) and 7.5 (SD 8.1), respectively. The east populations had substantially lower values than these means. Given the ancient genetic split between regions, it is expected that populations in the east region would have had opportunities for dispersal and development of genetic structure (as evident in west populations). Therefore, the low genetic diversity and population differentiation observed in the east region may be indicative of an historical bottleneck and a more recent range expansion/population growth event(s).
Differences in the availability of suitable refuge habitats (e.g. expansive swamps, lakes) between west and east regions during periods of extreme aridity (e.g. glacial maxima) could account for the contrasting patterns of genetic diversity observed in this study. Possible refuges in the west may have included several swamps and lakes in the western plains formed by extensive volcanic activity, e.g. associated with maars, claderas or lava flow blockages along river systems (Ollier and Joyce 1964). In contrast, refuge habitats for east region populations may have been scarce, e.g. confined largely to the Bassian paleo-lake (Harris et al. 2005). It is possible, however, that founder event occurred in the east region and only a small, isolated population existed for a long time prior to a recent range expansion. Although historical bottleneck or range expansion/population growth events were undetected using the COI data, it is recommended that these events be assessed more closely using additional mitochondrial and nuclear markers.
Anthropogenic impacts are unlikely to be a primary cause of contrasting genetic diversity between regions, as sites in both the east and west regions differ in their levels of human disturbance—some sites are in protected parks (e.g. Darby River, Glenelg River, Hammerhead Lagoon), others are surrounded by residential housing (e.g. Hallam Main Drain), while most are within catchments dominated by agricultural landscapes (particularly grazing).
The high F ST values between populations suggest that there is substantial genetic structure within both the east and west regions and this raises questions about the management of G. pusilla populations for conservation. Various definitions for deriving conservation management boundaries using molecular and ecological data have been proposed, with broad-scale evolutionary significant units (ESUs) and finer-scale management units (MUs) commonly discussed (e.g. Ryder 1986; Moritz 1994; Moran 2002; Hey et al. 2003; Wan et al. 2004). Under some of these definitions (Moritz 1994), the microsatellite and mtDNA data indicate that the ‘east’ and ‘west’ G. pusilla populations should be regarded as separate ESUs. Accordingly, independent conservation of populations in these regions is warranted. In addition, the translocation of individuals between ESUs is discouraged (Moritz 1994). To determine MUs within ESUs, however, a greater number of populations need to be sampled as well as larger sample sizes from each population. The observation of strong isolation-by-distance for populations indicates that, despite potential human intervention (e.g. accidental or deliberate translocation of individuals), drainage divisions provide a firm basis for the delineation of MUs. The derivation of MUs must refer to pre-European settlement drainage connections (~220 years ago), i.e. before anthropogenic barriers to gene flow such as reservoirs, weirs, swamp drainage, levees and channel realignment were created. To conserve the processes that maintain adaptive diversity and evolutionary potential, the goal of management should be to preserve the natural network of genetic connections between populations (Crandall et al. 2000), e.g. between the Cardinia Creek and Yallock Creek systems.
As intended, the sample sizes of this study are sufficient for describing the major genetic separation between the east and west regions and the broad genetic structure of G. pusilla across its range. Future genetic research on this species should focus on a finer-scale analysis of the 11 microsatellite loci with a larger number of individuals from a greater number of populations. In the context of the conservation status of G. pusilla, a non-destructive sampling technique (e.g. fin clipping) that reduces the impact on wild populations will be required for any expanded study. More samples from a broader spread of populations should provide more accurate estimates of genetic diversity, as well as estimates of gene flow along and between river systems. Targeted collection of samples from multiple populations within both ‘highly connected’ and ‘poorly connected’ river systems, may also provide insight into the potential genetic consequences of fragmentation and isolation of G. pusilla populations due to anthropogenic disturbance. The expanded genetic study would also help identify priority populations for protection or active management and enable the creation of MU boundaries. A detailed comparison using estimates of divergence times from multiple mtDNA genes, mapping of ancient drainage patterns and consideration of key geological events across south-eastern Victoria would improve estimates of dates and potential causes of the major east and west separation of G. pusilla populations. Collection of comparative morphometric, biological and ecological data would help clarify the taxonomic status of the two groupings of G. pusilla characterized here. A potential revision of this taxon would have significant implications for its conservation status and management—in particular, for fragmented populations in the east region where genetic diversity is lower.
References
Backhouse GN, Vanner RW (1978) Observations on the biology of the dwarf galaxiid, Galaxiella pusilla (Mack) (Pisces: Galaxiidae). Vic Nat 95:128–132
Balloux F, Lugon-Moulin N (2002) The estimation of population differentiation with microsatellite markers. Mol Ecol 11:155–165
Beck RG (1985) Field observation upon the dwarf galaxiid Galaxiella pusilla (Mack) (Pisces: Galaxiidae) in the South-east of South Australia, Australia. South Aust Nat 60:12–22
Belkhir K, Borsa P, Chikhi L, Raufaste N, Bonhomme F (2004) GENETIX 4.03, logiciel sous WindowsTM pour la génétique des populations. Laboratoire Génome, Populations, Interactions. Université de Montpellier II, Montpellier, France
Birch WD (ed) (2003) Geology of Victoria. Geological Society of Australia Special Publication 23. Geological Society of Australia (Victorian Division)
Brown WM, Prager EM, Wang A, Wilson AC (1982) Mitochondrial DNA sequences of primates: tempo and mode of evolution. J Mol Evol 18:225–239
Burridge CP, Craw D, Jack DC, King TM, Waters JM (2008) Does fish ecology predict dispersal across a river drainage divide? Evolution 62:1484–1499
Chilcott SJ, Humphries P (1996) Freshwater fish of northeast Tasmania with notes on the dwarf galaxias. Rec Queen Vic Mus Art Gallery 103:145–149
Chistiakov DA, Hellemans B, Volckaert FAM (2006) Microsatellites and their genomic distribution, evolution, function and applications: a review with special reference to fish genetics. Aquaculture 255:1–29
Crandall KA, Bininda-Emonds ORP, Mace GM, Wayne RK (2000) Considering evolutionary processes in conservation biology. Trends Ecol Evol 15:290–295
Currey DT (1964) The former extent of Lake Corangamite. Proc R Soc Vic 77:377–386
Danancher D, Izquierdo JI, Garcia-Vazquez E (2008) Microsatellite analysis of relatedness structure in young of the year of the endangered Zingel asper (Percidae) and implications for conservation. Freshw Biol 53:546–557
DeWoody JA, Avise JC (2000) Microsatellite variation in marine, freshwater and anadromous fishes compared with other animals. J Fish Biol 56:461–473
Douglas JG, Ferguson JA (eds) (1976) Geology of Victoria. Geological Society of Australia Special Publication No. 5. Geological Society of Australia (Victorian Division)
Dupanloup I, Schneider S, Excoffier L (2002) A simulated annealing approach to define the genetic structure of populations. Mol Ecol 11:2571–2581
Excoffier L, Smouse PE, Quattro JM (1992) Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics 131:479–491
Excoffier L, Laval G, Schneider S (2006) Arlequin ver 3.1: An integrated software package for population genetics data analysis. Computational and Molecular Population Genetics Laboratory. University of Berne, Switzerland
Fu YX (1997) Statistical tests of neutrality of mutations against population growth, hitchhiking and background selection. Genetics 147:915–925
Fu YX, Li WH (1993) Statistical tests of neutrality of mutations. Genetics 133:693–709
Gleeson DM, Howitt RLJ, Ling N (1999) Genetic variation, population structure and cryptic species within black mudfish, Neochanna diversus, an endemic galaxiid from New Zealand. Mol Ecol 8:47–57
Goudet J (2001) FSTAT, a program to estimate and test gene diversities and fixation indices (version 2.9.3). Available at http://www.unil.ch/izea/softwares/fstat.html
Guillot G, Mortier F, Estoup A (2005) GENELAND: a computer package for landscape genetics. Mol Ecol Notes 5:712–715
Guillot G, Santos F, Estoup A (2008) Analysing georeferenced population genetics data with Geneland: a new algorithm to deal with null alleles and a friendly graphical user interface. Bioinform 24:1406–1407
Guillot G, Santos F, Estoup A (2009) Population genetics analysis using R and Geneland. May 19
Hammer M (2001) Molecular systematics and conservation biology of the southern pygmy perch Nannoperca australis (Günther, 1861) (Teleostei: Percichthyidae) in south-eastern Australia. B.Sc. (Honours) thesis, Adelaide University
Hammer MP, Adams M, Unmack P, Walker KF (2007) A rethink on Retropinna: conservation implications of new taxa and significant genetic sub-structure in Australian smelts (Pisces: Retropinnidae). Mar Freshw Res 58:327–341
Harpending HC, Sherry ST, Rogers AR, Stoneking M (1993) The genetic structure of ancient human populations. Curr Anthropol 34:483–496
Harris P, Heap A, Passlow V, Sbaffi L, Fellows M, Porter-Smith R, Buchanan C, Daniell J (2005) Geomorphic features of the continental margin of Australia. Geoscience Australia, Record 2003/30
Hey J, Waples RS, Arnold ML, Butlin RK, Harrison RG (2003) Understanding and confronting species uncertainty in biology and conservation. Trends Ecol Evol 18:597–603
Hillis DM, Mable BK, Moritz C (1996) Applications of molecular systematics. In: Hillis DM, Moritz C, Mable BK (eds) Molecular systematics. Sinauer Associates, Sunderland, MA, pp 515–543
Hills ES (1975) Physiography of Victoria: an introduction to geomorphology, 5th edn. Whitcombe and Tombs, Australia
Hood G (2002) POPTOOLS. CSIRO, Canberra
Horwitz P (1988) Sea-level fluctuations and the distributions of some freshwater crayfishes of the genus Engaeus (Decapoda; Parastacidae) in the Bass Strait area. Aust J Mar Freshw Res 39:497–502
Huelsenbeck JP, Ronquist F (2001) MRBAYES: Bayesian inference of phylogeny. Bioinform 17:754–755
Jennings JN (1958) The submarine topology of Bass Strait. Proc R Soc Vic 71:49–72
Knaepkens G, Bervoets L, Verheyen E, Eens M (2004) Relationship between population size and genetic diversity in endangered populations of the European bullhead (Cottus gobio): implications for conservation. Biol Conserv 115:403–410
Koster W (2003) Threatened species of the world: Galaxiella pusilla (Mack 1936) (Galaxiidae). Environ Biol Fish 68:268
Lambeck K, Chappell J (2001) Sea level changes through the last glacial cycle. Science 292:679–686
Lewis PO, Zaykin D (2001) Genetic Data Analysis: a computer program for the analysis of allelic data. http://lewis.eeb.uconn.edu/lewishome/software.html
Mantel N (1967) The detection of disease clustering and generalized regression approach. Cancer Res 27:209–220
McDowall RM (1978) Sexual dimorphism in an Australian galaxiid. Aust Zool 19:309–314
McDowall RM, Frankenberg RS (1981) The galaxiid fishes of Australia. Rec Aust Mus 33:443–605
McDowall RM, Waters JM (2004) Phylogenetic relationships in a small group of diminutive galaxiid fishes and the evolution of sexual dimorphism. J R Soc N Z 34:23–57
Moran P (2002) Current conservation genetics: building an ecological approach to the synthesis of molecular and quantitative genetic methods. Ecol Freshw Fish 11:30–55
Moritz C (1994) Defining ‘Evolutionary Significant Units’ for conservation. Trends Ecol Evol 9:373–375
Ollier CD, Joyce EB (1964) Volcanic physiography of the western plains of Victoria. Proc R Soc Vic 77:357–376
Park SDE (2001) Trypanotolerance in West African cattle and the population genetic effects of selection. Ph.D. thesis. University of Dublin, Dublin, Ireland
Peakall R, Smouse PE (2006) GENALEX 6: genetic analysis in Excel. Population genetics software for teaching and research. Mol Ecol Notes 6:288–295
Posada D, Crandall KA (1998) Modeltest: testing the model of DNA substitution. Bioinform 14:817–818
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
RO CSI (2007) Climate change in Australia. Technical report 2007 by the Commonwealth Scientific and Industrial Research Organisation (CSIRO) and the Australian Bureau of Meteorology. CSIRO Atmospheric Research, Melbourne, Australia
Rogers AR, Harpending H (1992) Population-growth makes waves in the distribution of pairwise genetic-differences. Mol Biol Evol 9:552–569
Rogers AR, Fraley AE, Bamshad MJ, Watkins WS, Jorde LB (1996) Mitochondrial mismatch analysis is insensitive to the mutational process. Mol Biol Evol 13:895–902
Ronquist F, Huelsenbeck JP (2003) MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinform 19:1572–1574
Rousset F (1997) Genetic differentiation and estimation of gene flow from F-statistics under isolation by distance. Genetics 145:1219–1228
Rozas J, Sanchez-De I, Barrio JC, Messeguer X, Rozas R (2003) DnaSP, DNA polymorphism analyses by the coalescent and other methods. Bioinform 19:2496–2497
Rozen S, Skaletsky HJ (2000) Primer3 on the WWW for general users and for biologist programmers. In: Krawetz S, Misener S (eds) Bioinformatics methods and protocols: methods in molecular biology. Humana Press, Totowa, NJ, pp 365–386
Rugman-Jones PF, Weeks AR, Hoddle MS, Stouthamer R (2005) Isolation and characterization of microsatellite loci in the avocado thrips Scitothrips perseae (Tysanoptera: Thripidae). Mol Ecol Notes 5:644–646
Ryder OA (1986) Species conservation and systematics: the dilemma of subspecies. Trends Ecol Evol 1:9–10
Salgueiroa P, Carvalho G, Collares-Pereiraa MJ, Coelhoa MM (2003) Microsatellite analysis of genetic population structure of the endangered cyprinid Anaecypris hispanica in Portugal: implications for conservation. Biol Conserv 109:47–56
Schultz MB, Ierodiaconou DA, Smith SA, Horwitz P, Richardson AMM, Crandall KA, Austin CM (2008) Sea-level changes and paleo-ranges: reconstruction of ancient shorelines and river drainages and the phylogeography of the Australian land crayfish Engaeus sericatus Clark (Decapoda: Parastacidae). Mol Ecol 17:5291–5314
Sinnott RW (1984) Virtues of the Haversine. Sky Teles 68:159
Slatkin M (1995) A measure of population subdivision based on microsatellite allele frequencies. Genetics 139:457–462
Sokal RR, Rohlf FJ (1995) Biometry: the principles and practice of statistics in biological research. W.H. Freeman, New York
Swofford DL (1998) PAUP*: Phylogenetic Analysis Using Parsimony (* and Other Methods). Version 4. Sinauer Associates, Sunderland, MA
Tajima F (1989) Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 123:585–595
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Thacker CE, Unmack PJ, Matsui L, Rifenbark N (2007) Comparative phylogeography of five sympatric Hypseleotris species (Teleostei: Eleotridae) in south-eastern Australia reveals a complex pattern of drainage basin exchanges with little congruence across species. J Biogeogr 34:1518–1533
Unmack PJ (2001) Biogeography of Australian freshwater fishes. J Biogeogr 28:1053–1089
Van Oosterhout C, Hutchinson WF, Wills DPM, Shipley P (2004) MICRO-CHECKER: software for identifying and correcting genotyping errors in microsatellite data. Mol Ecol Notes 4:535–538
Vrijenhoek RC (1998) Conservation genetics of freshwater fish. J Fish Biol 53(Supplement A):394–412
Wager R (1996) Galaxiella pusilla. In: IUCN 2008. 2008 IUCN Red List of Threatened Species. www.iucnredlist.org. Downloaded on 26 January 2009
Walsh PS, Metzgar DA, Higuschi R (1991) Chelex 100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. Biotechniques 10:506–513
Wan QH, Wu H, Fujihara T, Fang SG (2004) Which genetic marker for which conservation genetics issue? Electrophoresis 25:2165–2176
Ward RD, Tyler SZ, Innes BH, Last PR, Herbert PDN (2005) DNA barcoding Australia’s fish species. Philos Trans R Soc Biol Sci 1462:1847–1857
Waters JM, Wallis GP (2001) Cladogenesis and loss of the marine life-history phase in freshwater Galaxiid fishes (Osmeriformes: Galaxiidae). Evolution 55:587–597
Webb SA, Jefferson AG, Macias-Garcia C, Magurran AE, Foighil D, Ritchie MG (2004) Molecular phylogeny of the livebearing Goodeidae (Cyprinodontiformes). Mol Phylogenet Evol 30:527–544
Weir BS, Cockerham CC (1984) Estimating F-statistics for the analysis of population structure. Evolution 38:1358–1370
Wong BBM, Keogh JS, McGlashan DJ (2004) Current and historical patters of drainage connectivity in eastern Australia inferred from population genetic structuring in a widespread freshwater fish Pseudomugil signifier (Pseudomugilidae). Mol Ecol 13:391–401
Wright S (1943) Isolation by distance. Genet 28:114–138
Zane L, Bargelloni L, Patarnello T (2002) Strategies for microsatellite isolation: a review. Mol Ecol 11:1–16
Zhu D, Degnan S, Moritz C (1998) Evolutionary distinctiveness and status of the endangered Lake Eacham rainbowfish (Melanotaenia eachamensis). Conserv Biol 12:80–93
Acknowledgements
We thank Melbourne Water Corporation for funding this research. Thanks also to members of CESAR for ongoing assistance, particularly Nancy Endersby, Andrew Weeks, Paul Mitrovski, Paul Umina and Rob Good for advice on laboratory methods, and Andrew Weeks, Mark Blacket and Elise Furlan for advice on data analysis. Thanks to Edward Tsyrlin and to Megan, James and Harry Coleman for assistance in the field and to Peter Unmack and Michael Hammer for correspondence regarding the progress and outcomes of their dwarf galaxias study. Adam Mattinson is thanked for his assistance with the map and finally, thanks to John McGuckin and Ron Lewis for ongoing practical discussions regarding survey techniques and potential survey locations. Constructive comments on a draft of this manuscript from three reviewers (including Chris Burridge) are also greatly appreciated. Research was carried out with Melbourne University animal ethics approval (AEC 0705391) and relevant State research permits for threatened species, national parks and fisheries permits for South Australia (PIRSA 9902114), Victoria (DPI RP916, DSE 10004233) and Tasmania (IFS 2008-7, TFA 08090).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Coleman, R.A., Pettigrove, V., Raadik, T.A. et al. Microsatellite markers and mtDNA data indicate two distinct groups in dwarf galaxias, Galaxiella pusilla (Mack) (Pisces: Galaxiidae), a threatened freshwater fish from south-eastern Australia. Conserv Genet 11, 1911–1928 (2010). https://doi.org/10.1007/s10592-010-0082-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10592-010-0082-z