Abstract
Purpose
In previous studies, we demonstrated that green tea (Camellia sinensis, CS) water extract had potent anti-tumor and anti-metastasis effects in the 4T1 mouse breast cancer xenograft model, and the metronomic regimen (0.0125 mg/kg twice a week for 4 weeks) of zoledronic acid (ZOL) was also effective in decreasing tumor burden and metastasis when compared with the conventional regimen. This study aimed to investigate the combined use of CS water extract and metronomic ZOL against tumor metastasis and bone destruction in MDA-MB-231-TXSA human breast cancer.
Methods
Female nude mice were injected with MDA-MB-231-TXSA cells into the marrow space of tibia and were treated with CS water extract and/or metronomic ZOL for 4 weeks. Tumor growth and metastasis to lungs and livers were assessed by in vivo bioluminescence imaging. Abilities of migration and invasion of MDA-MB-231-TXSA cells were also evaluated in vitro.
Results
Our results demonstrated that combination of CS and ZOL had the most potent effects on tumor burden and metastasis to bone, lung and liver, while treatment with CS or ZOL alone significantly protected the bone from cancer-induced osteolysis. In vitro, the combined use of CS + ZOL significantly inhibited MDA-MB-231-TXSA cell migration and invasion. Mechanistic zymography studies showed that the enzyme activities of MMP-9 and MMP-2 were significantly suppressed by CS and CS + ZOL.
Conclusions
The combination of CS plus metronomic ZOL demonstrated potent anti-tumor, anti-metastasis and anti-osteolysis effects against breast cancer, suggesting the potential clinical application against breast cancer patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Zoledronic acid (ZOL), the potent third-generation nitrogen-containing bisphosphonate, is commonly used for the prevention and treatment of various bone diseases characterized by increased bone resorption, which was also effective in treatment of bone destruction caused by bone metastases (Labrinidis et al. 2010). Besides, ZOL exhibited direct and indirect anti-tumor effects in both in vitro and in vivo models (Labrinidis et al. 2010). ZOL dose-dependently inhibited proliferation of breast cancer, prostate cancer and osteosarcoma cells in vitro and induced apoptosis in these tumor cells (Evdokiou et al. 2003; Ottewell et al. 2009). ZOL also inhibited the proliferation of human endothelial cells and modulated endothelial cell adhesion and invasion (Wood et al. 2002). The combination of ZOL and meloxicam reduced bone loss and tumor growth in an orthotopic mouse model of bone-invasive oral squamous cell carcinoma (Martin et al. 2013). Zoledronic acid also produced combinatory anti-tumor effects with cisplatin on mesothelioma by increasing p53 expression levels (Okamoto et al. 2013). Recently, some reports showed that ZOL administrated at a metronomic way could potentiate its anti-tumor effects. Metronomic way means lower doses given more frequently on a prolonged schedule (Pasquier et al. 2011). Clinical studies showed that the metronomic use of ZOL appeared to be more effective in the reduction of biomarkers of VEGF and NTx in breast cancer patients as compared to conventional regimen (Zhao et al. 2010). The metronomic regimen of ZOL also exhibited greater anti-tumor effects in a breast cancer mouse model than the conventional regimen (Facchini et al. 2010). In addition, a recent study demonstrated that metronomic administration of ZOL and taxotere combination showed promising anti-tumor activity in castration-resistant prostate cancer patients (Facchini et al. 2010). In our previous studies, we have demonstrated that metronomic ZOL (0.0125 mg/kg i.p. injected twice a week for 4 weeks) was more effective than the conventional regimen (0.1 mg/kg i.p. injected once only) in reducing breast cancer tumor burden and decreasing lung and liver metastasis in both primary and metastatic breast cancer (Luo et al. 2013). However, few studies reported on the drug–herb interaction between ZOL and Chinese herbal extract on breast cancer.
Green tea (Camellia sinensis, CS), a kind of Chinese tea commonly consumed as a healthy beverage, was demonstrated to have various biological activities, including anti-oxidation, anti-obesity and anticancer (Forester and Lambert 2011; Chacko et al. 2010; Bettuzzi et al. 2006). Green tea extract may act as an antioxidant reagent by up-regulating phase II antioxidant enzymes indirectly (Forester and Lambert 2011). Epidemiological studies have shown that the ingestion of green tea and tea polyphenols leads to a reduction in body fat (Chacko et al. 2010). Besides, there are also a number of reports demonstrating the anti-tumor effects of CS and its polyphenolic components. CS extract or tea polyphenol induces apoptosis and results in significant inhibition of tumor growth in a variety of cancer types including prostate (Bettuzzi et al. 2006) and breast cancer (Gu et al. 2013). In addition, tea polyphenol EGCG from CS was used as adjuvant therapy in combination with other chemotherapies. For example, EGCG induced chemosensitization of cancer cells through additive or synergistic effects with anticancer drugs evidenced in a number of in vitro and in vivo studies. The effect of chemotherapeutic drugs including 5-fluorouracil (Qiao et al. 2011), doxorubicin (Liang et al. 2010), or tamoxifen (Farabegoli et al. 2011) is significantly increased when combined with EGCG, in a variety of cancer types. In our previous studies, we demonstrated that CS water extract inhibited 4T1 mouse breast cancer cell proliferation and induced apoptosis in 4T1 cells which was concomitant with activation of caspase-8, caspase-3 and PARP cleavage, while also inhibited 4T1 cell migration and invasion (Luo et al. 2014). In animal studies, CS water extract was effective in decreasing the tumor burden, lung and liver metastasis in mice-bearing 4T1 tumors, and inhibited breast cancer-induced bone destruction significantly (Luo et al. 2014). However, few reports investigated the combination between CS extract and conventional anticancer agents.
More recently, patients with cancer frequently use herbal medicine along with the conventional medical treatment, hoping to enhance the efficacy of drugs, ameliorate the unwanted side effects and obtain some additional protections. For example, patients with breast cancer treated with chemotherapy, in combination with Chinese medicinal mushroom, Coriolus versicolor (Yun Zhi) preparation, showed a significant survival advantage compared with the standard conventional anticancer treatment alone (Eliza et al. 2012). There is an increasing trend in cancer treatment and research adopting combination therapy approach for advanced cancers (Eliza et al. 2012; Lam et al. 2009). It was reported that over half (53.9 %) of cancer patients in Hong Kong took Chinese herbal medicines together with chemotherapeutic agents (Lam et al. 2009).
ZOL is a potent clinical drug in prevention and treatment of bone destruction as well as tumor metastasis to bone. However, some reports showed that long term use of ZOL could lead to osteonecrosis of jaw and femoral insufficiency fracture (Ryan et al. 2009; Sellmeyer 2010). Hence, the present study aimed to investigate the combined use of metronomic ZOL and Chinese herbal medicine CS against tumor growth, metastasis and bone destruction. We hypothesize that the herbal extract CS can cooperate synergistically with the metronomic regimen of ZOL in inhibiting the growth and metastasis of breast tumor. The metronomic dose of ZOL used in this study was 0.0125 mg/kg, intraperitoneally injected 8 times in 4 weeks, and the total amount was the same as the single conventional treatment of 0.1 mg/kg, but the metronomic way decreased the dose and increased the frequency. In this study, we assessed the anti-tumor, anti-metastasis and anti-osteolysis effects of the combination of CS and metronomic ZOL in the intratibial breast cancer-induced osteolysis model. Furthermore, the anti-migration and anti-invasion abilities of the combined use of CS and ZOL on MDA-MB-231-TXSA cells were also assessed in vitro. Gelatin zymography analysis was employed to determine the underlying mechanism of anti-metastasis activities of CS + ZOL.
Materials and methods
Aqueous extract preparation and its chemical analysis
The dried leaves of C. sinensis (CS) were purchased from the herbal supplier of Hong Kong, and the sources of origin were from Hainan province in China, and a voucher specimen was kept in the museum of the Institute of Chinese Medicine. The voucher specimen was numbered as 2011–3336. One kilogram dried leaves of CS was soaked in boiled water for 15 min each for two times. Following the filtration, the water extracts were combined and evaporated under reduced pressure at 60 °C to dryness.
The chemical composition of CS extract was analyzed by HPLC (Agilent, USA) as described (Peng et al. 2008). Two-gradient elution system including mobile phase A (85 % ortho-phosphoric acid and water (0.05:99.95, v:v)) and mobile phase B (acetonitrile) was introduced. The gradient was running as: 0–4 min, 2 % B; 4–21 min, linear gradient from 2 to 9 % B; 21–32 min, linear gradient from 9 to 23 % B; and 32–45 min, 23 % B. The concentration of catechins in CS extract was determined by HPLC.
Cells and reagents
The bioluminescent human breast cancer cell line MDA-MB-231-TXSA was kindly provided by Prof. Andreas Evdokiou (Basil Hetzel Institute, The Queen Elizabeth Hospital, Adelaide, Australia) and was cultured in RPMI-1640 medium containing 10 % (v/v) fetal bovine serum (FBS) and 1 % (v/v) penicillin–streptomycin (Life Technologies, USA) at 37 °C in 5 % CO2 humidified incubator.
Zoledronic acid (ZOL) was purchased from Novartis Pharma Stein, Switzerland. Transwell plates for transwell migration assay were purchased from Corning Incorporated, USA. Creatine kinase (CK), alanine transaminase (ALT), aspartate transaminase (AST) and alkaline phosphatase (ALP) kits were purchased from Stanbio, USA. Gelatin and 3-(4,5-dimethylthiazol-z-yl)-2,5-diphenyl tetrazolium bromide (MTT) were obtained from Sigma, USA. D-luciferin was purchased from Biosynth, Switzerland.
Intratibial breast cancer-induced osteolysis model
Female BALB/c nude mice (4 weeks of age) were provided by Laboratory Animal Services Center, The Chinese University of Hong Kong, and were housed under pathogen-free conditions. The experiments were approved by the Animal Experimentation Ethics Committee of The Chinese University of Hong Kong. After the mice were anaesthetized, MDA-MB-231-TXSA cells (1 × 106) resuspended in 10 μl PBS, were injected into the marrow space of the proximal tibia with a 27-gauge needle coupled to a Hamilton syringe. After cancer cell implantation, mice were divided randomly into four groups (n = 10): untreated control group (orally fed with distilled water daily), CS group (0.6 g/kg CS extract, orally fed daily), ZOL group (0.0125 mg/kg ZOL, i.p. injected twice a week), CS + ZOL group (0.6 g/kg CS, orally fed daily + 0.0125 mg/kg ZOL, i.p. injected twice a week). Naive mice without tumor and treatment were set as normal standard. During treatment, body weight and bioluminescence measurement were performed once a week. After 4-week treatment, mice were killed, lungs and livers were removed for bioluminescence imaging and quantification of tumor burden. Both the tibias of each animal were removed for X-ray and μ-CT analysis as previously described (Luo et al. 2013).
In vivo bioluminescence imaging
Bioluminescence measurement of mice was determined using in vivo imaging system (IVIS) 200 bioluminescence system (Xenogen, USA), and tumor growth in live animals was assessed. Bioluminescence images were taken 30 min after the D-luciferin injection, acquired for 1–30 s and the photon emission was quantified using the software, Living image 3.2 (Xenogen, USA), and graphed according to the average radiance (photons/s/cm2/sr). The lungs and livers were removed for IVIS imaging to assess the tumor metastasis. Images were acquired for less than 3 min, and the signal emission was quantified using the Living image 3.2 and graphed according to the average radiance.
X-ray and μ-CT analysis
Tibias removed from tumor-bearing mice were scanned with X-ray (MX-20, Faxitron X-ray, WI, USA) and a high-resolution microtomographic system, μ-CT 40 (Scanco Medical, Switzerland). The tibia specimens were measured at room temperature and placed inside the X-ray chamber of the X-ray machine. The voltage and exposure time of the X-ray were 32 kV and 10 s, respectively. Then, the samples were exposed to the μ-CT. Each three-dimensional image data were consisted of approximately 500 μ-CT slide image (8 μm/slide) starting from the growth plate of tibial interface and moving down the tibia. The bone density was expressed as percentage of bone volume (BV)/tissue volume (TV), which was generated and compared with each group using the formula: (BV/TV) × 100 % (Daubine et al. 2007).
Histological analysis
Lungs and livers of tumor-bearing mice were fixed in 10 % buffered formalin for 7 days at room temperature. For each tissue sample embedded in the parafilm block, sections at 5 μm from three levels (slice intervals spaced 500 microns apart) were collected and stained with hematoxylin and eosin (H&E). Then, the stained slides were photographed using Olympus IX71 microscope (Japan) and SPOT advanced software (version 3.5.6). The ImageJ software (NIH, USA) was used to measure the tumor area and the organ area in each slide. Then, the tumor area and the organ area on one slide were added together and the % tumor area to lung or liver was generated as tumor area divided by the lung or liver area. The position and direction of the organs in the parafilm block during the embedding process have been ensured, and the distance of each sectioning levels has been standardized. The tumor burden in lung or liver was calculated from the average percentage of tumor area to lung or liver area from three levels of each slide of a total of 10 animals (i.e., 30 tissue sections for each group). These data should be representative for the actual tumor growth in animals under different treatments.
Cells viability assay
MDA-MB-231-TXSA cells (1 × 104/well) in 100 µl medium were seeded in 96-well plates (Corning, USA) and incubated with CS (0, 0.05, 0.1, 0.2 and 0.4 mg/ml) and/or ZOL (0, 20, 40 and 60 µM) at various concentrations for 48 h. Following incubation, 30 µl of MTT solution (5 mg/ml in PBS) was added to each well and the plate was incubated at 37 °C for another 4 h. Then, the medium was discarded and 150 µl of DMSO was added to dissolve the formazan crystals. The absorbance of each sample was read at 540 nm using a microplate reader (Biotek μ-Quant, USA). Results were expressed as percentage of cell viability with respect to untreated control cells (as 100 %). Only the most effective dose of ZOL would be chosen in further studies when combined with CS at various concentrations (0, 0.1 and 0.2 mg/ml).
Scratch wound healing assay
MDA-MB-231-TXSA cells (1 × 105/well) in 1 ml culture medium were seeded in 24-well plates and incubated at 37 °C for 24 h. After starved in medium without FBS for 24 h, cells were scraped with crosses using 200-µl pipette tips. The medium was then replaced with fresh medium with CS (0, 0.05 and 0.1 mg/ml) and ZOL (40 µM). Cells were incubated for 9 h, and each well was photographed under a microscope (Nikon Eclipse TS100). The percentages of open wound area were measured and calculated using the TScratch software. Motility was determined by the decrease in open wound area (Luo et al. 2013).
Transwell migration assay
MDA-MB-231-TXSA cells (5 × 104 in 100 μl) were added into each transwell filter chamber with 1 % v/v FBS. At the same time, 100 μl of medium containing CS (0, 0.1 and 0.2 mg/ml) and ZOL (40 µM) (with 1 % v/v FBS) was added to the upper chamber. Then, 500 μl complete medium (with 10 % v/v FBS), served as chemoattractant medium, was added into the lower chamber. The cells were allowed to migrate through the Boyden chamber membrane to the lower chamber for 6 h at 37 °C, 5 % CO2. After incubation, cells were fixed with methanol, stained with hematoxylin and photographed under microscope (Nikon Eclipse TS100). The non-migrated cells on the top surface of the filter membrane were scraped with cotton swab. Stained filters were photographed under microscope (Nikon Eclipse TS100). The migrated cells were quantified by manual counting in blinded manner. Changes in cell numbers were represented as a percentage of control values (as 100 %) (Luo et al. 2013).
Gelatin zymography
MDA-MB-231-TXSA cells (1 × 105/well) in 1 ml medium were seeded in 24-well plate and incubated with CS (0, 0.1 and 0.2 mg/ml) and ZOL (40 µM) at 37 °C for 24 h. The supernatant was collected and stored at −80 °C. Protein sample (20 μg) from the supernatant was fractionated in 10 % SDS–polyacrylamide gel with 0.1 % gelatin substrate. Following electrophoresis, the gels were washed three times in 2.5 % Triton X-100 in PBS for 30 min at room temperature. The gels were then incubated overnight at room temperature in developing buffer (50 mM Tris base, 200 mM NaCl, 0.005 mM ZnCl2, 5 mM CaCl2·2H2O, and 0.02 % NaN3, pH 7.5) and then stained with 0.125 % (w/v) Coomassie brilliant blue for 20–30 min and destained in destain buffer (10 % acetic acid and 5 % ethanol in distilled water) for 1–2 days. Visualization of bands was performed on a Bio-RAD, XBS+ imaging system (Bio-Rad, USA).
Statistical analysis
All data were expressed as mean ± SD for in vitro studies, or mean ± SEM for in vivo studies. Statistical analysis was performed using one-way ANOVA, with p value (p) < 0.05 as considered statistically significant.
Results
HPLC analysis of polyphenols of CS aqueous extract
The chemical composition of tea polyphenols in CS extract was analyzed by HPLC. CS water extract contained large amount (around 25 %) of tea polyphenol, with EGCG as the most abundant, followed by EGC, ECG and EC (Luo et al. 2014).
Effects of combined use of CS and ZOL on MDA-MB-231-TXSA tumor growth
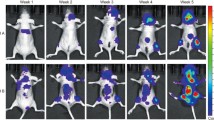
To evaluate the efficacy of CS and/or metronomic ZOL against tumor growth within the bone, breast cancer-induced osteolysis and tumor metastasis, MDA-MB-231-TXSA cells were injected directly into the tibial marrow cavity of nude mice. After treatment for 4 weeks, no significant body weight loss was found in all treatment groups (data not shown). As shown in Fig. 1a, the signal emission (expressed as average radiance) increased from day 7 onwards and associated with an increase in tumor burden in bone. Treatment with CS or ZOL as single agents or in combination resulted in no obvious effect in tumor growth in bone before day 21. While a slight inhibition of tumor growth was noted in CS or ZOL treatment groups from day 21 to day 28, indicating the suppression of tumor growth in bone, this was not statistically significant. However, the combined use of CS and ZOL inhibited the tumor growth significantly at day 28, and a significant difference was shown when compared with control (p < 0.05) (Fig. 1b). The combination therapy showed the most potent anti-tumor result among the three treatment groups.
Tumor burden change during CS and/or metronomic ZOL treatment in intratibial breast cancer-induced osteolysis model. a Representative images of tumor burden obtained from each group at different time points as assessed by IVIS system. b Graph showed the bioluminescence measurements according to the average radiance. Data were expressed as mean ± SEM, n = 15. *p < 0.05, as compared with control. “Ctl” means control group; “CS” means CS water extract treatment group in which mice were treated with 0.6 g/kg CS extract, orally fed daily; “ZOL” means metronomic ZOL treatment group in which mice were treated with 0.0125 mg/kg ZOL, i.p. injected twice a week for 4 weeks; “CS + ZOL” means the combination treatment group in which mice were treated with CS plus ZOL, and the dose was the same as the individual treatment of CS or ZOL
Effects of combined use of CS and ZOL on breast cancer-induced osteolysis
To assess the effect of the combined use of CS and metronomic ZOL on breast cancer-induced osteolysis, the tibiae of mice were resected for X-ray and μ-CT analysis. The X-ray and μ-CT images showed that by day 28 which coincided with the end of the experiment, the tumor-bearing tibiae from the control group had extensive osteolysis with only 6.9 % of the bone volume (%BV/TV), when compared with the non-tumor-bearing tibiae which were intact (Fig. 2a, b). Treatment with CS, ZOL and CS + ZOL resulted in a significant protection against breast cancer-induced osteolysis with the bone volume density increased to 16.2, 24 and 32.5 %, respectively (Fig. 2d). Consistent with the known anabolic effects of ZOL treatment, our results showed that there was a significant increase in bone volume density in the contralateral non-tumor-bearing tibiae in ZOL and CS + ZOL treatment groups (Fig. 2c). Therefore, the combination of CS plus ZOL showed superior anti-osteolysis effect in the tumor-bearing tibiae, when compared to individual treatment of CS or ZOL.
Qualitative and quantitative assessment of bone structure in both tibias after administration of CS and/or metronomic ZOL. a, b Representative X-ray, μ-CT 3D images, section cut and growth plate of a non-tumor-bearing and b tumor-bearing tibias obtained from different groups. c, d Graphs showed the percentage of bone volume to tissue volume (% BV/TV) of c non-tumor-bearing and d tumor-bearing tibias. Data were expressed as mean + SEM, n = 10. **p < 0.01 and ***p < 0.001, as compared with control; ### p < 0.001, as compared between groups indicated
Effects of combined use of CS and ZOL on lung and liver metastasis
At the end of experiment, lung and liver metastasis were evaluated by bioluminescence imaging and histological analysis. As shown in Fig. 3a, the signal emission of lungs in control group was very strong, and the frequency of lung metastasis in this group was 14/15, showing the highest incidence of lung metastasis among the four groups. Treatment with CS, ZOL and CS + ZOL decreased the signal emission (expressed as average radiance) of bioluminescent MDA-MB-231-TXSA tumor cells in lung. The combination of CS + ZOL decreased the frequency of lung metastasis and also showed the lowest signal emission from MDA-MB-231-TXSA cells among the three treatment groups. A significant difference was shown between the untreated control and CS + ZOL groups (p < 0.05) (Fig. 3b. Similar results were found in histological analysis (Fig. 3c, d). Tumor burden in lung decrease by 52.3 % (lung metastasis decreased from 3.2 to 1.5 %) and 70.6 % (lung metastasis decreased from 3.2 to 0.9 %) in CS- and (CS + ZOL)-treated groups, respectively. The combined use of CS + ZOL showed the best result in decreasing lung metastasis among the three treatment groups.
Effects of CS and/or metronomic ZOL treatment on lung metastasis in intratibial breast cancer-induced osteolysis model. a Representative images of lungs obtained from different groups at end point of IVIS scan, and the fractional number revealed the frequency of lung metastasis. b Graph showed the bioluminescence measurements (expressed as average radiance) in lungs. c Photographs were the representative H&E-stained sections of lungs from different groups with arrows showing the MDA-MB-231-TXSA tumor nodules. d Graph showed the tumor burden in lungs as assessed by histological analysis and expressed as an average percentage of tumor area to lung area per group. Data were expressed as mean + SEM, n = 15; *p < 0.05, **p < 0.01 and ***p < 0.001, as compared with control. “Ctl” means control group; “Naive” means normal mice without tumor and treatment
The tumor burden in liver was also assessed, and similar results were found. Bioluminescence measurement showed that the livers in control group had the strongest signal emission from tumor cells, with the highest frequency of liver metastasis among the four groups (Fig. 4a). Treatment with CS, ZOL and CS + ZOL decreased the liver metastasis, and the combination of CS and ZOL showed the best result in decreasing liver metastasis and frequency among the three groups, and significant difference was shown (p < 0.05) (Fig. 4b). The result was further confirmed by histological analysis. As shown in Fig. 4c, d, treatment with CS + ZOL decreased the tumor burden in liver by 89.5 % (liver metastasis decreased from 0.22 to 0.023 %), and significant difference against control group was shown (p < 0.01).
Effects of CS and/or ZOL treatment on liver metastasis in intratibial breast cancer-induced osteolysis model. a Representative images of livers obtained from different groups at end point of IVIS scan, and the fractional number in each group revealed the frequency of metastasis. b Graph represented the bioluminescence measurements (expressed as average radiance) in livers. c Photographs were the representative H&E-stained sections of mouse livers with arrows showing the tumor nodules. d Graph represented the tumor burden in livers as assessed by histological analysis. Data were expressed as mean + SEM, n = 15. *p < 0.05 and **p < 0.01, as compared with control. “Ctl” means control group; “Naive” means normal mice without tumor and treatment
Effects of the combined use of CS and ZOL on MDA-MB-231-TXSA cell viability
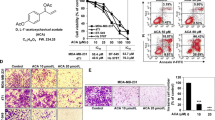
MTT assay was performed to assess the effect of ZOL as a single agent and in combination with CS at different concentrations on cell viability of MDA-MB-231-TXSA cells, and then, the most effective dose of combination would be chosen for further studies. As shown in Fig. 5, the combination of CS and ZOL inhibited cell viability significantly. The combination of ZOL and CS at various concentrations was lower than CS alone. The combination of ZOL and CS demonstrated additive cytotoxic effects, especially at the doses of 40 μM ZOL plus 0.1 mg/ml CS, which produced an inhibition of 26.6 % cell viability, while ZOL and CS added alone caused inhibition rates of 12.9 and 4.2 %, respectively. The IC50 values of CS, ZOL and CS + ZOL treatments in MDA-MB-231-TXSA cells are listed in Table 1. Besides, the combined use of ZOL (20 μM) and CS (0.1 mg/ml) on MDA-MB-231-TXSA cells also demonstrated additive cytotoxic effects. Since the combination of ZOL (40 μM) and CS (0.1 mg/ml) has the best additive inhibition capacity of cell viability, such dose of combination was selected in further studies.
Effects of combined use of CS and ZOL on MDA-MB-231-TXSA cell migration and invasion
Treatment with CS alone resulted in no obvious effect in inhibition of MDA-MB-231-TXSA cell migration, and ZOL (40 μM) alone induced no significant change in the open wound area. However, ZOL enhanced the inhibitory effect of CS on MDA-MB-231-TXSA cell migration, and significant difference was shown in the combination of ZOL (40 μM) and CS (0.2 mg/ml) (p < 0.05) (Fig. 6a, b).
Effect of CS or CS + ZOL on the MDA-MB-231-TXSA cell migration and invasion. a Representative images of the wounded cell monolayers of MDA-MB-231-TXSA cells after incubated with various concentrations of CS and fixed amount of ZOL (40 μM). b Quantitative analysis of the migration activity of cells after 9 h treatment of CS or CS + ZOL. c Representative images of the stained MDA-MB-231-TXSA cells. d Quantitative analysis of the invasion activity of cells after treated with CS or CS + ZOL. Data were expressed as mean + SD (n = 3). *p < 0.05 and ***p < 0.001, as compared with corresponding untreated control
In order to determine the efficacy of CS and ZOL against cancer cell invasion in vitro, the transwell migration assay was included. As shown in Fig. 6c, d, treatment with CS (0.2 mg/ml) resulted in inhibition of cell invasion in MDA-MB-231-TXSA cells. ZOL (40 μM) alone also induced significant suppression of MDA-MB-231-TXSA cell invasion. Besides, the combination of ZOL (40 μM) and CS (0.1 or 0.2 mg/ml) showed significant inhibitory effect against MDA-MB-231-TXSA cell invasion. However, no significant difference was shown between the combination and individual treatments.
Effect of the combined use of CS and ZOL on MMP-9, MMP-2 activities
MMP-2 and MMP-9 play important roles in extracellular matrix and basement membrane degradation, which are critical for cancer cell metastasis (Terranova et al. 1986). As shown in Fig. 7a, MMP-9 and MMP-2 in MDA-MB-231-TXSA cell culture supernatant were detected in the gel at molecular weight of 92 and 72 kDa, respectively. The MMP-9 and MMP-2 digested the gelatin substrate significantly, and clear bands were presented in control. Treatments with CS or CS + ZOL resulted in the reduction of the bands, indicating the suppression of the enzyme activities of MMP-9 or MMP-2. The activities of MMP-9 and MMP-2 were significantly suppressed by CS and CS + ZOL. However, no significant difference was shown between the combination of CS + ZOL and the individual treatment of CS alone (Fig. 7b, c).
Discussion
This study investigated the combined use of CS water extract and metronomic ZOL on anti-tumor, anti-metastasis and anti-osteolysis activities using an intratibial breast cancer-induced osteolysis mouse model. A limitation in measuring tumor burden in bone is that it is impossible to evaluate the progression of tumor growth in bone accurately by caliper measurements, especially before the soft tissue tumors break through the cortical bone. The noninvasive and sensitive bioluminescence imaging approach we have adopted here using the IVIS 200 enabled in vivo tracking of tumor growth in bone and its metastatic spread to lungs and livers in live animals.
The intratibial breast cancer-induced osteolysis model closely mimics breast cancer cells metastasized to bone and developed severe bone destruction (Zinonos et al. 2009), so the bone architecture was assessed using X-ray and μ-CT analysis. The bone volume density (% BV/TV) in both of the tibias was assessed. Our results demonstrated that treatment with ZOL and CS + ZOL resulted in significant increase of percentage of bone volume density in both tumor-bearing and contralateral non-tumor-bearing tibiae, and the bone structure was protected, indicating the combination therapy had both direct anti-osteoclast and anti-tumor effect. Since the bone protection effect of treatments may arise from either direct anti-osteolysis effect by suppressing osteoclast, or from the indirect anti-tumor effect and then decreased the cancer cell metastasis to bone, the increase of bone volume density in the former one was reflected in both tibiae, while in the latter one was reflected only in tumor-bearing tibia. The remarkable anti-osteolysis effect of the combined use of CS and metronomic ZOL may mainly due to ZOL. Previous report showed that ZOL is a potent clinical drug for prevention and treatment of various bone diseases by preventing prenylation of GTPases and thus inhibiting osteoclastic bone resorption (Zhang and Casey 1996). On the other hand, CS may also have partly contributed to the anti-osteolysis of the combination therapy. Previous reports have demonstrated that tea polyphenol EGCG in CS had significant effect in inhibiting osteoclasts formation and differentiation both in vitro and in vivo (Lee et al. 2010, 2012). Our previous report also demonstrated that CS water extract suppressed the bone structure from breast cancer-induced osteolysis in 4T1 mouse mammary tumor model (Luo et al. 2014).
The metronomic dose of ZOL used in this study was 0.0125 mg/kg, administered intraperitoneally 8 times over 4 weeks, where the cumulative total dose was the same as the single conventional dose of 0.1 mg/kg. Our previous study demonstrated that metronomic dose of ZOL was more effective than the single conventional dose in reducing breast cancer tumor burden and decreasing lung and liver metastasis in the same model mentioned above (Luo et al. 2013). In the present study, the signal emission from bioluminescent cells in lungs and livers was found to be the lowest in the combination CS + ZOL treatment group, suggesting the combination treatment was more effective in decreasing the level of lung and liver metastasis. The individual treatment of metronomic ZOL was effective in bone protection, while CS alone was effective in decreasing lung and liver metastasis, and the combined use of CS and metronomic ZOL worked additively and exerted the advantages of the two and showed significant anti-tumor, anti-metastasis and anti-osteolysis effects. Combination therapy, in which one or more medication or therapies are used at the same time, is the proven cure for cancer treatment (Fitzgerald et al. 2006). An optimal combination therapy of anti-tumor agents is expected to produce some synergistic or additive therapeutic efficacies, including increased therapeutic efficacy, decreased side effects and minimal or delayed drug resistance (Yeh and Kishony 2007). Previous report demonstrated that green tea polyphenol EGCG acted synergistically in combination with clinical anticancer drugs cisplatin and designed trans-palladiums in ovarian cancer cells (Mazumder et al. 2012). Our results presented the first evidence on the anti-tumor, anti-metastasis and anti-osteolytic effects of the combined use of herbal medicine CS and metronomic ZOL in mouse model.
Apart from the in vivo studies, the in vitro studies on MDA-MB-231-TXSA cell migration and invasion were also assessed. Greater level of inhibition on cell migration was observed when the cells were incubated with CS + ZOL, indicating that ZOL could potentiate the inhibitory effect of CS on cell migration of MDA-MB-231-TXSA cells. Previous reports showed that ZOL and CS water extract could significantly prevent cell migration in breast cancer cells MDA-MB-231 cells (Rachner et al. 2010) and colon cancer SW620 cells (Zhou et al. 2012), respectively. Our results showed that the combination of CS and ZOL had greater level of inhibition on MDA-MB-231-TXSA cell migration than the individual treatment of CS or ZOL. In addition, the enzymes activities of MMP-9 and MMP-2 were significantly suppressed by CS and CS + ZOL, indicating the anti-metastasis effect of the combined use of CS and ZOL. However, in invasion assay, no additive effect was shown in the combined use of CS and ZOL on cell invasion. The possible reason for the similarity between CS and CS + ZOL groups was that the dose of CS used in transwell migration and zymography assay might be a bit high. Treatment of CS alone (0.2 mg/ml) resulted in significant decrease in transwell migration as well as MMP-9 and MMP-2 activities, and therefore, the combined use of CS + ZOL had similar data as CS alone. Nevertheless, we found that ZOL strengthened the effect of CS when CS was used at a relative low dose of 0.1 mg/ml. Individual treatment of CS (0.1 mg/ml) had no significant effect on transwell migration and MMP-9 activities, while the combination of CS + ZOL resulted in significant decrease on both. Similar results were also found in MMP-2 activities, CS + ZOL showed better effect than CS treatment alone at the dose of 0.1 mg/ml. The underlying mechanism of the anti-tumor and anti-metastasis effects of the combined use of CS + ZOL was not included in our study, and there are accumulated reports demonstrated the apoptosis induction and anti-metastasis effects of CS or ZOL. Green tea extract was shown to induce apoptosis in human breast cancer MDA-MB-231 and MCF-7 cells by increasing the ratio of Bax-to-Bcl-2 (Thangapazham et al. 2007; Hsuuw and Chan 2007). Besides, ZOL was shown to induce apoptosis in MDA-MB-231 cells via activation of caspase-3 and caspase-7 and enhance the TNF-related apoptosis-inducing ligand (TRAIL) to osteoprotegerin (OPG) ratio in this cell line (Rachner et al. 2010).
In conclusion, our results present the first evidence of combined use of a standard chemotherapy (ZOL) plus a herbal extract (CS) against tumor growth, metastasis and bone destruction in a breast cancer-induced osteolysis mouse model. The synergistic and/or additive effects were further elucidated in our in vitro cell model. The present findings could play roles in the future production of CS as a supplement in breast cancer treatment, and the combination of CS and metronomic ZOL suggested promising application in breast cancer patients clinically.
References
Bettuzzi S, Brausi M, Rizzi F, Castagnetti G, Peracchia G, Corti A (2006) Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of principle study. Cancer Res 66:1234–1240
Chacko SM, Thambi PT, Kuttan R, Nishigaki I (2010) Beneficial effects of green tea: a literature review. Chin Med 5:13
Daubine F, Le Gall C, Gasser J, Green J, Clezardin P (2007) Antitumor effects of clinical dosing regimens of bisphosphonates in experimental breast cancer bone metastasis. J Natl Cancer Inst 994:322–330
Eliza WL, Fai CK, Chung LP (2012) Efficacy of Yun Zhi (Coriolus versicolor) on survival in cancer patients: systematic review and meta-analysis. Recent Pat Inflamm Allergy Drug Discov 6(1):78–87
Evdokiou A, Labrinidis A, Bouralexis S, Hay S, Findlay DM (2003) Induction of cell death of human osteogenic sarcoma cells by zoledronic acid resembles anoikis. Bone 33:216–228
Facchini G, Caraglia M, Morabito A, Marra M, Piccirillo MC, Bochicchio AM, Striano S, Marra L, Nasti G, Ferrari E, Leopardo D, Vitale G, Gentilini D, Tortoriello A, Catalano A, Budillon A, Perrone F, Iaffaioli RV (2010) Metronomic administration of zoledronic acid and taxotere combination in castration resistant prostate cancer patients: phase I ZANTE trial. Cancer Biol Ther 10:543–548
Farabegoli F, Papi A, Orlandi M (2011) (−)-Epigallocatechin-3-gallate down-regulates EGFR, MMP-2, MMP-9 and EMMPRIN and inhibits the invasion of MCF-7 tamoxifen-resistant cells. Biosci Rep 31(2):99–108
Fitzgerald JB, Schoeberl B, Nielsen UB, Sorger PK (2006) Systems biology and combination therapy in the quest for clinical efficacy. Nat Chem Biol 22(3):1737–1754
Forester SC, Lambert JD (2011) The role of antioxidant versus pro-oxidant effects of green tea polyphenols in cancer prevention. Mol Nutr Food Res 55(6):844–854
Gu JW, Makey KL, Tucker KB, Chinchar E, Mao X, Pei I, Thomas EY, Miele L (2013) EGCG, a major green tea catechin suppresses breast tumor angiogenesis and growth via inhibiting the activation of HIF-1α and NFκB, and VEGF expression. Vasc Cell 5(1):9
Hsuuw YD, Chan WH (2007) Epigallocatechin gallate dose-dependently induces apoptosis or necrosis in human MCF-7 cells. Ann N Y Acad Sci 1095:428–440
Labrinidis A, Hay S, Liapis V, Findlay DM, Evdokiou A (2010) Zoledronic acid protects against osteosarcoma-induced bone destruction but lacks efficacy against pulmonary metastases in a syngeneic rat model. Int J Cancer 127:345–354
Lam YC, Cheng CW, Peng H, Law CK, Huang XZ, Bian ZX (2009) Cancer patients’ attitudes toward Traditional Chinese Medicine: a Hong Kong survey. Chinese Med 4:25
Lee JH, Jin H, Shim HE, Kim HN, Ha H, Lee ZH (2010) Epigallocatechin-3-gallate inhibits osteoclastogenesis by down-regulating c-Fos expression and suppressing the nuclear factor-kappaB signal. Mol Pharmacol 77(1):17–25
Lee SH, Kim BJ, Choi HJ, Cho SW, Shin CS, Park SY, Lee YS, Lee SY, Kim HH, Kim GS, Koh JM (2012) (−)-Epigallocathechin-3-gallate, an AMPK activator, decreases ovariectomy-induced bone loss by suppression of bone resorption. Calcif Tissue Int 90(5):404–410
Liang G, Tang A, Lin X, Li L, Zhang S, Huang Z, Tang H, Li QQ (2010) Green tea catechins augment the antitumor activity of doxorubicin in an in vivo mouse model for chemoresistant liver cancer. Int J Oncol 37(1):111–123
Luo KW, Ko CH, Yue GGL, Lee MY, Siu WS, Lee JK, Shum WT, Fung KP, Leung PC, Li G, Evdokiou A, Lau CBS (2013) Anti-tumor and anti-osteolysis effects of the metronomic use of zoledronic acid in primary and metastatic breast cancer mouse models. Cancer Lett 339(1):42–48
Luo KW, Ko CH, Yue GGL, Lee JKM, Lee M, Li G, Fung KP, Leung PC, Lau CBS (2014) Green tea (Camellia sinensis) extract inhibits both the metastasis and osteolytic components of mammary cancer 4T1 lesions in mice. J Nutr Biochem 25(4):395–403
Martin CK, Dirksen WP, Carlton MM, Lanigan LG, Pillai SP, Werbeck JL, Simmons JK, Hildreth BE, London CA, Toribio RE, and Rosol TJ (2013) Combined zoledronic acid and meloxicam reduced bone loss and tumour growth in an orthotopic mouse model of bone-invasive oral squamous cell carcinoma. Vet Comp Oncol. doi:10.1111/vco.12037
Mazumder ME, Beale P, Chan C, Yu JQ, Huq F (2012) Epigallocatechin gallate acts synergistically in combination with cisplatin and designed trans-palladiums in ovarian cancer cells. Anticancer Res 32(11):4851–4860
Okamoto S, Jiang Y, Kawamura K, Shingyoji M, Fukamachi T, Tada Y, Takiguchi Y, Tatsumi K, Shimada H, Hiroshima K, Kobayashi H, Tagawa M (2013) Zoledronic acid produces combinatory anti-tumor effects with cisplatin on mesothelioma by increasing p53 expression levels. PLoS One 8(3):e60297
Ottewell PD, Woodward JK, Lefley DV, Evans CA, Coleman RE, Holen I (2009) Anticancer mechanisms of doxorubicin and zoledronic acid in breast cancer tumor growth in bone. Mol Cancer Ther 8:2821–2832
Pasquier E, Kavallaris M, Andre N (2011) Metronomic chemotherapy: new rationale for new directions. Nat Rev Clin Oncol 7:455–465
Peng L, Song XH, Shi XG, Li JX, Ye CX (2008) An improved HPLC method for simultaneous determination of phenolic compounds, purine alkaloids and theanine in Camellia species. J Food Compost Anal 21:559–563
Qiao J, Gu C, Shang W, Du J, Yin W, Zhu M, Wang W, Han M, Lu W (2011) Effect of green tea on pharmacokinetics of 5-fluorouracil in rats and pharmacodynamics in human cell lines in vitro. Food Chem Toxicol 49(6):1410–1415
Rachner TD, Singh SK, Schoppet M, Benad P, Bornhauser M, Ellenrieder V, Ebert R, Jakob F, Hofbauer LC (2010) Zoledronic acid induces apoptosis and changes the TRAIL/OPG ratio in breast cancer cells. Cancer Lett 287:109–116
Ryan P, Saleh I, Stassen LF (2009) Osteonecrosis of the jaw: a rare and devastating side effect of bisphosphonates. Postgrad Med J 85:674–677
Sellmeyer DE (2010) Atypical fractures as a potential complication of long-term bisphosphonate therapy. JAMA 304(13):1480–1484
Terranova VP, Hujanen ES, Martin GR (1986) Basement membrane and the invasive activity of metastatic tumor cells. J Natl Cancer Inst 77:311–316
Thangapazham RL, Passi N, Maheshwari RK (2007) Green tea polyphenol and epigallocatechin gallate induce apoptosis and inhibit invasion in human breast cancer cells. Cancer Biol Ther 6(12):1938–1943
Wood J, Bonjean K, Ruetz S, Bellahcene A, Devy L, Foidart JM, Castronovo V, Green JR (2002) Novel antiangiogenic effects of the bisphosphonate compound zoledronic acid. J Pharmacol Exp Ther 302:1055–1061
Yeh P, Kishony R (2007) Networks from drug–drug surfaces. Mol Syst Biol 3:85
Zhang FL, Casey PJ (1996) Protein prenylation: molecular mechanisms and functional consequences. Annu Rev Biochem 65:241–269
Zhao X, Xu X, Guo L, Ragaz J, Guo H, Wu J, Shao Z, Zhu J, Guo X, Chen J, Zhu B, Wang Z, Hu X (2010) Biomarker alterations with metronomic use of low-dose zoledronic acid for breast cancer patients with bone metastases and potential clinical significance. Breast Cancer Res Treat 124:733–743
Zhou F, Zhou H, Wang T, Mu Y, Wu B, Guo DL, Zhang XM, Wu Y (2012) Epigallocatechin-3-gallate inhibits proliferation and migration of human colon cancer SW620 cells in vitro. Acta Pharmacol Sin 33(1):120–126
Zinonos I, Labrinidis A, Lee M, Liapis V, Hay S, Ponomarev V, Diamond P, Zannettino AC, Findlay DM, Evdokiou A (2009) Apomab, a fully human agonistic antibody to DR5, exhibits potent antitumor activity against primary and metastatic breast cancer. Mol Cancer Ther 8:2969–2980
Acknowledgments
This study was supported by Focused Innovations Scheme (Major Area Scheme A—Phase 2) of The Chinese University of Hong Kong. The authors would like to thank Dr. Sammy Siu, Ms. Anita Shum and Mr. Jimmy Cheng for their technical support on the IVIS and micro-CT analysis.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Luo, KW., Ko, CH., Yue, G.GL. et al. The combined use of Camellia sinensis and metronomic zoledronic acid in a breast cancer-induced osteolysis mouse model. J Cancer Res Clin Oncol 141, 1025–1036 (2015). https://doi.org/10.1007/s00432-014-1882-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-014-1882-1