Abstract
Bone is one of the most common sites for metastasis in breast cancer (BC). Micro-metastasis in bone marrow was detected in 30 % of patients with stage I, II, or III BC at primary surgery and is a strong indicator of poor prognosis. The role dietary soy isoflavones play in BC with bone micro-metastasis is unclear. In this study, we examined the effects of genistein, daidzein, (−)-equol or a mixture of soy isoflavones on BC with bone micro-metastasis using an experimental model of murine mammary cancer 4T1 cells engineered with luciferase. A small number (1000) of 4T1 cells were injected into the tibia of female Balb/c mice to establish micro-tumors in bone. Soy isoflavones were supplemented in the AIN-93G diet at 750 mg/kg and were provided to mice from 3 weeks before to 3 weeks after cell injection. Bioluminescent imaging was conducted on day 2 (D2), D6, D8, D16 and D20 post cell injection and the results indicated dietary soy isoflavones enhanced the growth of bone micro-tumors on D8. Furthermore, dietary soy isoflavones stimulated metastatic tumor formation in lungs and increased Ki-67 protein expression in these metastasized tumors. In vitro, soy isoflavones (<10 µM) had limited effects on the growth, motility or invasion of 4T1 cells. Thus, the in vivo stimulatory effect could be likely due to systemic effects between the host, 4T1 tumors and soy isoflavones. In conclusion, soy isoflavones stimulate BC with bone micro-metastasis in mice and further investigations are needed regarding their consumption by BC survivors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer metastasis is a complex multistep process wherein cancer cells first proliferate at a primary site, leave the site by invading the basement membrane and the stroma, and then pass through the circulatory and/or lymphatic systems, finally establish a secondary tumor at a distant organ site [1]. Breast cancer (BC) is one of the most common cancers affecting women in the United States with up to 30 % BC survivors relapsing within 5 years with metastatic tumors [2, 3].
Metastatic BC is the leading cause of death in BC patients [4, 5] and bone is one of the most common sites for metastasis in BC. After death from BC, approximately 70 % of these individuals had metastatic tumors in bone [6]. Recent studies have shown that micro-metastasis in bone marrow was detected in 30 % of patients with stage I, II, or III BC at primary surgery and the presence of micro-metastasis in the bone marrow is associated with a poor prognosis [7]. This is very important because it indicates that micro-metastasis in bone marrow exists before stage IV metastatic breast cancer when metastases can be detected by routine techniques. Therefore, BC survivors with presence of micro-metastasis in bone marrow can live for years, but have a poor survival when compared to patients without bone marrow micro-metastasis. To mimic this clinical setting, we established, in the present study, an experimental model by injecting a small number (1000) of murine mammary cancer 4T1 cells into the tibial marrow of mice to produce a lesion representative of an early micro-metastasis tumor in bone.
Since BC survivors with bone micro-metastasis can live for years, diet could play an important role in BC progression. Additionally, dietary supplements, including soy isoflavones, could impact progression of BC metastasis. To date, investigations of the association between soy isoflavones and BC are mainly focused on formation and growth of primary tumors, not on BC metastasis progression. For example, epidemiologic studies have shown that, in general, consumption of soy foods in Asian countries is associated with a reduction of BC [8, 9], and preclinical studies confirm that early life exposure to soy is preventative for BC [10]. On the other hand, we have demonstrated that feeding mice with diets high in isoflavones to produce blood levels of isoflavones similar to those observed in women consuming soy products, can stimulate pre-existing estrogen (E2)-dependent breast tumor growth in mice [11], while, clinically, approximately 75 % of BC survivors are using alternative disease-modulating treatments, including soy isoflavones, in most cases, without their oncologists’ knowledge [12–14]. Therefore, it is important to evaluate the role of dietary supplements on BC metastasis in an experimental model.
Genistein and daidzein are the major isoflavones in soy, mostly as glycosides with a small percentage of aglycones [15], and daidzein is metabolized to (−)-equol by intestinal microflora [16, 17]. Currently, studies have shown that soy isoflavones could inhibit BC metastasis potential in vitro at concentrations higher than 10 μM. For example, genistein, daidzein and equol (50 μM) inhibited human BC MDA-MB-231 cell invasion [18]. This inhibition could possibly be related to down-regulation of MMP-9 and up-regulation of TIMP1 [19]. In preclinical models, soy isoflavones exhibit differential effects on BC metastasis. For example, genistein inhibited osteolytic bone metastasis followed by intracardiac injection of MDA-MB-231 cells [20]. Daidzein increased lung and heart metastasis, while genistein decreased bone and liver metastasis following subcutaneous injection of MDA-MB-435 cells [21]. Therefore, whether dietary soy isoflavones have beneficial or adverse effects on BC metastasis is still uncertain, and whether they have impact, especially, on BC patients with bone micro-metastasis is unknown and remains as a critical question. In the present study, we evaluated the effect of these three soy isoflavones on BC with bone micro-metastasis. We injected a small number (1000) of murine 4T1 mammary cancer cells into the tibial marrow of mouse to mimic BC with bone micro-metastasis. With this model, we were able to evaluate the effect of dietary genistein, daidzein, (−)-equol and a mixture of soy isoflavones on BC with bone micro-metastasis.
Materials and methods
Material
Dulbecco’s Modified Eagle’s Medium (DMEM) was obtained from the Cell Media Facility, University of Illinois at Urbana-Champaign (Urbana, IL). Heat-Inactivated Fetal Bovine Serum (HI-FBS) was purchased from Atlanta Biological (Lawrenceville, GA). MatrigelTX was purchased from BD Biosciences (San Jose, CA). d-luciferin potassium was purchased from Regis Technologies (Morton Grove, IL). Mixed isoflavones were a mixture of soy isoflavones containing mainly genistein (aglycone + conjugated) and daidzein (aglycone + conjugated), and were purchased from the United States Department of Agriculture (Washington, DC). The composition of the mixed isoflavones was listed in Supplemental Table 1. Genistein (98 % by HPLC) and daidzein (98 % by HPLC) were purchased from Allway Chem-Pharm International (Xi’an, China). (−)-Equol (98 % by chiral HPLC) was purchased from Obiter Research (Champaign, IL).
Cell culture
ER-negative murine 4T1 cells engineered with firefly luciferase were originally provided by Dr. David Piwnica-Worms from Washington University (St. Louis, MO), and have been grown and maintained in DMEM supplemented with 10 % HI-FBS, 100 unit/mL penicillin and 100 μg/mL streptomycin (culture media) at 37 °C in 5 % CO2 humidified air. Cells were harvested at 70 % confluence, counted and suspended in Matrigel™ for inoculation.
Proliferation, wound healing and invasion (see supplemental materials)
RNA isolation
ER-negative murine 4T1 cells (2 × 105/well) were seeded into 6-well plates in culture media and cultured for 24 h. Media were then changed to 0.1 % HI-FBS and incubated overnight. Cells were treated with genistein, daidzein or (−)-equol in 0.1 % HI-FBS media at 0.1, 1 or 10 µM respectively for 24 h (n = 3). Experiments were repeated for 3 times. Total RNA was collected using TRI Reagent (Sigma-Aldrich) and isolated according to the manufacturer’s instructions as described previously [22]. RNA concentrations were measured using SmartSpec Plus Spectrophotometer (BioRad, Hercules, CA). cDNA was synthesized from RNA using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) in a Thermal Cycler 2720 (Applied Biosystems). In each reaction system, 2 µg of total RNA were used in a 20 µL mixture containing 1× RT buffer, 1× random primers, 4 mM dNTPs and 2.5 U/µL multiScribe Reverse Transcriptase. cDNA was synthesized following the program: 25 °C for 10 min, 37 °C for 2 h, and 85 °C for 5 s.
Quantitative PCR analysis
Synthesized cDNA for measuring gene expression was analyzed by quantitative PCR in a 7300 thermal cycler (Applied Biosystems). In each reaction, 25 ng of synthesized cDNA was used in a 20 µL volume containing 10 µL of SYBR Green master mix (2×, Applied Biosystems) and 0.25 µM of each primer. PCR was performed using the following program: 95 °C for 10 min, followed by 35 cycles of 95 °C for 15 s and 60 °C for 1 min. After PCR, melting curves were acquired stepwise from 55 to 95 °C to ensure that a single product was amplified in the reaction. The primers used in this study are listed in Supplemental Table 2.
Animals
Female BALB/c mice were purchased from Charles River Laboratories (Wilmington, MA) at 4 weeks of age. Animals were single-caged in a controlled environment and had free access to food and water. Artificial light was provided in a 12-hour light/dark cycle. Experimental procedures were conducted under protocols approved by the Institutional Animal Care and Uses Committee (IACUC) at the University of Illinois at Urbana-Champaign (UIUC).
Study design
Upon arrival, mice were switched to the AIN-93G pellet diet in 1 week, and then aged to 4 months old. Mice were then randomly assigned into the following groups with 10–11 mice in each group: (1) the control group, (2) the genistein group, (3) the daidzein group, (4) the (−)-equol group and (5) the mixed isoflavone group. As Supplemental Table 3 shows, mice in the control group received the AIN-93G powder diet at 5 g/day/mouse. Soy isoflavones were mixed with the AIN-93G powder diet at 750 mg/kg and the mixed diets were provided to each mouse in their respective group at 5 g/day. The dose in the mixed isoflavone group was normalized to 750 mg/kg genistein equivalent with 318 mg/kg daidzein equivalent. The AIN-93G semi-purified diet (Research Diets, Brunswick, NJ) was selected as the base diet as it has been established as meeting the nutritional requirements of mice [23]. The 5 g of diets were provided to mice in respective groups at the same time on each day. Body weights were measured weekly after mice were on diets supplemented with soy isoflavones. Mice were then injected with 4T1 cells after 3 weeks on AIN-93G diets supplemented with soy isoflavones. Bioluminescence imaging was conducted to monitor tumor progression for 3 weeks post cell injection. Mice were then sacrificed and internal organs were collected. The study design was summarized in Supplemental Fig. 1.
Intra-tibial cell inoculation
During the surgery, each mouse was placed face-up and the patellar tendon of the right tibia was exposed with a small incision. A 26-gauge needle was inserted into the joint surface of the tibia through the patellar tendon into the bone marrow cavity to create a pilot hole. A 25 μL Hamilton microsyringe with a 27-gauge needle was used to deliver 1000 4T1 cells suspended in 2.5 μL Matrigel™ through the pilot hole. The incision site was closed with a surgical staple. General anesthesia was maintained with isoflurane/oxygen gas throughout the surgery.
Bioluminescence imaging (BLI)
BLI was performed using a custom-made imaging system (Stanford Photonics, Palo Alto, CA) with a dual micro-channel plate ICCD camera. Potassium d-luciferin (0.15 g/kg) was freshly dissolved in sterile PBS and injected intraperitoneally to each mouse 3 min prior to imaging. d-luciferin reacted with live, luciferase-labeled 4T1 cells and this reaction emitted bioluminescence, which was then captured by the BLI unit. Photon emission was allowed to accumulate for 3 min under Movie mode using the software Piper Control (Stanford Photonics, Palo Alto, CA). Bioluminescence was visualized as pseudo-color and post-processed using ImageJ (NIH, Bethesda, MD) and Photoshop Elements (Adobe, San Jose, CA). General anesthesia was maintained throughout the imaging process.
Quantification of serum isoflavones
At necropsy, blood was drawn from the vena cava of each mouse and serum was separated and kept at −20 °C until analysis. Serum levels of total isoflavones (aglycones and conjugated forms) were determined after complete enzymatic hydrolysis with a H. pomatia preparation containing glucuronidase, sulfatase, and glucosidase using a previously validated LC-ES/MS/MS method based on isotope dilution quantification of genistein, daidzein, and equol [24]. Method detection limits (s/n ratio of 3) for genistein, daidzein, and equol were approximately 0.005 μM for aliquots of 10 µl. Intra- and inter-day precisions were in the range of 3–13 % relative standard deviation, and accuracy was in the range of 88–99 %. Quality control analyses were also performed for every sample set, including the analysis of blank and spiked serum samples (glucuronidase/sulfatase), blank injections, and injections of authentic standards.
India ink staining
At necropsy, an incision was made on each mouse from the rib cage to the jaw to expose trachea and lungs. India ink solution (85 %) was slowly infused into the lungs through the trachea. The infused lungs were kept in Fekete’s solution (90 % of 70 % ethanol, 9 % of 37 % formaldehyde and 1 % of glacial acetic acid) for de-staining [25, 26]. When injected from trachea, India ink only infuses the respiratory tract, which results in normal lung tissues staining black while the tumor nodules remaining white. The white tumor nodules were counted and the results were analyzed.
H&E staining
After the white tumor nodules on the surface of the lungs were counted, lungs were embedded in paraffin, sectioned and stained with H&E. Slides stained with H&E were observed under an AxioSkop 40 microscope (Carl Zeiss, Thornwood, NY) and photographed. Metastasis was quantified by counting tumor colonies and measuring tumor area in the sectioned lung tissue as described previously [27, 28].
Ki-67 expression
Four mice were randomly selected from each group for evaluation of Ki-67 protein expression on the metastatic tumors on lungs. Sectioned lung tissue slides were first incubated with a primary Ki-67 antibody (1:3000) (Pharmingen, San Diego, CA) at 4 °C overnight, and then with a biotinylated anti-mouse secondary antibody (VECTASTAIN Elite ABS reagent, Vector Laboratories Inc. Burlingame, CA) for 30 min at room temperature. Positive cells were stained brown with 3,3′-diaminobenzidine tetrahydrachloride (DAB) and negative cells were stained blue with hematoxylin [29, 30]. Both positive and negative cells were counted in a given field under the AxioSkop 40 microscope. Results were presented as percentage of positive cells of a given field of tumor cells.
Statistical analysis
Data were analyzed using either SAS™ 9.2 (SAS Institute Inc., Cary, NC) or OriginPro® 8.5 (OriginLab Corporation, Northampton, MA). Tumor occurrence rate was analyzed using Fisher’s exact test. Continuous data (body weight, BLI and Ki-67) were analyzed using two-sample t test (OriginPro® 8.5). Count data (India ink and H&E staining) were analyzed using SAS PROC GENMOD. Comparison of data in different concentrations of the same treatment (cell growth, motility, gene expression) was conducted using one-way ANOVA with post hoc Tukey’s test (OriginPro® 8.5). P < 0.05 was considered indicative of significant differences between means of two experimental groups.
Results
Dietary soy isoflavones did not affect mice body weights in the duration of the study
Genistein, daidzein, or (−)-equol were supplemented in the AIN-93G powder diet at 750 mg/kg diet. The composition of mixed isoflavones was listed in Supplemental Table 1, and the dose of mixed isoflavones was normalized to 750 mg/kg genistein equivalent, with 318 mg/kg daidzein equivalent. Mice were fed on their respective diets starting from 3 weeks before cell injection for a total of 6 weeks. Body weights were recorded weekly (Supplemental Fig. 2a). Body weight changes were calculated for the duration of week 0 (cell injection) to week 3 (the end of the study). No significant differences in body weight change were observed when comparing dietary soy isoflavone groups to the control group, indicating that dietary soy isoflavones did not affect body weight in mice with tumor burdens (Supplemental Fig. 2b).
Monitoring of metastatic progression of 4T1 tumors in bone using bioluminescent imaging
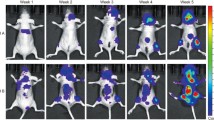
We were able to monitor the growth and progression of micro-tumors in bone in real time using BLI, because the murine 4T1 cells were engineered with luciferase. In the present study, no bioluminescence in bone was detected on day 2 (D2) after cell injection. Bioluminescence from tumors in the bone was detectable on D6. Signals in the control and the mixed isoflavone groups continued to increase until the end of the study, while signals in the genistein, daidzein and equol groups peaked on D16, and then reduced on D20 (Fig. 1a), likely due to tumor necrosis and subsequently reduced luciferin uptake by necrotic tumor cells. In addition to bioluminescence from the bone micro-tumors, bioluminescence in the lung area, produced by tumor cells metastasized from bone marrow, were observed on D8, which then continued to increase until the end of the study. Most animals with metastatic tumor burdens followed a similar time course, suggesting that 4T1 cells first formed a micro-tumor in the bone after intra-tibial injection and then subsequently metastasized to the lungs in mice.
Monitoring of metastasis progression from 4T1 micro-tumors in bone to lungs using bioluminescence imaging. a Integrated density of bioluminescence from 4T1 micro-tumors in bone from each experimental group over time. CTL control, n = 9; GEN genistein, n = 10; DDZ daidzein, n = 10; EQL (−)-equol, n = 10; MIX mixed isoflavone, n = 10. Mice without bioluminescent signals in the bone area at the end of the study were excluded. b Integrated density of bioluminescence from 4T1 micro-tumors in bone from each experimental group on day 8 after cell injection. Data are presented as mean ± SEM and analyzed using two-sample t-test. c Representative bioluminescent images of mice from each experimental group recorded on day 8. Bioluminescence was presented in pseudocolors
To examine the effects of dietary soy isoflavones on tumor growth in the bone, we measured the integrated density of bioluminescence from micro-tumors in the bone. In this circumstance, unsaturated bioluminescent signals from engineered 4T1 tumors are fairly quantifiable, because tumors are located in the same position in each mouse with defined depth in bone. The absence of detectable bioluminescence in the tibia at the end of the study was considered as a failed injection and then was excluded from analysis. Fig. 1a shows the strength of bioluminescent signals over time from 4T1 tumors in bone. On D8, genistein (P = 0.03) and (−)-equol (P = 0.04) significantly increased the strength of bioluminescent signals from 4T1 tumors in bone when compared to the control group. Daidzein (P = 0.06) treatment showed a trend to approach significance in bioluminescence strength, while mixed isoflavone did not show difference when compared to the control group (Fig. 1b). No significant difference, other than on D8, in bioluminescent signals from tumors in bone was observed between the control group and the soy isoflavone-supplemented groups. Fig. 1c shows representative bioluminescence images of mice recorded on D8.
Effects of dietary soy isoflavones on 4T1 tumor metastasis from bone to lung
Monitoring 4T1 tumor progression using BLI indicated that 4T1 tumors metastasized from bone marrow to lungs. However, the analysis of bioluminescence in the lung area provided a less accurate quantification, mainly because not all mice with 4T1 tumors in bone developed lung metastasis detectable by BLI in the present study. This resulted in large variation in integrated density measurement with 0 for undetectable bioluminescent signal or higher than 106 for intense bioluminescent signals. Therefore, we used gross examination and histological analysis of the collected lungs, instead of integrated density of bioluminescent signals, for accurate quantification of metastasis. To calculate the metastasis occurrence rate in each experimental group, we counted mice with 4T1 tumor nodules on lungs. The metastasis occurrence rate for each group was, the control group, 6/9 (the number of mice with lung metastasis/the number of mice for the group), the genistein group, 9/10, the daidzein group, 10/10, the (−)-equol group, 7/10, and the mixed isoflavone group, 10/10. No significant differences between experimental groups were observed, indicating that dietary soy isoflavones did not affect metastasis occurrence rates.
To examine the effects of soy isoflavones on metastatic tumor formation on the surface of lungs in mice, we counted the number of white tumor nodules formed by 4T1 cells on the lung surface. Fig. 2a shows that mice in the control group had an average of 2 tumor nodules on the lung surface, while mice in the dietary soy isoflavone-treated groups had a significant increase in tumor nodule count with an average of 6 tumor nodules on the lung surface (P < 0.05). Fig. 2b shows representative pictures of tumor-bearing lungs infused with India ink. To examine microscopic metastasis inside the lungs, sectioned lung tissues were stained with H&E. Tumor colonies in the sectioned lung tissue were counted and tumor area was measured. Fig. 3a shows that mice in the control group had an average of 2 tumor colonies per lung section, while mice in the dietary soy isoflavone-treated groups had a significant increase in tumor colony count with an average of 7 (genistein), 6 (daidzein), 9 [(−)-equol] or 11 (mixed isoflavones) tumor colonies (P < 0.05). Fig. 3b shows that tumor colonies in the mixed isoflavone and (−)-equol groups had a large average size when compared to the control group, while the genistein and daidzein groups did not. The two variables, tumor colony count and tumor area per lung section, showed a significant correlation (Pearson’s r = 0.86, P < 0.001), indicating similar patterns when used to represent lung metastasis inside lung tissues. Fig. 3c shows representative pictures of H&E stained lung sections from each experimental group. These results indicate that dietary soy isoflavones enhance the metastasis of 4T1 tumors from bone to lungs.
Dietary soy isoflavones increase the formation of metastatic tumor nodules on the lung surface. a Data are present as mean ± SEM and analyzed using SAS PROC GENMOD. Asterisk indicates significant difference when compared to the group (P < 0.05). b Representative images of lungs infused with India ink from each experimental group. Arrows indicate white 4T1 tumor nodules on the lung surface
Dietary soy isoflavones increase the formation of metastatic tumor colonies inside the lungs. a Tumor colony count and b Tumor area measurement on sectioned lung tissues. Data are presented as mean ± SEM and analyzed using SAS PROC GENMOD. (*P < 0.05) and (**P < 0.01) indicate significant difference when compared to the control group. c Representative images of sectioned lung tissues from each experimental group. Normal lung tissues remained black after being infused with India ink at necropsy. Tumors were stained blue/red with H&E. Yellow circles with a T inside indicate tumor colonies inside sectioned lung tissues. T tumors, Magnification ×50
To investigate the possible biological basis for the stimulatory effect of soy isoflavones on metastatic tumor formation on lungs, Ki-67 immunohistochemical staining was conducted on sectioned lung tissues. Fig. 4a shows that mice in the control group had 19.1 ± 2.8 % of tumor cells positive for Ki-67 expression, while mice in the dietary soy isoflavone-treated groups had a significant increase in tumor cells positive for Ki-67 expression with 28.6 ± 1.9 % in the genistein group, 35.9 ± 2.1 % in the daidzein group, 28.6 ± 1.8 % in the (−)-equol group or 34.3 ± 1.7 % in the mixed isoflavones group, indicating that dietary genistein, daidzein, (−)-equol and the mixed isoflavones increase proliferating cells in the metastatic tumors in lungs. Fig. 4b shows representative pictures of Ki-67 stained tumor cells in sectioned lungs from each experimental group.
Dietary soy isoflavones increase percentage of cells expressing Ki-67 in tumors metastasized to lungs. a Percentage of tumor cells positive for Ki-67 expression. Data are presented as mean ± SEM and analyzed using two-sample t-test. (*P < 0.05) and (**P < 0.01) indicate significant difference when compared to the control group. b Representative images of sectioned lung tissues from each experimental group. Normal lung tissues were remained black after being infused with India ink at necropsy. Tumor cells positive for Ki-67 were stained brown and negative tumor cells were counterstained with hematoxylin. Magnification ×400
Quantification of serum levels of soy isoflavones
To determine levels of total soy isoflavones in blood, serum samples were hydrolyzed by a H. pomatia preparation and analyzed using LC/MS/MS with a previously validated method [24]. Fig. 5 shows the blood levels of total soy isoflavones in experimental groups: 1.2 ± 0.2 μM genistein in the genistein group; 1.5 ± 0.3 μM daidzein and 5.0 ± 0.6 μM equol in the daidzein group; 5.6 ± 1.1 μM equol in the (−)-equol group. Mice in the mixed isoflavone group contained 1.2 ± 0.3 μM genistein, 0.9 ± 0.2 μM daidzein and 1.0 ± 0.2 μM equol. The presence of equol in the daidzein and the mixed isoflavone groups is due to intestinal metabolism of daidzein. The percentages of aglycones were not measured directly in this study, but our previous studies of genistein, daidzein, and equol in BALB/c mice have shown them to be approximately 5 % of the respective total isoflavone concentration [31].
Effects of soy isoflavones on cell growth, wound healing, invasion and gene expression of 4T1 cells in vitro
We evaluated the effects of genistein, daidzein, (−)-equol in 4T1 cells in vitro. Since the main components of mixture isoflavones are isoflavone glycosides, we did not evaluate this sample in cultured 4T1 cells. Genistein, daidzein and (−)-equol at the concentration 1 and 10 µM had no effect on 4T1 cell growth, while inhibitory effects occurred at 100 µM due to toxic effects (Supplemental Fig. 3a). Genistein, daidzein and (−)-equol at 1 µM stimulated the motility of 4T1 cells, while the stimulatory effects disappeared with 10 µM genistein or (−)-equol and the effects became inhibitory when the concentration increased to 100 µM (Supplemental Fig. 3b). Genistein, daidzein or (−)-equol at 1 µM did not show any effect on 4T1 cell invasion (Supplemental Fig. 3c). Therefore, genistein, daidzein and (−)-equol showed limited ability to alter 4T1 cell growth, motility or invasion at 1 µM, while strong inhibitory effects of these compounds on cultured 4T1 cells occurred at very high concentration (100 µM), which was very likely unachievable in vivo.
To further determine the mechanisms how soy isoflavones stimulated BC metastasis, we examined the effects of genistein, daidzein and (−)-equol on the expression of genes relative to cell cycle, cancer metastasis or growth factor pathways in cultured 4T1 cells using quantitative PCR. The expression of MMP-2 and two of E2-responsive genes (TFF1 (PS2), WISP2) was very low in both non-treated and treated 4T1 cells (data not shown). The expression of TIMP1, CAV1, MYO1b, CCND1 (cyclin D1), CXCL1, EGFR, VEGFa and VEGFb gene was unchanged with genistein, daidzein or (−)-equol at the tested concentration range (Supplemental Fig. 4). The expression of BCL2 gene was unchanged with 0.1 or 1 µM genistein, daidzein or (−)-equol, and then down-regulated when the concentration increased to 10 µM (Fig. 6). The expression of VEGFc was down-regulated with genistein at 10 µM and with daidzein at 1 or 10 µM, while (−)-equol did not affect its expression at the tested concentration range (Fig. 6). The expression of MMP9 was down-regulated with daidzein at 1 or 10 µM and with (−)-equol at 10 µM, while genistein did not regulate its expression at the tested concentration range (Fig. 6). The expression of TIMP2 gene was down-regulated by genistein and (−)-equol at 0.1 and 10 µM, while by daidzein at 1 and 10 µM (Fig. 6). Therefore, genistein, daidzein and (−)-equol showed limited ability to modulate gene expression in cultured 4T1 cells at 0.1 or 1 µM, while 10 µM of the tested isoflavones showed stronger down-regulation effect on gene expression.
Effects of soy isoflavones on gene expression of cultured 4T1 cells. Experiments were conducted in triplicates and repeated 3 times. Results were the average of the 3 independent experiments. Data are presented as mean ± SEM and analyzed using one-way ANOVA with post hoc Tukey’s test. Different letters indicate significant difference between the means of two groups of the same treatment set (P < 0.05)
Discussion
Bone is one of the most common sites for BC metastasis in women [32] and the presence of bone micro-metastasis in stage I, II and III BC patients at primary surgery is a strong indicator of poor prognosis [7]. In the present study, we successfully established an experimental model in mice to mimic BC with bone micro-metastasis by injecting 1000 murine 4T1 cells into the tibial bone marrow of mice. We observed micro-tumors formed in the tibial marrow, which then subsequently metastasized to the lungs. The presence of lung metastases makes this model more powerful in mimicking BC with bone micro-metastasis, because clinically the presence of bone micro-metastasis in BC patients at primary surgery is associated with higher systemic relapse and death [33] and shorter distant disease - free survival [34].
One limitation of this model relates to the rapid tumor growth in the bone. Three weeks after cell inoculation, micro-tumors in the bone became visible and this tumor burden caused mice unable to bear weight on the injected limb. However, this limitation can be overcome by using BLI to detect early changes when tumors are still in micro status, as we showed in the present study. Dietary soy isoflavones increased the growth of 4T1 micro-tumors in the bone and the subtle stimulatory effect occurred at early time resulted in more lung metastases. The disappearance of the stimulatory effect on micro-tumors in the bone later on was probably due to signal saturation, which resulted from rapid tumor growth and the limited space of bone marrow for tumor growth. However, based on the limited and mixed preclinical results, how soy isoflavones impact BC metastasis is still unclear. For example, a study has shown that dietary genistein reduced lung metastasis in a postsurgical orthotopic model of MDA-MB-435/HAL cells [35], while daidzein and a mixture of genistein, daidzein, glycitein increased visceral metastasis from MDA-MB-435 (GFP tagged) cells injected subcutaneously into mice [21]. The mixed conclusions from published reports are likely due to differences in cell lines, models, administration methods etc. utilized in studies. Therefore, caution must be taken when interpreting preclinical results.
To understand how soy isoflavones affect BC metastasis, we evaluated their effect on the growth, motility and invasion of cultured 4T1 cells. Cell motility is an important property of malignant cells, which they need to migrate from a primary tumor to secondary organs [36]. Invasion of the basement membrane is another critical step in the metastasis cascade, because the basement membrane is the largest barrier between malignant cells and the blood stream, and has to be traversed for malignant cells to enter circulation [1]. Interestingly, we observed genistein, daidzein and (−)-equol at concentrations exceeding those likely in the in vivo phase of the study had no effects on proliferation and invasion of 4T1 cells, while the stimulatory effect on 4T1 cell motility was minimal. At 100 μM, these 3 compounds showed significantly inhibitory effects on proliferation and motility of 4T1 cells. This inhibitory effect was similar to previous reports describing that soy isoflavones inhibited cell motility and invasion in cancer cells in vitro at high concentrations of 20–50 μM [18, 37]. However, these concentrations would be too high for typical dietary exposure to achieve. Blood levels of total isoflavones in mice in the present study are 1–5 μM, similar to the levels observed in humans consuming typical soy foods or supplements [38, 39]. Blood levels of isoflavone aglycones would be even lower, because aglycones comprise only 0.1–5 % of the total isoflavones present in serum [31]. Even though tissue levels of aglycones often exceed those in serum [40], their concentrations in mouse lung would be far lower than those inducing effects in vitro. Therefore, the in vitro results obtained in the presence of high concentration of aglycone isoflavones should be interpreted with caution relative to data from an in vivo model.
The results of gene expression were similar to the results of proliferation and motility tests. The regulation of tested gene expression by soy isoflavones at low concentrations was minimal. Therefore, the stimulatory effect of dietary isoflavones on BC metastasis in the 4T1 intratibial model could likely be due to systemic interactions between the host, the tumor cells, and the dietary isoflavones as opposed to direct effects on the tumor cells. It has been shown that a systemic effect of the steroid hormone, estradiol, plays a role in ER negative BC metastasis [41]. Recently, we have also demonstrated that estradiol stimulates ER-negative BC metastasis in a 4T1 tail vein injection model [42]. Since soy isoflavones are phytoestrogens, they may share similar pathways with estrogen via systemic responses to stimulate BC metastasis. Investigation in this direction is currently undergoing in our laboratory.
In summary, the present study examined the effect of dietary genistein, daidzein, (−)-equol or a mixture of soy isoflavones using an experimental model of BC bone micro-metastasis by injecting 1000 murine 4T1 cells engineered with luciferase into the tibial bone marrow of mice to mimic BC patients who have micro-metastasis existing in the bone marrow cavity. We demonstrated that dietary soy isoflavones enhanced 4T1 tumor growth in bone on D8 post cell inoculation, and more importantly, increased lung metastasis from the 4T1 tumors in bone. In addition, we showed that dietary soy isoflavones had limited ability on growth, motility or invasion of 4T1 cells in vitro. Therefore, the stimulatory in vivo effect could be likely due to systemic effects between the host system, 4T1 tumors and soy isoflavones. Due to differences between humans and rodents, the findings in the study need to be interpreted carefully for the consumption of dietary isoflavones by BC survivors.
Abbreviations
- BLI:
-
Bioluminescence imaging
- BC:
-
Breast cancer
- H&E:
-
Hematoxylin and eosin
- GEN:
-
Genistein
- DDZ:
-
Daidzein
- EQL:
-
Equol
- MIX:
-
Mixed isoflavones
- HI-FBS:
-
Heat-Inactivated Fetal Bovine Serum
References
Pavese JM, Farmer RL, Bergan RC (2010) Inhibition of cancer cell invasion and metastasis by genistein. Cancer Metastasis Rev 29(3):465–482
Rosen PR, Groshen S, Saigo PE, Kinne DW, Hellman S (1989) A long-term follow-up study of survival in stage I (T1N0M0) and stage II (T1N1M0) breast carcinoma. J Clin Oncol 7(3):355–366
Lee YT (1985) Patterns of metastasis and natural courses of breast carcinoma. Cancer Metastasis Rev 4(2):153–172
Hagemeister FB Jr, Buzdar AU, Luna MA, Blumenschein GR (1980) Causes of death in breast cancer: a clinicopathologic study. Cancer 46(1):162–167
Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ (2003) Cancer statistics. CA Cancer J Clin 53(1):5–26
Coleman RE (2006) Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res 12(20 Pt 2):6243s–6249s. doi:10.1158/1078-0432.CCR-06-0931
Braun S, Vogl FD, Naume B, Janni W, Osborne MP, Coombes RC, Schlimok G, Diel IJ, Gerber B, Gebauer G, Pierga JY, Marth C, Oruzio D, Wiedswang G, Solomayer EF, Kundt G, Strobl B, Fehm T, Wong GY, Bliss J, Vincent-Salomon A, Pantel K (2005) A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med 353(8):793–802. doi:10.1056/NEJMoa050434
Lee HP, Gourley L, Duffy SW, Esteve J, Lee J, Day NE (1991) Dietary effects on breast-cancer risk in Singapore. Lancet 337(8751):1197–1200
Wu AH, Yu MC, Tseng CC, Pike MC (2008) Epidemiology of soy exposures and breast cancer risk. Br J Cancer 98(1):9–14
Messina M, Hilakivi-Clarke L (2009) Early intake appears to be the key to the proposed protective effects of soy intake against breast cancer. Nutr Cancer 61(6):792–798. doi:10.1080/01635580903285015
Allred CD, Allred KF, Ju YH, Virant SM, Helferich WG (2001) Soy diets containing varying amounts of genistein stimulate growth of estrogen-dependent (MCF-7) tumors in a dose-dependent manner. Cancer Res 61(13):5045–5050
Lloyd KB, Hornsby LB (2009) Complementary and alternative medications for women’s health issues. Nutr Clin Pract 24(5):589–608
Tempfer CB, Froese G, Heinze G, Bentz EK, Hefler LA, Huber JC (2009) Side effects of phytoestrogens: a meta-analysis of randomized trials. Am J Med 122(10):939–946 e939
van Tonder E, Herselman MG, Visser J (2009) The prevalence of dietary-related complementary and alternative therapies and their perceived usefulness among cancer patients. J Hum Nutr Diet 22(6):528–535
Setchell KD (1998) Phytoestrogens: the biochemistry, physiology, and implications for human health of soy isoflavones. Am J Clin Nutr 68(6 Suppl):1333S–1346S
Yuan JP, Wang JH, Liu X (2007) Metabolism of dietary soy isoflavones to equol by human intestinal microflora–implications for health. Mol Nutr Food Res 51(7):765–781
Bowey E, Adlercreutz H, Rowland I (2003) Metabolism of isoflavones and lignans by the gut microflora: a study in germ-free and human flora associated rats. Food Chem Toxicol 41(5):631–636
Magee PJ, McGlynn H, Rowland IR (2004) Differential effects of isoflavones and lignans on invasiveness of MDA-MB-231 breast cancer cells in vitro. Cancer Lett 208(1):35–41
Shao ZM, Wu J, Shen ZZ, Barsky SH (1998) Genistein exerts multiple suppressive effects on human breast carcinoma cells. Cancer Res 58(21):4851–4857
Zhang Y, Zhu G, Gu S, Chen X, Hu H, Weng S (2010) Genistein inhibits osteolytic bone metastasis and enhances bone mineral in nude mice. Environ Toxicol Pharmacol 30(1):37–44. doi:10.1016/j.etap.2010.03.016
Martinez-Montemayor MM, Otero-Franqui E, Martinez J, De La Mota-Peynado A, Cubano LA, Dharmawardhane S (2010) Individual and combined soy isoflavones exert differential effects on metastatic cancer progression. Clin Exp Metastasis 27(7):465–480. doi:10.1007/s10585-010-9336-x
Zhang Y, Li Q, Chen H (2013) DNA methylation and histone modifications of Wnt genes by genistein during colon cancer development. Carcinogenesis 34(8):1756–1763. doi:10.1093/carcin/bgt129
Reeves PG, Nielsen FH, Fahey GC Jr (1993) AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 123(11):1939–1951
Twaddle NC, Churchwell MI, Doerge DR (2002) High-throughput quantification of soy isoflavones in human and rodent blood using liquid chromatography with electrospray mass spectrometry and tandem mass spectrometry detection. J Chromatogr B Analyt Technol Biomed Life Sci 777(1–2):139–145
Hong X, Liu Y, Hu G, Zhao D, Shen J, Shen F, Cao X, Wang Q (2009) EBAG9 inducing hyporesponsiveness of T cells promotes tumor growth and metastasis in 4T1 murine mammary carcinoma. Cancer Sci 100(5):961–969
Lewis JD, Shearer MH, Kennedy RC, Bright RK (2005) Surrogate tumor antigen vaccination induces tumor-specific immunity and the rejection of spontaneous metastases. Cancer Res 65(7):2938–2946
Hiraga T, Williams PJ, Ueda A, Tamura D, Yoneda T (2004) Zoledronic acid inhibits visceral metastases in the 4T1/luc mouse breast cancer model. Clin Cancer Res 10(13):4559–4567
Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verastegui E, Zlotnik A (2001) Involvement of chemokine receptors in breast cancer metastasis. Nature 410(6824):50–56
Ju YH, Fultz J, Allred KF, Doerge DR, Helferich WG (2006) Effects of dietary daidzein and its metabolite, equol, at physiological concentrations on the growth of estrogen-dependent human breast cancer (MCF-7) tumors implanted in ovariectomized athymic mice. Carcinogenesis 27(4):856–863
Jun JY, Griffith JW, Bruggeman R, Washington S, Demers LM, Verderame MF, Manni A (2008) Effects of polyamine depletion by alpha-difluoromethylornithine on in vitro and in vivo biological properties of 4T1 murine mammary cancer cells. Breast Cancer Res Treat 107(1):33–40
Allred CD, Twaddle NC, Allred KF, Goeppinger TS, Churchwell MI, Ju YH, Helferich WG, Doerge DR (2005) Soy processing affects metabolism and disposition of dietary isoflavones in ovariectomized BALB/c mice. J Agric Food Chem 53(22):8542–8550
Weiss L (1992) Comments on hematogenous metastatic patterns in humans as revealed by autopsy. Clin Exp Metastasis 10(3):191–199
Wiedswang G, Borgen E, Karesen R, Kvalheim G, Nesland JM, Qvist H, Schlichting E, Sauer T, Janbu J, Harbitz T, Naume B (2003) Detection of isolated tumor cells in bone marrow is an independent prognostic factor in breast cancer. J Clin Oncol 21(18):3469–3478. doi:10.1200/JCO.2003.02.009
Braun S, Pantel K, Muller P, Janni W, Hepp F, Kentenich CR, Gastroph S, Wischnik A, Dimpfl T, Kindermann G, Riethmuller G, Schlimok G (2000) Cytokeratin-positive cells in the bone marrow and survival of patients with stage I, II, or III breast cancer. N Engl J Med 342(8):525–533. doi:10.1056/NEJM200002243420801
Vantyghem SA, Wilson SM, Postenka CO, Al-Katib W, Tuck AB, Chambers AF (2005) Dietary genistein reduces metastasis in a postsurgical orthotopic breast cancer model. Cancer Res 65(8):3396–3403
Chen J, Thompson LU (2003) Lignans and tamoxifen, alone or in combination, reduce human breast cancer cell adhesion, invasion and migration in vitro. Breast Cancer Res Treat 80(2):163–170
Farina HG, Pomies M, Alonso DF, Gomez DE (2006) Antitumor and antiangiogenic activity of soy isoflavone genistein in mouse models of melanoma and breast cancer. Oncol Rep 16(4):885–891
Xu X, Wang HJ, Murphy PA, Cook L, Hendrich S (1994) Daidzein is a more bioavailable soymilk isoflavone than is genistein in adult women. J Nutr 124(6):825–832
Mathey J, Lamothe V, Coxam V, Potier M, Sauvant P, Pelissero CB (2006) Concentrations of isoflavones in plasma and urine of post-menopausal women chronically ingesting high quantities of soy isoflavones. J Pharm Biomed Anal 41(3):957–965
Chang HC, Churchwell MI, Delclos KB, Newbold RR, Doerge DR (2000) Mass spectrometric determination of Genistein tissue distribution in diet-exposed Sprague-Dawley rats. J Nutr 130(8):1963–1970
Banka CL, Lund CV, Nguyen MT, Pakchoian AJ, Mueller BM, Eliceiri BP (2006) Estrogen induces lung metastasis through a host compartment-specific response. Cancer Res 66(7):3667–3672
Yang X, Belosay A, Du M, Fan TM, Turner RT, Iwaniec UT, Helferich WG (2013) Estradiol increases ER-negative breast cancer metastasis in an experimental model. Clin Exp Metastasis 30(6):711–721. doi:10.1007/s10585-012-9559-0
Acknowledgments
This project was made possible by the National Cancer Institute Grant Number [CA77355] and [P50AT006268] to (WGH) from the National Center for Complementary and Alternative Medicines (NCCAM), the Office of Dietary Supplements (ODS) and the National Cancer Institute (NCI). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCCAM, ODS, NCI or the National Institutes of Health. In addition, the views expressed in this paper do not necessarily reflect those of the U.S. Food and Drug Administration.
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the study involving animals were in accordance with the ethical standards of the IACUC of the UIUC.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, X., Belosay, A., Hartman, J.A. et al. Dietary soy isoflavones increase metastasis to lungs in an experimental model of breast cancer with bone micro-tumors. Clin Exp Metastasis 32, 323–333 (2015). https://doi.org/10.1007/s10585-015-9709-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-015-9709-2