Abstract
Spatial and temporal oxygen heterogeneity exists in most solid tumour microenvironments due to an inadequate vascular network supplying a dense population of tumour cells. An imbalance between oxygen supply and demand leads to hypoxia within a significant proportion of a tumour, which has been correlated to the likelihood of metastatic dissemination in both rodent tumour models and human patients. Experimentally, it has been demonstrated that near-anoxic in vitro exposure results in transiently increased metastatic potential in some tumour cell lines. The purpose of this study was to examine the effect of graded low oxygen conditions on the invasive phenotype of human tumour cells using an in vitro model of basement membrane invasion, in which we measured oxygen availability directly at the invasion surface of the transwell chamber. Our results show a relationship between culture vessel geometry and time to achieve hypoxia which may affect the interpretation of low oxygen experiments. We exposed the human tumour cell lines, HT1080 and MDA MB231, to graded normobaric oxygen (5% O2–0.2% O2) either during or prior to in vitro basement membrane invasion to simulate conditions of intravasation and extravasation. A secondary aim was to investigate the potential regulation of matrix metalloproteinase activity by oxygen availability. We identified significant reductions in invasive ability under low oxygen conditions for the HT1080 cell line and an increase in invasion at intermediate oxygen conditions for the MDA MB231 cell line. There were differences in the absolute activity of the individual matrix metalloproteinases, MMP-2, -9, -14, between the two cell lines, however there were no significant changes following exposure to hypoxic conditions. This study demonstrates cell line specific effects of graded oxygen levels on invasive potential and suggests that intermediate levels of low oxygen may increase metastatic dissemination.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The concentration of oxygen within a solid tumour is generally lower than in the corresponding normal tissue and spatial oxygen heterogeneity has been extensively demonstrated in human tumours [1]. Recently, temporal oxygen heterogeneity has also been documented in spontaneous canine tumours [2], small animal tumours [3–5] and human xenograft models [6] with oxygen fluctuations in the range of 0–10 mmHg and periods on the order of 3–45 min. The median pO2 values in the preclinical models are often very low, with a significant fraction of the measurements below 5 mmHg. Thus tumour cells are exposed to a range of low oxygen conditions including normal physiological levels to anoxic levels, which may stimulate oxygen-regulated molecular responses such as the regulation of mRNA transcription by the hypoxia-inducible factor (HIF) protein [7].
Current studies of oxygen-regulated cellular mechanisms have generally examined a single low concentration of oxygen, however early reports demonstrated that HIF-1α protein stability and DNA-binding activity of the HIF complex varied as a complex function of oxygen concentration, increasing at marginally decreased oxygen concentrations (as high as 5% O2) to a maximum at 0.5% O2, followed by a sharp decline as anoxia was reached [8]. In contrast the maximal expression of VEGF mRNA, which is regulated by the HIF transcription factor in response to low oxygen conditions, was observed to occur at 1.5–2.5% O2, depending on cell type [9]. The expression of many genes is altered by hypoxic conditions and not all are HIF-regulated [10–13]. Our present understanding of the molecular responses to low oxygen is not consistent with the view that there is a single critical value of low oxygen that dominates the cellular hypoxic response.
Tumour hypoxia has been shown to predict for poor disease-free survival and overall survival in head and neck carcinomas, cervical carcinomas, and soft tissue sarcomas, irrespective of treatment modality [14–19]. Furthermore some studies have identified a relationship between tumour hypoxia and distant metastatic disease [14, 16, 20, 21]. In vitro studies have also shown an increase in experimental metastatic potential following in vitro exposure to near anoxic conditions [22–26]. The metastatic dissemination of a tumour cell requires a number of phenotypic abilities, such as the ability to degrade biological barriers including basement membrane and an ability to initiate movement through such barriers [27]. These processes are necessary during the early stages of metastasis, as the tumour cell invades through the local matrix of the primary tumour into a lymphatic or vascular vessel (intravasation) and again, when the tumour cell invades out of the vasculature through the local basement membrane into the stroma of the secondary site (extravasation).
The experiments presented in this study were designed to investigate how exposure to a range of low oxygen conditions might alter the process of tumour cell migration and invasion. We assumed that within an established primary solid tumour the initial events of tumour cell dissemination and intravasation could occur under low oxygen conditions. We developed our assay to investigate invasion across the basement membrane matrix, Matrigel, under both hypoxic conditions (to model intravasation) and under normoxic conditions following hypoxic pre-exposure (to model extravasation). We examined a range of oxygen concentrations, from normal physiological levels (∼5% O2) to near-anoxic levels (0.2% O2). Since matrix metalloproteinases are considered to be required for the degradation of stromal and basement membrane matrix [28, 29], we also examined the expression and activity of MMPs -2 and -9, recently reported to be hypoxia-induced [30–32], and a key player in metastatic dissemination (MMP-14 or MT1–MMP) which may also be regulated by hypoxia [33]. It is not clear if migration and invasion varies directly with changing oxygen concentrations or if it is altered at a critical concentration of oxygen. Here we demonstrate cell-line specific changes in invasion with respect to a range of oxygen concentrations, using two different human tumour cell lines, HT1080 fibrosarcoma and MDA MB231 breast carcinoma, which vary in their intrinsic invasive ability. Matrix metalloproteinase activity was not altered and did not associate with the invasive phenotype.
Materials and methods
Cell lines and culture conditions
Early passage HT1080 and MDA MB231 cell cultures were routinely cultured in α-MEM (Gibco BRL, Burlington, ON) (containing penicillin/streptomycin) supplemented with 10% FBS and passaged regularly. For maintenance, cells were kept at 37°C in a humidified atmosphere of air + 5% CO2. Normobaric hypoxic conditions were achieved by placing cell culture vessels in a plastic modular incubator chamber (Billups-Rothenberg, Del Mar, CA, USA) under normal atmospheric conditions and subsequently flushing the chamber with defined gas mixtures containing proportions of O2, 5% CO2, and balanced with N2 (Praxair, Mississauga, ON). The gas flush was initiated at 12 l/min for 8 min, followed by continuous flushing at 12 l/h for the remaining period of exposure.
The pO2 was routinely measured during experiments using a fibre-optic fluorescent oxygen sensor (OxyLite 4000; Oxford Optronix, Oxford, UK). Probes were placed as needed to measure the incubator chamber atmosphere (using a thinly wetted probe in a shallow dish) or placed within the medium of the culture vessel at the cell surface layer.
For isolation of enzymatically active MMPs, cell cultures were grown in 10 cm dishes containing serum-free α-MEM supplemented with 1x ITS Liquid Media Supplement (Sigma, St. Louis, MO, USA).
VEGF and MT1-MMP ELISAs
Conditioned media was collected from cell cultures exposed to low oxygen conditions for 24 h, centrifuged to remove potential debris, and stored frozen at −20°C until assayed. Ten to forty-fold dilutions of conditioned media were prepared in α-MEM (minus FBS) and 200 μl of diluted conditioned media was assayed in duplicate per experiment. The human VEGF Quantikine ELISA kit (R&D Systems, Minneapolis, MN, USA) was used to quantify the amount of vascular endothelial growth factor contained within the conditioned media. Total cellular protein lysates were also prepared from the cultures and the total cellular protein was determined (BCA Protein Assay; Pierce Biotechnology, Rockford, IL, USA) and used to calculate the cell concentration of the culture with the aid of previously derived standard curves of total protein to cell number specific to each cell line. Concentrations of VEGF were calculated as pg VEGF per 104 cells.
Quantification of active MMP-14 was performed by detection of a colorimetric substrate cleavage product processed by a detection enzyme that was itself activated by immuno-captured active MMP-14 present in the assayed conditioned medium (MMP-14 Biotrak Activity Assay; GE Healthcare Life Sciences, Baie d’Urfe, QC). Total protein cellular lysates were prepared using the manufacturer’s supplied extraction buffer at a protein concentration of 2.6 μg/100 μl extraction buffer and assayed in duplicate following the manufacturer’s instructions.
Western blots and immunodetection
Following treatments, adherent cells were washed with PBS and harvested in 1 ml lysis buffer [34] supplemented with protease inhibitors (Complete Mini Tablets; Roche Applied Science, Laval, QC). Total protein concentration of the cell lysates was determined using the BCA protein assay (Pierce Biotechnology, Rockford, IL, USA). Total protein lysates (10 μg/lane) were resolved on 8% SDS-polyacrylamide gels and protein was transferred to a nitrocellulose membrane via electroblotting. Nitrocellulose membranes were blocked with 5% (w/v) nonfat milk powder in TBST for 1 h at room temperature. The antibodies used were as follows: anti-human α-MT1 [donkey] (a kind gift provided by Gillian Murphy, University of Cambridge, UK) and anti-β-actin [rabbit] (Rockland Immunochemicals, Gilbertsville, PA, USA). The blots were incubated at RT with the indicated antibodies for a period of 1–3 h and thoroughly washed, then incubated with horseradish peroxidase-conjugated secondary antibodies. Detection was by enhanced chemiluminescence according to the manufacturer’s instructions (Immobilon Western Chemiluminescent HRP Substrate; Millipore, Billerica, MA, USA)
For detection of HIF1α, cell cultures (≤60% confluent) that had been exposed to controlled low oxygen conditions for 24 h were washed with ice-cold PBS and harvested in 600 μl lysis solution (0.5 M NaCl, 1.5 mM MgCl2, 10 mM Tris [pH-8.0], 5 mM EDTA, 0.1% NP-50) supplemented with protease inhibitors (Complete Mini Tablets) and a phosphatase inhibitor (1 mM Na3VO4). Total protein concentration of the cell lysates was determined as described above. Total protein lysates (60 μg/lane) were resolved as above and transferred to nitrocellulose membranes. Nitrocellulose membranes were blocked using 1:1 solution of PBS:blocking buffer (Odyssey Blocking Buffer; Li-Cor Biosciences, Lincoln, NB, USA) for 1 h at room temperature. Antibodies against specific proteins were as follows: anti-human HIF1α [mouse] and anti-β-actin [donkey]. The blots were incubated at 4°C O/N and thoroughly washed, then incubated with infrared-sensitive fluorescent dye-labeled secondary antibodies (Li-Cor Biosciences). Detection was performed using the Odyssey Infrared Imaging System (Li-Cor Biosciences). Images shown in the figure are 8-bit .jpg images derived from the original 16-bit .tif scans.
Gelatin zymography
Standard SDS-PAGE gels were prepared with the addition of 1 mg/ml gelatin [35]. Conditioned media samples, corresponding to a proportional volume from 3.0 × 105 cells, were concentrated using Centricon-30 centrifugal filters (30 kDa NMWL) (Millipore, Billerica, MA, USA) and the final retentate was resuspended in 16 μl MMP dilution buffer. Just prior to sample loading, 4 μl of 4x gel loading buffer was added. Positive controls were also loaded to each gel (1 ng each of MMP-2 and MMP-9). Gels were run under standard conditions until the bromophenol blue dye front had just run off the end of the gel (∼100 min). Gel sandwiches were disassembled and the gel was placed in wash buffer (2 × 30 min washes). Subsequently, the gels were placed in activity buffer and incubated for 18 h at 37°C in a humidified incubator. Following this, gels were quickly rinsed with ddH2O and then stained with 0.01% Coomassie Blue R-350 stain (PlusOne Coomassie PhastGel Blue R-350; GE Healthcare Life Sciences, Baie d’Urfe, QC) for 3 h. A destain step was unnecessary and by omission it was ensured that the gels were equivalently stained for all experiments [36]. Gels were placed O/N in a gel storage solution and scanned using a flat-bed scanner set in the transillumination mode (Agfa DuoScan T1200; AgfaPhoto GmbH, Leverkusen, Germany).
Digital scans were quantified using Scion Image for Windows (Scion Corporation, Frederick, MD, USA) previously calibrated for percent transmission (calibrated against a density standard step tablet scanned on the Agfa DuoScan T1200). Mean integrated transmission was calculated by integrating the area under the curve. Zymogram quantification was performed according to methods previously described by Kleiner and Stetler-Stevenson [37].
Matrigel invasion
Transwell invasion was performed in a modified Boyden chamber 24-well transwell plate [38, 39]. Matrigel (BD Biosciences, Mississauga, ON) was diluted to a final concentration of 1 mg/ml with α-MEM (− FBS) and 100 μl/well was added to each transwell membrane and dried O/N in a biological safety cabinet. Dried Matrigel layers were reconstituted with 100 μl α-MEM (− FBS) at 37°C for 2 h. During this period, tumour cells were prepared for seeding by detachment from culture vessels using trypsin treatment. Detached cells were collected and washed 2x in medium containing 2% FBS. Cell concentrations were quantified by a Coulter particle counter (Beckman Coulter Canada, Mississauga, ON) and then diluted to concentrations of 5.0 × 104 to 5.0 × 105 cells/ml using α-MEM + 2% FBS. Following rehydration, excess α-MEM was aspirated from the transwell chambers and then 100 μl of diluted cell suspension was seeded to the upper wells of each transwell. The lower well was filled with 600 μl of α-MEM + 10% FBS. Transwell plates (24-well), with the covering lid removed, were then placed in a plastic incubator chamber (Billups-Rothenberg, Del Mar, CA, USA) for either hypoxic exposure or oxic control. Following the specified period of invasion, Matrigel and non-invaded cells were scrubbed from the upper surface and invaded cells (attached to the lower membrane surface) were fixed and stained (Dif-Qik; Dade Behring, Deerfield, IL, USA). Membranes to be quantified were removed and permanently mounted on microscope slides. The complete surface of each membrane was visually scanned with the aid of an eye-piece indexed grid graticule (Electron Microscopy Sciences; Hatfield, PA, USA) at 125× and individual cells were counted.
Statistical analysis
The nonparametric Kruskal-Wallis test was used to test for differences in expression of VEGF protein over the oxygen conditions, followed by individual comparisons between specific oxygen conditions using the Dunn’s multiple comparison test. The data for transwell invasion experiments was calculated as an invasion ratio by comparing the number of cells invaded in each of 6 replicate transwells per condition to the mean number of cells invading under oxic conditions. The mean invasion ratios for each of three independent experiments per oxygen condition were compared to matched oxic control mean invasion ratios using Student’s t-test.
Results
In vitro hypoxia
The geometry of the multi-well transwell culture dish requires a relatively large volume of media (600 μl) to wet the lower surface of the upper well plus a small volume (100 μl) to hydrate the upper reservoir, resulting in a column of media 6 mm tall within each well with a relatively small surface area, ∼120 mm2 available for gas exchange with the experimental atmosphere (see diagram e in Fig. 1). Therefore, to determine the optimal timing of the low oxygen treatments, we investigated the change in oxygen partial pressure within the transwell chambers during continuous flushing of the treatment chamber with defined low-oxygen containing gas mixtures. Equilibration of the culture medium pO2 with the defined low oxygen atmosphere was dependent on the geometry of the culture vessel (Fig. 1). Exposure of 10 cm round culture dishes to low oxygen atmospheres resulted in equilibration within 2–4 h but in the 24-well transwell chambers, the pO2 only reached that of the experimental low oxygen atmosphere after 14–16 h. Based on these observations, experiments examining transwell invasion during hypoxic exposure were performed for a period of 36 h to ensure that the culture was exposed to low oxygen conditions for a minimum of 20–22 h.
pO2 levels in the cultures during exposure to defined low oxygen conditions. (a) A 24-well transwell culture dish exposed to 1.0% O2 under continuous flow. The upper curves are traces obtained from an Oxylite probe placed at the porous membrane of a transwell chamber (see Fig. 1 e). The lower traces are corresponding measures of the chamber atmosphere. (3 independent experiments shown) (b) pO2 traces of cell cultures grown in 10 cm dishes exposed to 1.0% O2 under continuous flow. Simultaneously measured traces of the culture dish and the chamber atmosphere are shown. (2 independent experiments shown). (c) Representative transwell pO2 profiles of independent experiments exposed to continuous flow flushing with gas mixtures containing 5.0, 1.0 and 0.2% O2 respectively. (d) Representative 10 cm dish pO2 profiles of independent experiments exposed to continuous flow flushing with gas mixtures containing 5.0, 1.0 and 0.2% O2 respectively. (e) pO2 in the chamber atmosphere was measured using an Oxylite 4000 with an individual fibre optic probe placed in a thin-layer wetted dish and within the culture vessel at the surface closest to the adherent cell population with additional fibre optic probes
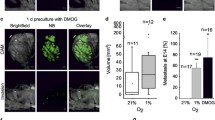
The response of the two cell lines to hypoxic exposure was examined through an analysis of soluble VEGF protein production and expression/stabilization of the HIF1α protein (Fig. 2). Both cell lines expressed VEGF protein under oxic control conditions (21% O2) but the HT1080 cell line expressed higher levels compared to the MDA MB231 cell line (27 ± 5.4 pg/104 cells vs. 7 ± 1.1 pg/104 cells). MDA MB231 expressed a significantly larger amount of VEGF at a pO2 of 2.0% or lower relative to an oxic control, whereas the increase in VEGF production at 2.0% for HT1080 was not significant. Increased levels of HIF1α protein occurred in both cell lines at oxygen levels as high as 5% O2 (Fig. 2b, d). Decreasing levels of oxygen resulted in a further increase of HIF1α, with a maximum level observed at 0.2% O2 in the MDA MB231 cell line and 1% O2 in HT1080 cell line. In related studies, we have also observed increased expression of the hypoxia-responsive gene, CA9, in the HT1080 cell line as oxygen levels decrease from normoxic conditions to 0.2% O2 (data not shown). Exposure to oxygen concentrations as low as 0.2% O2 did not have any effect on the cell viability/clonogenicity of either cell line (see Supplementary Fig. 1).
Molecular hypoxic response to 24 h exposure to defined low oxygen conditions. VEGF protein was quantified from the conditioned medium of cell cultures exposed to low oxygen conditions for 24 h using an enzyme-linked immunoassay kit. (a) MDA MB231 breast carcinoma VEGF protein production and (c) HT1080 fibrosarcoma VEGF protein. Results are shown as mean +/− standard error (n = 3). HIF1α expression and stabilization was examined using immunoblotting of total cellular protein lysates of cell cultures exposed to low oxygen conditions for 24 h. Total cellular lysates from an unrelated cell line were used as positive (3 h hypoxic exposure) and negative (normoxic conditions) controls. (b) MDA MB231 breast carcinoma cells and (d) HT1080 fibrosarcoma
Transwell invasion under hypoxic conditions
The ability of tumour cells to invade through a Matrigel layer is dependent on layer thickness and time. In preliminary experiments (data not shown) we established that 100 μg of Matrigel deposited onto the porous membrane acted as a suitable barrier to the cell lines tested such that 1–10% of the seeded cells could invade in the specified time period (24–36 h) under oxic conditions. The HT1080 cell line was more invasive than the MDA MB231 cell line, with approximately 100 in 1,000 seeded cells regularly invading (10%), whereas only ∼1% of seeded MDA MB231 cells possessed invasive ability (data not shown).
Our initial experiments examined invasion through Matrigel layers for 24 h showing 2 to 11-fold enhanced invasion of MDA MB231 cells under low oxygen conditions involving gassing with 1% O2 compared to an oxic control (Table 1). The HT1080 cell line showed invasion under 1% O2 of 0.7–1.9 fold. However, our oxygen measurements had demonstrated that the time taken for the Matrigel surface, where invasion occurs, to equilibrate with the 1% O2 gas phase was 14 to 16 h. Therefore, to ensure that any effect on invasion was due to the specific low oxygen condition being tested, we extended the time of the assay to 36 h, resulting in > 50% of the total invasion period corresponding to a stable and defined low oxygen concentration.
Under low oxygen conditions (≤3% O2), invasion of MDA MB231 cells was moderately increased relative to the oxic control but showed a parabolic response trend with a minimum level, similar to the oxic control, at 1–2% O2 (Fig. 3a). Although the overall hypoxic data set showed a significant increase relative to the oxic control (P < 0.05), of the individual oxygen levels only the data at 4% O2 was significantly increased (P < 0.05). In the HT1080 cell line, the invasion pattern under hypoxia showed a similar parabolic response but was depressed relative to the oxic control. At the individual oxygen levels, the data for 1% or 2% O2 was significantly decreased relative to the oxic control (P < 0.05) (Fig. 3c).
Matrigel transwell invasion assays. (a, b MDA MB231 breast carcinoma and c, d HT1080 fibrosarcoma) cells were seeded to the upper wells of transwell chambers coated with 100 μg Matrigel and (a, c) exposed to hypoxic conditions (see Figure for % O2) for 36 h or oxic control conditions (21% O2) or (b, d) treated under low oxygen conditions (% O2 shown on x-axis) for 24 h and then seeded, under oxic conditions to the upper wells of the transwell chambers for 36 h. Following the invasion period, invaded cells were stained and quantified. Counts of invaded cells were normalized against the mean of the oxic control for each exposure and are plotted as the mean + S.E.M. Data is reported from a minimum of three independent experiments containing 6 replicates each. Matched control cultures invading under oxic conditions were prepared for each experiment and are summarized in the figure by the open bar
Transwell invasion following hypoxic exposure
To simulate extravasation in the transwell invasion assay, we exposed the tumour cell lines (MDA MB231 and HT1080) to low-oxygen conditions within an environmental chamber and then isolated the cells under oxic conditions and seeded them to the transwell chambers to examine invasion under well-oxygenated conditions for a period of 36 h. The invasive ability of MDA MB231 and HT1080 cell lines did not show any significant change, relative to a matched oxic control, under all low oxygen pre-exposure conditions tested (Fig. 3b–d). The mean invasion ratio fluctuated between 1.0 and 2.0, with relatively large variability. HT1080 cells demonstrated a trend towards increasing invasion following exposure to oxygen levels less than or equal to 2.0%. However, for the individual oxygen concentrations this was not significant compared to the oxic control.
Effect of low oxygen conditions on the activity of matrix metalloproteinases
MMPs -2 and -9 are believed to be key mediators in invasion and metastatic progression [40] and have been reported to be up-regulated by hypoxia. As shown in Fig. 4a-b, HT1080 cells express MMP-2 in relative abundance when compared to MDA MB231. Furthermore, HT1080 cells express MMP-2 primarily in the active form, whereas active MMP-2 was undetectable in MDA MB231 cells even though a faint zymogen form of MMP-2 was present. In contrast, MMP-9 levels were relatively similar between the two cell lines, although expression of MMP-9 in HT1080 cells was mainly in the inactive zymogen form, whereas in the MDA MB231 cells, the protein was observed primarily in its active form. Relative quantification of MMP-2 and -9 active and total expression in the two cell lines across the low oxygen condition series is shown in Fig. 4c–f, demonstrating variable expression across the low oxygen range, lacking a clear pattern, with the possible exception of active MMP-9, which showed a parabolic response with a minima observed at 2–3% O2. However, these changes were not statistically significant.
MMP-2 and -9 expression and activity under low oxygen conditions. Conditioned medium from cultures of MDA MB231 or HT1080 cells exposed to low oxygen conditions was used for gelatin zymography. (a, b) A representative zymogram of MDA MB231 and HT1080 samples. MMP controls were loaded at 1 ng/well (c–f) Quantification of gelatin zymography from three independent hypoxia-series. Bars represent total MMP-9 levels (c, MDA MB231; d, HT1080) or MMP-2 levels (e, MDA MB231; f, HT1080) quantified by integrating the transmission of all three forms of the enzyme (zymogen, pre, and active bands). The points (+ line) represent the ratio of active:total forms of each enzyme. Data are normalized to that of the 21% control sample
Recent studies have suggested a link between the expression of MMP-14 (MT1-MMP), a membrane-bound member of the matrix metalloproteinase family, and metastatic progression [41]. In addition, MMP-14 is involved in the activation of MMP-2 at the cell surface through interaction with TIMP-2 [42, 43]. We investigated the regulation of MMP-14 under low oxygen conditions by analyzing both protein expression and enzymatic activity (Fig. 5). There was a slight increase in relative MMP-14 expression in the MDA MB231 cell culture but this was not apparent in the HT1080 cell line. The activity of MMP-14 across the range of low oxygen conditions also showed no clear differences compared to the oxic control. However, the overall activity of MMP-14 in HT1080 cells (6 ng/ml per μg total protein) was consistently higher than that of MDA MB231 cells (3 ng/ml per μg total protein).
MMP-14 (MT1-MMP) activity following low oxygen exposure. Total cellular protein lysates were assayed for expression and activity of the membrane-bound matrix metalloproteinase, MMP-14. Representative western blots of MMP-14 expression in (a) MDA MB231 and (b) HT1080 cells exposed for 24 h to various low oxygen conditions. (c, d) Quantification of MMP-14 protein expression from three independent hypoxic exposures of MDA MB231 or HT1080 cultures, respectively. Quantification of Western blots was performed by digital densitometry and values are reported relative to β-actin and normalized to the oxic control sample. (e, f) Quantification by enzyme linked immunoassay analysis of active MMP-14 in MDA MB231 and HT1080 cellular lysates, respectively
Discussion
The presence of hypoxia in solid tumours has been identified as a factor that can affect the metastatic potential of tumour cells [18]. However, although oxygen heterogeneity within solid tumours has also been observed, relatively little is known about the effects of exposure to a range of oxygen conditions on the invasive potential of tumour cells. Furthermore, in some of the studies which have examined the effects of hypoxia on invasion and metastasis, the exact levels of oxygen to which the cells are exposed are not well controlled. For these reasons we investigated changes in tumour cell invasion under a range of oxygen conditions from near anoxia to normal physiological levels. Our use of the in vitro transwell invasion assay was based on the expectation that the process of intravasation within an established primary solid tumour would likely occur under poor oxygen conditions, while the process of extravasation by previously hypoxic tumour cells would occur under well-oxygenated conditions.
We made careful measurements of oxygen levels in the culture environment because efficient oxygen exchange between gas and liquid phases depends on a number of factors including liquid depth, differences in oxygen content, and agitation [44, 45]. The material of the transwell culture plates is also critical as plastics can contribute to back-diffusion, acting as an oxygen reservoir and releasing oxygen into the tissue culture as the media O2 concentration declines [46, 47]. Our results identified differences in time taken to achieve low oxygen equilibration depending on the specific geometry of the tissue culture vessel. In particular, the transwell chambers, which have a low surface area to volume ratio, achieved equilibration only after 14–16 h, whereas in a tissue culture dish equilibration was achieved in 2–5 h. To account for this period of equilibration we extended our experiments to 36 h of total invasion time, to provide a period of 20–22 h of stability at the desired oxygen concentration.
We also demonstrated that the two tumour cell lines display a biological response to low oxygen conditions. An analysis of the expression and stabilization of the HIF1α protein in both cell lines showed a clear increase in the amount of protein as the oxygen levels declined from normoxic conditions (21% O2) down to levels of 1–0.2% O2. VEGF mRNA is up-regulated by low oxygen conditions via increases in mRNA transcription and mRNA stabilization leading to an increase in protein production [48–50]. The breast carcinoma cell line (MDA MB231) increased expression of VEGF protein upon exposure to low oxygen conditions, whereas the fibrosarcoma cell line (HT1080) did not demonstrate a significant change in soluble protein. Maximum induction of VEGF protein was observed at 2.0% O2, similar to a quantitative study of the relationship between oxygen levels and VEGF mRNA production in cervical carcinoma cell lines, where half-maximal levels of mRNA induction were observed between 13.0 and 27.0 μM O2, which is approximately equivalent to 1.5–3.0% O2 [9] Interestingly, both cell lines examined in the current study have constitutive expression of VEGF under conditions of abundant oxygen (atmospheric air, 21% O2) suggesting misregulation of VEGF expression in the two cell lines. The survival of both cell lines, as demonstrated by plating efficiency, was unchanged under all conditions tested. Only at very low, near anoxic conditions, was the cell survival of the HT1080 cell line reduced by 50%. These findings suggest that both cell lines can adapt the expression of hypoxia-sensitive molecules enabling the maintenance of homeostatic conditions, which result in cell survival and growth across a range of low oxygen conditions.
We did not observe significant differences in invasion under oxic conditions following low oxygen pre-conditioning for either cell line but our results indicate that invasive ability across basement membrane matrices under low oxygen conditions is tumour cell line specific. Very low oxygen conditions significantly inhibited the invasive ability of the HT1080 cell line and, for both cell lines, we observed an interesting parabolic relationship between oxygen level and invasion with minima at 1–2% O2. In both cell lines, invasion at 4.0 and 5.0% O2 was increased relative to the oxic control and this increase was significant in the MDA MB231 cell line at 4.0% O2.
The differences we observed between the cell lines with respect to invasion at very low oxygen levels (≤2.0% O2) may be due to differences in hypoxia tolerance. A study of malignant glioma cell lines showed that prolonged exposure to low oxygen conditions resulted in marked differences among the cell lines in their ability to down-regulate oxygen consumption. Differences between the cell lines were also identified in the expression of VEGF mRNA following hypoxic exposure [51]. A further study [52] found differences in mitochondrial membrane potential and ATP production between hypoxia-sensitive and hypoxia-tolerant glioma cell lines. It was suggested that cells which were able to down-regulate energy consumption were able to preserve ATP stores and were thus more likely to be hypoxia-tolerant. By maintaining an energy balance under hypoxic conditions the cell may be able to carry out energy dependent processes such as cell movement and invasion, although low oxygen levels had no effect on plating efficiency in either cell line. Our results also show that chronic exposure to low oxygen conditions does not permanently alter invasive ability. The invasive ability of the HT1080 cell line was inhibited under low oxygen conditions but recovered within a short period of time under oxic conditions to levels at or above that of the oxic control.
Our results are in contrast to reported findings of enhanced invasion of MDA MB231 cells during exposure to 1.0% O2 for 24 h [30], although our initial experiments did show increased invasion after 24 h at 1% O2 [26,31]. The experimental systems used to expose the cultures to low oxygen conditions in the previous studies were different than ours and, unfortunately, direct measurements of the oxygen within the media layer were not performed in these earlier studies. However, given the long time period needed for oxygen to decline from normoxic to hypoxic levels in our transwell system, it is very possible that a stable period of hypoxic exposure was achieved only for 8–10 h during a 24 h exposure in the previous studies and the majority of invasion may have occurred during the period of relative higher oxygen concentrations.
We also examined the activity of MMPs-2, -9, and -14 (MT1-MMP), key molecules involved in the metastatic process [53], following exposure to low oxygen conditions. In the MDA MB231 cells we observed relatively little change in the expression of MMP-9 or the ratio of active:total enzyme. The expression of MMP-14 was variable across the oxygen range, however any change in activity was not significant. The expression profiles of MMP-9 and MMP-14 matrix metalloproteinases did not follow the patterns observed in the invasion profile of MDA MB231 tumour cells across the oxygen series. In the HT1080 cell line the ratio of active:total MMP-2 enzyme was relatively unchanged across the hypoxic range although the quantification of total enzyme generally decreased as the oxygen concentration was lowered. The MMP-9 profile demonstrated a parabolic shape, decreasing in activity to a minima observed at 3.0% O2 and then increasing as the oxygen concentration was further lowered. The shape of this profile was similar to that observed for invasion although the minima did not match exactly. Whether these small changes in individual MMP activities can act synergistically in reducing the invasive ability of the HT1080 cell line as oxygen levels decrease is unclear.
Earlier reports suggested that the activity of MMP-9 was increased upon exposure of cells to 1% O2 [30] in contrast to our findings. A more recent publication suggests that an observed hypoxia-enhanced invasion at 1% O2, of MDA MB231, was due to changes in cellular localization of MMP-14 as opposed to increases in mRNA expression or protein production [31]. We did not observe enhanced invasion at 1.0% O2, but it is possible that this discrepancy may be due to the differences between the experimental systems used to induce low oxygen conditions in the culture medium. Other groups have reported that they have been unable to find correlations and/or have found a decrease in specific MMPs upon hypoxic exposure [54–59].
The observation of repressed tumour cell invasion at low oxygen conditions with the recovery or increase of invasion at intermediate oxygen concentrations suggests that small reductions in oxygen from normal physiological levels may preferentially lead to the dissemination of tumour cells. Intra-tumoural oxygen heterogeneity is a pathological feature of solid tumours and there is increasing evidence of temporal oxygen heterogeneity (often referred to as acute hypoxia) within solid tumours [2–6]. With temporal oxygen heterogeneity, it is possible that tumour cells nearer to the imperfect vasculature of a solid tumour may be exposed to small decreases in oxygen that would enhance the regulation of invasive processes, leading to dissemination of tumour cells into the vasculature. Cells further from the vascular system would be at a disadvantage for invasion not only because of the greater distance to be traversed but also due to exposure to lower oxygen conditions which could inhibit invasive processes.
In support of this view, in vivo tumour models exposed to repeated cycles of hypoxic exposure lead to increased metastatic dissemination [60, 61]. Furthermore, a recent study by Rofstad et al [62] using two human melanoma xenograft models, suggested that the acute hypoxic fraction of cells within an experimental tumour contributes more to metastatic potential than the chronic hypoxic cell fraction. The effects of temporal oxygen heterogeneity and its potential contribution to metastatic dissemination is currently being addressed in both animal models and cell culture models. Experimental control of in vitro models with respect to absolute oxygen level and time of exposure is needed to clarify the range of oxygen which is most conducive to metastatic processes and to further elucidate if temporal fluctuations in oxygen concentrations that occur within solid tumours can influence invasion differently from the patterns demonstrated here. Such a study would require different experimental techniques than those here since it is not practical to achieve rapid changes in oxygen concentration in transwell chambers as demonstrated in Fig. 1.
In summary, we have demonstrated that low oxygen conditions of an intermediate level below average normal arterio-venous oxygenation values (∼40 mmHg) are most effective in enabling or enhancing the invasion of tumour cells through basement membrane matrices (simulating intravasation) but that prior exposure to different low oxygen levels had relatively little effect on invasion under oxic conditions (simulating extravasation). The changes in invasive ability do not correlate strongly with a change in any one matrix metalloprotease molecule. An important observation is the finding that the invasive capacity of the HT1080 cell declines at low oxygen levels. Together with the observation that intermediate oxygen levels show a small enhancement in invasion, this result suggests that hypoxic cell populations relatively closer to the vasculature may represent the primary source of metastatic cells and may be a critical target population for successful disease treatment.
Abbreviations
- HIF:
-
Hypoxia inducible factor
- ITS:
-
Insulin, transferrin, sodium selenite
- mmHg:
-
Millimeters of mercurcy
- MMP:
-
Matrix metalloproteinase
- MT-MMP:
-
Membrane type matrix metalloproteinase
- pO2 :
-
Partial pressure of oxygen
- TIMP:
-
Tissue inhibitor of MMP
- VEGF:
-
Vascular endothelial growth factor
References
Vaupel P, Kelleher DK, Hockel M (2001) Oxygen status of malignant tumors: pathogenesis of hypoxia and significance for tumor therapy. Semin Oncol 28(2 Suppl 8):29–35
Brurberg KG, Skogmo HK, Graff BA et al (2005) Fluctuations in pO2 in poorly and well-oxygenated spontaneous canine tumors before and during fractionated radiation therapy. Radiother Oncol 77(2):220–226
Cardenas-Navia LI, Yu D, Braun RD et al (2004) Tumor-dependent kinetics of partial pressure of oxygen fluctuations during air and oxygen breathing. Cancer Res 64(17):6010–6017
Cardenas-Navia LI, Braun R, Lewis K et al (2003) Comparison of fluctuations of oxygen tension in FSA, 9L, and R3230AC tumors in rats. Adv Exp Med Biol 510:7–12
Lanzen J, Braun RD, Klitzman B et al (2006) Direct demonstration of instabilities in oxygen concentrations within the extravascular compartment of an experimental tumor. Cancer Res 66(4):2219–2223
Brurberg KG, Thuen M, Ruud EB et al (2006) Fluctuations in pO2 in irradiated human melanoma xenografts. Radiat Res 165(1):16–25
Semenza GL (2003) Targeting HIF-1 for cancer therapy. Nat Rev Cancer 3(10):721–732
Jiang BH, Semenza GL, Bauer C et al (1996) Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Am J Physiol 271(4 Pt 1):C1172–C1180
Chiarotto JA, Hill RP (1999) A quantitative analysis of the reduction in oxygen levels required to induce up-regulation of vascular endothelial growth factor (VEGF) mRNA in cervical cancer cell lines. Br J Cancer 80(10):1518–1524
Koong AC, Denko NC, Hudson KM et al (2000) Candidate genes for the hypoxic tumor phenotype. Cancer Res 60(4):883–887
Lal A, Peters H, St Croix B et al (2001) Transcriptional response to hypoxia in human tumors. J Natl Cancer Inst 93(17):1337–1343
Scandurro AB, Weldon CW, Figueroa YG et al (2001) Gene microarray analysis reveals a novel hypoxia signal transduction pathway in human hepatocellular carcinoma cells. Int J Oncol 19(1):129–135
Yoon DY, Buchler P, Saarikoski ST et al (2001) Identification of genes differentially induced by hypoxia in pancreatic cancer cells. Biochem Biophys Res Commun 288(4):882–886
Brizel DM, Scully SP, Harrelson JM et al (1996) Tumor oxygenation predicts for the likelihood of distant metastases in human soft tissue sarcoma. Cancer Res 56(5):941–943
Hockel M, Schlenger K, Aral B et al (1996) Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res 56(19):4509–4515
Fyles A, Milosevic M, Hedley D et al (2002) Tumor hypoxia has independent predictor impact only in patients with node-negative cervix cancer. J Clin Oncol 20(3):680–687
Brizel DM, Dodge RK, Clough RW et al (1999) Oxygenation of head and neck cancer: changes during radiotherapy and impact on treatment outcome. Radiother Oncol 53(2):113–117
Subarsky P, Hill RP (2003) The hypoxic tumour microenvironment and metastatic progression. Clin Exp Metastasis 20(3):237–250
Milosevic M, Fyles A, Hedley D et al (2004) The human tumor microenvironment: invasive (needle) measurement of oxygen and interstitial fluid pressure. Semin Radiat Oncol 14(3):249–258
Pitson G, Fyles A, Milosevic M et al (2001) Tumor size and oxygenation are independent predictors of nodal diseases in patients with cervix cancer. Int J Radiat Oncol Biol Phys 51(3):699–703
Sundfor K, Lyng H, Rofstad EK (1998) Tumour hypoxia and vascular density as predictors of metastasis in squamous cell carcinoma of the uterine cervix. Br J Cancer 78(6):822–7
Young SD, Marshall RS, Hill RP (1988) Hypoxia induces DNA overreplication and enhances metastatic potential of murine tumor cells. Proc Natl Acad Sci USA 85(24):9533–9537
Young SD, Hill RP (1990) Effects of reoxygenation on cells from hypoxic regions of solid tumors: anticancer drug sensitivity and metastatic potential. J Natl Cancer Inst 82(5):371–380
Rofstad EK, Danielsen T (1999) Hypoxia-induced metastasis of human melanoma cells: involvement of vascular endothelial growth factor-mediated angiogenesis. Br J Cancer 80(11):1697–1707
Zhang L, Hill RP (2004) Hypoxia enhances metastatic efficiency by up-regulating Mdm2 in KHT cells and increasing resistance to apoptosis. Cancer Res 64(12):4180–4189
Erler JT, Bennewith KL, Nicolau M et al (2006) Lysyl oxidase is essential for hypoxia-induced metastasis. Nature 440(7088):1222–1226
Chambers AF, Groom AC, MacDonald IC (2002) Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer 2(8):563–572
Deryugina EI, Quigley JP (2006) Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev 25(1):9–34
Coussens LM, Werb Z (1996) Matrix metalloproteinases and the development of cancer. Chem Biol 3(11):895–904
Canning MT, Postovit LM, Clarke SH et al (2001) Oxygen-mediated regulation of gelatinase and tissue inhibitor of metalloproteinases-1 expression by invasive cells. Exp Cell Res 267(1):88–94
Munoz-Najar UM, Neurath KM, Vumbaca F et al (2006) Hypoxia stimulates breast carcinoma cell invasion through MT1-MMP and MMP-2 activation. Oncogene 25(16):2379–2392
Osinsky SP, Ganusevich II, Bubnovskaya LN et al (2005) Hypoxia level and matrix metalloproteinases-2 and -9 activity in Lewis lung carcinoma: correlation with metastasis. Exp Oncol 27(3):202–205
Petrella BL, Lohi J, Brinckerhoff CE (2005) Identification of membrane type-1 matrix metalloproteinase as a target of hypoxia-inducible factor-2 alpha in von Hippel-Lindau renal cell carcinoma. Oncogene 24(6):1043–1052
Lohi J, Lehti K, Westermarck J et al (1996) Regulation of membrane-type matrix metalloproteinase-1 expression by growth factors and phorbol 12-myristate 13-acetate. Eur J Biochem 239(2):239–247
Heussen C, Dowdle EB (1980) Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal Biochem 102(1):196–202
Leber TM, Balkwill FR (1997) Zymography: a single-step staining method for quantitation of proteolytic activity on substrate gels. Anal Biochem 249(1):24–28
Kleiner DE, Stetler-Stevenson WG (1994) Quantitative zymography: detection of picogram quantities of gelatinases. Anal Biochem 218(2):325–329
Albini A, Iwamoto Y, Kleinman HK et al (1987) A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res 47(12):3239–3245
Albini A, Benelli R, Noonan DM et al (2004) The “chemoinvasion assay”: a tool to study tumor and endothelial cell invasion of basement membranes. Int J Dev Biol 48(5–6):563–571
Chambers AF, Matrisian LM (1997) Changing views of the role of matrix metalloproteinases in metastasis. J Natl Cancer Inst 89(17):1260–1270
Sato H, Takino T, Miyamori H (2005) Roles of membrane-type matrix metalloproteinase-1 in tumor invasion and metastasis. Cancer Sci 96(4):212–217
Strongin AY, Collier I, Bannikov G et al (1995) Mechanism of cell surface activation of 72-kDa type IV collagenase. Isolation of the activated form of the membrane metalloprotease. J Biol Chem 270(10):5331–5338
Lehti K, Lohi J, Valtanen H et al (1998) Proteolytic processing of membrane-type-1 matrix metalloproteinase is associated with gelatinase A activation at the cell surface. Biochem J 334(Pt 2):345–353
Koch CJ (1984) A thin-film culturing technique allowing rapid gas-liquid equilibration (6 sec) with no toxicity to mammalian cells. Radiat Res 97(2):434–442
Boag JW (1969) Oxygen diffusion and oxygen depletion problems in radiobiology. In: Ebert M, Howard A (eds), Current topics in radiation research. North-Holland Publishing Co., Amsterdam, pp 141–195
Chapman JD, Sturrock J, Boag JW et al (1970) Factors affecting the oxygen tension around cells growing in plastic Petri dishes. Int J Radiat Biol Relat Stud Phys Chem Med 17(4):305–328
Chapman JD, Sturrock J, Boag JW et al (1969) The oxygen tension around mammalian cells growing on plastic Petri dishes and its effect on cell survival curves. Br J Radiol 42(497):399
Levy AP, Levy NS, Wegner S et al (1995) Transcriptional regulation of the rat vascular endothelial growth factor gene by hypoxia. J Biol Chem 270(22):13333–13340
Levy AP, Levy NS, Goldberg MA (1996) Hypoxia-inducible protein binding to vascular endothelial growth factor mRNA and its modulation by the von Hippel-Lindau protein. J Biol Chem 271(41):25492–25497
Forsythe JA, Jiang BH, Iyer NV et al (1996) Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol 16(9):4604–4613
Allalunis-Turner MJ, Franko AJ, Parliament MB (1999) Modulation of oxygen consumption rate and vascular endothelial growth factor mRNA expression in human malignant glioma cells by hypoxia. Br J Cancer 80(1–2):104–109
Turcotte ML, Parliament M, Franko A et al (2002) Variation in mitochondrial function in hypoxia-sensitive and hypoxia-tolerant human glioma cells. Br J Cancer 86(4):619–624
Egeblad M, Werb Z (2002) New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2(3):161–174
Himelstein BP, Koch CJ (1998) Studies of type IV collagenase regulation by hypoxia. Cancer Lett 124(2):127–133
Saed GM, Zhang W, Diamond MP (2000) Effect of hypoxia on stimulatory effect of TGF-beta 1 on MMP-2 and MMP-9 activities in mouse fibroblasts. J Soc Gynecol Investig 7(6):348–354
Chen PS, Zhai WR, Zhou XM et al (2001) Effects of hypoxia, hyperoxia on the regulation of expression and activity of matrix metalloproteinase-2 in hepatic stellate cells. World J Gastroenterol 7(5):647–651
Zhang J, Salamonsen LA (2002) Expression of hypoxia-inducible factors in human endometrium and suppression of matrix metalloproteinases under hypoxic conditions do not support a major role for hypoxia in regulating tissue breakdown at menstruation. Hum Reprod 17(2):265–274
Ben Yosef Y, Lahat N, Shapiro S et al (2002) Regulation of endothelial matrix metalloproteinase-2 by hypoxia/reoxygenation. Circ Res 90(7):784–791
Fahling M, Perlewitz A, Doller A et al (2004) Regulation of collagen prolyl 4-hydroxylase and matrix metalloproteinases in fibrosarcoma cells by hypoxia. Comp Biochem Physiol C Toxicol Pharmacol 139(1–3):119–126
Cairns RA, Kalliomaki T, Hill RP (2001) Acute (cyclic) hypoxia enhances spontaneous metastasis of KHT murine tumors. Cancer Res 61(24):8903–8908
Cairns RA, Hill RP (2004) Acute hypoxia enhances spontaneous lymph node metastasis in an orthotopic murine model of human cervical carcinoma. Cancer Res 64(6):2054–2061
Rofstad EK, Galappathi K, Mathiesen B et al (2007) Fluctuating and Diffusion-Limited Hypoxia in Hypoxia-Induced Metastasis. Clin Cancer Res 13(7):1971–1978
Acknowledgements
We would like to thank Gillian Murphy for the kind gift of antibody against MMP-14 and Jane English for helpful discussion regarding the gelatin zymography method. Research by the authors is supported by NCI Canada with funds raised by the Terry Fox Run.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10585_2007_9139_MOESM1_ESM.eps
Plating efficiency following low oxygen exposure. ( a) MDA MB231 and (b) HT1080 tumour cells were exposed to various low oxygen conditions for 24 h and assayed for plating efficiency under normal atmospheric conditions. Four replicate plates at each of two different seeding densities were used to calculate average plating efficiency for each experiment. n = (#) shows the number of separate experiments at each low oxygen condition. (EPS 873 kb)
Rights and permissions
About this article
Cite this article
Subarsky, P., Hill, R.P. Graded hypoxia modulates the invasive potential of HT1080 fibrosarcoma and MDA MB231 carcinoma cells. Clin Exp Metastasis 25, 253–264 (2008). https://doi.org/10.1007/s10585-007-9139-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-007-9139-x