Abstract
Natural killer (NK) cells play a role in the innate and adaptive antitumor immune responses. The activity of NK cells is regulated by functionally opposing, activating and inhibitory receptors whose balance ultimately determines whether target cells will be susceptible to NK cell mediated lysis. As melanoma is an immunogenic tumor, the effect of immunomodulating agents is consistently investigated. In this study in 79 metastatic melanoma (MM) patients and 52 controls NK activity, expression of activating NKG2D and CD161 receptors and KIR receptors, CD158a and CD158b, on freshly isolated PBL and NK cells were evaluated. Native NK cell activity of melanoma patients in clinical stage I–III and MM patients was determined against NK sensitive K562, NK resistant Daudi, human melanoma FemX, HeLa and HL 60 target tumor cell lines. In addition, predictive pretherapy immunomodulating effect after 18 h in vitro treatments of PBL of MM patients with rh IL-2, IFN-α (IFN), 13-cis retinoic acid (RA) and combination IFN-α and RA was evaluated with respect to NK cell lyses against K562 and FemX cell lines. In this study we show for the first time that low expression of CD161 and activating NKG2D receptors, without increased expression of KIR receptors CD158a and CD158b, as well as a decrease in the cytotoxic, CD16bright NK cell subset, is associated with a significant impairment in NK cell activity in MM patients. Furthermore, the predictive pretherapy finding that IL-2, IFN, IFN and RA, unlike RA alone, can enhance NK cell activity of MM patients against FemX melanoma tumor cell line can be of help in the design and development of therapeutic regimens, considering that it has recently been shown that low-dose combination of different immunomodulators represents the most promising approach in the therapy of MM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The first line of antitumor immune defense is mediated by natural killer (NK) cells, as the main effector subpopulation of the innate immune system. NK cells, unlike tumor specific T cells, are defined by their capacity to kill certain tumor-target cells without prior sensitization or MHC-restriction [1]. Aside from this, by producing IFNγ, TNFα, GM-CSF and chemokines [2], NK cells regulate adaptive T-cell mediated immune response. They are characterized by a CD3-CD16+ phenotype, CD16 (FcγRIII) being the low affinity receptor for IgG, expressed on the majority of NK cells that is involved in antibody dependent cell-mediated cytotoxicity (ADCC).
Natural killer cells are equipped with multiple activating and inhibitory cell surface receptors whose engagement by cognate ligands on target tumor cells allows NK cells to discriminate between normal and infected or transformed cells. Activating receptors include recently characterized C-dependent lectin like receptor, NKG2D, natural cytotoxicity receptors (NCR) [3–5], CD16 and CD161 with activating and inhibitory potential [6], that cooperate and play a major role in NK-cell cytotoxicity against transformed cells [7].
It has been well established that healthy cells are protected from NK-cell mediated lysis by expression of major histocompatibility (MHC) class I molecules, while malignant cells, that have during malignant transformation, lost MHC class I ligands, are susceptible to their lysis. The identification of killer cell inhibitory, i.e., immunoglobulin, receptors (KIR), type I membrane glycoproteins that are responsible for the inhibition of NK-cell mediated lysis of target cells that express defined groups of MHC class I glycoproteins, proves this “missing-self” hypothesis [8, 9].
Natural killer cell activity is down-regulated in advanced malignancies including melanoma [10–12]. Several cytokines, among them IL-2 and IFNα, have been most extensively studied. Considering that metastatic melanoma (MM) is an immunogenic tumor with poor response to chemotherapy, immunomodulating agents have been introduced in the therapy, most notably IL-2 and IFN, that affect NK cell activity, have shown therapeutic benefit [13, 14]. In MM IL-2, as a T cell growth factor and NK cell activator, offers a small but significant advantage in overall survival, whereas IFN, member of type I interferon family, also supports protective antitumor cellular immune reactions by activating NK cells, inducing proliferation and differentiation of T cells and increasing tumor antigenicity. A new biological agent, 13-cis retinoic acid (RA), that with antiproliferative, pro-differentiating and immunomodulating activity [14], when given alone or in combination with IFNα, upon binding its non-steroid nuclear receptors (RAR and RXR) has shown numerous effects, including induction of innate immune cytokines, IFNγ, IL-12 and IL-15 which augment NK cell activity [15, 16]. To date the most promising therapeutic results for MM are obtained in combined trials that aside from chemo biotherapy include low-dose immunomodulators, IFN-α, IL-2 and other agents, including RA [14, 17].
In this study we give extensive new data related to the percentage and absolute numbers in peripheral blood of CD3-CD16+CD56+ NK, CD3+CD161+ and CD3+γδ subsets and other major phenotypic characteristics of NK cells as effector cells of innate immunity, we supplement the scarce data of the expression of activating and KIR receptors on PBL and NK cells of healthy controls, and even more so, of MM patients and give comparative analysis of predictive, pretherapy, in vitro modulating potential of NK cell activity with IL-2, IFNα, RA and IFNα and RA combination for MM patients.
Materials and methods
In this study 79 patients with histologically proven MM in clinical stage IV from 24 to 69 years (37 males and 42 females) were evaluated for NK cell lysis, while of these patients, 44 patients (24 males and 20 females) in this stage, with median age of 51 years and 9 non-MM patients in clinical stage I–III, according to modified AJCC/UICC staging system, were included for other evaluated parameters. Performance status was evaluated by the ECOG Scale from 0 to 4, where grade 0 represents fully active patients, grade 1 patients restricted in physically strenuous activity, grade 2 patients unable to carry out any work activities, grade 3 patients confined to bed or chair more than 50% of waking hours and grade 4 completely disabled patients (Table 1). Fifty two healthy volunteers, age and sex matched, with no evidence of any disease or infection was evaluated for NK cell lysis and other immunological parameters. Heparinized blood samples were obtained from patients before initiation of treatment.
Peripheral blood lymphocyte isolation (PBMC)
Peripheral blood lymphocyte were isolated using Lymphoprep (Nypacon, Norway) density gradient, centrifuged at 500 g, 40 min, and washed 3 times in RPMI 1640 culture medium (CM, Gibco, UK) supplemented with 10% FCS (Sigma, USA).
Flow cytometric analysis
Surface phenotype of freshly isolated peripheral blood lymphocytes (PBL) were identified using the following combinations of directly labeled monoclonal antibodies (mAbs): CD45FITC/CD14PE (Leucogate), and lymphocyte subsets were identified using CD3PerCP/CD16FITC/CD56PE, CD3FITC/CD161PE and CD3PE/TCRγδFITC (Becton Dickinson, San Jose, USA). Also CD16FITC/CD161PE, CD16PE/CD158aFITC, CD16FITC/CD158bPE (BD Pharmingen, US) and CD16FITC/NKG2DPE subsets (R&D, US) were analyzed. The samples were prepared as previously described [18]. Briefly, 1.0 × 105 freshly isolated PBL in 100 μl RPMI 1640 supplemented with 10% FCS, were incubated for 30 min at 4°C with 20 μl of appropriate mAb combination, washed twice with ice-cold PBS and fixed with 1% paraformaldehyde prior to FACS analyses. Surface marker expression was quantified on FACSCalibur flow cytometer (Becton Dickinson, San Jose, USA). A total of 10,000–50,000 gated events verified as PBL, according to, both, their physical characteristics (FSC and SSC) and were collected per sample and analyzed using CellQUEST software. Exclusion of non-specific fluorescence was based on matched isotype mAb combinations conjugated with FITC, PE and PerCP (Becton Dickinson, San Jose, USA). In order to precisely define the expression of any receptor on CD16+ NK cell subset for each individual the Flow cytometry data of the percent of a double positive subset (e.g., CD16+/CD161+) was divided by the percent of CD16+ NK cells and multiplied by 100, according to the following formula:
as previously reported [19].
NK cell assay
Natural killer cell specific lysis was determined using standard cytotoxicity assay [20]. 100 μl of freshly isolated or in vitro stimulated PBL, as effector cells, at concentration of 4.0 × 106/ml of CM and two 1:1 dilutions, were mixed with 100 μl of the erythromyeloid cell line K562 for all healthy controls and all melanoma patients, as well as against human melanoma cell line FemX, human Burkitt lymphoma derived B-cell line, Daudi, human promyelocytic leukemia cell line HL60 and cervical carcinoma cell line, HeLa as target cells for minimum of 5–10 healthy controls and MM patients, at concentration of 0.05 × 106/ml, (prelabelled with radioactive 51Chromium (Na2CrO4, As = 3.7MBq, Amersham, UK), to form triplicates of three effector cell (E) to target cell (T) ratios (E:T), 80:1, 40:1 and 20:1. The assay was performed in 96 round bottom microwell plates (Falcon, USA) which were incubated at 37°C in a humidified atmosphere containing 5% CO2. Plates were, then centrifuged for 3 min at 200 g and the supernatant from each well was used for determination of the amount of released 51Chromium from the lysed target tumor cells in a gamma counter (Berthold, FRG) and expressed in counts per minute (cpm). The mean percent cytotoxicity was calculated using the following formula:
Maximal release was obtained by incubation of target K562 and/or FemX, Daudi, HL60 and HeLa tumor cell lines at the same concentration in the presence of 5% Triton X-100, and spontaneous release was obtained by incubation of appropriate target tumor cell line in CM, alone.
In vitro treatment of PBMC with IL-2, interferon-α, RA and IFN-α and RA combination
Peripheral blood lymphocyte isolated from MM patients and healthy controls were cultivated for 18 h in RPMI 1640 CM, alone, CM supplemented with IL-2 200 U /ml (BD Pharmingen, US), IFN 250 IU/ml (Sigma, US), RA (10−6 M) (Sigma, US) and their combination, IFN and RA, in 6-well plates (Nunc, Danmark) at 37°C and 5% CO2 in humid atmosphere. After incubation, NK cell cytotoxicity was determined by the 51-chromium-release assay using K562 and FemX as tumor target cell lines.
Results
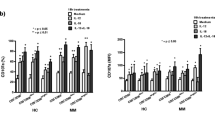
The evaluation of NK cell activity of freshly isolated PBL of patients with early (I–III) and metastatic, (IV) clinical stage of melanoma, prior to therapy shows that MM patients have significantly impaired NK cell activity compared to healthy controls (31.26 ± 3.69% vs. 43.06 ± 4.86 respectively, p < 0.05, Man–Whitney test) evaluated against K562 tumor target cell line. Melanoma patients in early clinical stage do not show significant difference in NK cell activity compared to healthy controls (36.12 ± 2.69%) (Fig. 1a).
(a) NK cell specific lysis of healthy controls, non-MM patients (clinical stage I–III, n = 9) and MM patients (n = 79) evaluated against K562 tumor target cell line (E:T, 80:1) shows significant (p > 0.05) impairment in clinical stage IV; (b) NK cell specific lysis of MM patients and healthy controls against K562, FemX and Daudi cell lines, unlike HL60 and HeLa target tumor cell lines (E:T, 80:1) shows a significant decrease; (c) the percentage of subsets with CD161 and NKG2D receptor expression in PBL shows a significant decrease in the CD161+ subset, only in MM patients; (d) the relative percentage of CD16+ subsets with CD161 and NKG2D receptor expression shows a significant decrease in MM patients compared to healthy controls
Evaluation of NK cell lysis of MM patients against different tumor targets, including standard NK-sensitive, K562 cell line, human melanoma FemX cell line, NK-resistant Daudi cell line, HL60 and HeLa tumor cell lines, shows that, compared to healthy controls, it is, for MM patients, as shown in Fig. 1, significantly decreased against K562 tumor cell line. NK cell lysis against NK-resistant Daudi target cell line has the same trend as for K562 but on a significantly lower level for both controls and MM patients. NK cell lysis against melanoma FemX cell line is even more impaired, although, unlike against HeLa and HL 60 target tumor cell lines, there is a small difference between controls and MM patients (Fig. 1b).
The expression of CD161 receptor on freshly isolated PBL of MM patients was significantly lower than in controls (10.66 ± 1.06% vs. 14.07 ± 1.51%, respectively, p < 0.05) (Fig. 1c). However, we also show that CD161 expression on CD16+ NK cell subset, only, in MM patients (26.18 ± 3.42%) in comparison to healthy controls (39.41 ± 5.60%) is significantly lower (p < 0.05, Man–Whitney test) (Fig. 1d). Furthermore, we show, a significant decrease in the expression of NKG2D activating receptor on the population of CD16+ NK cells in MM patients (66.98 ± 7.61%), compared to healthy controls (92.72 ± 1.52%), (p < 0.05, Man–Whitney test), while there was no significant difference between MM patients and healthy controls in the expression of this activating receptor when analyzed in the entire PBL population (39.00 ± 3.95%, vs. 43.2 ± 2.66%, respectively) (Fig. 1c). The relative percent of the expression of these receptors was obtained by calculation from Flow cytometry dot plot data as described in Material and methods. Given representative Flow cytometry dot plots show decreased expression of CD161 (Fig. 2b) and NKG2D on CD16+ NK cells (Fig. 2d) of an MM patient compared to a healthy control (Fig. 2a, c).
In this study we give new data related to KIR expression in MM patients, as well as healthy controls. We show that in PBL or CD16+ NK cells there is no significant difference between MM patients and controls in the expression of KIR receptor CD158a (2.83 ± 0.47% vs. 1.76 ± 0.37% in PBL, and 11.77 ± 1.77% vs. 7.78 ± 2.21% on CD16+NK cell subset) and CD158b (6.10 ± 0.73% vs. 8.12 ± 1.19% in PBL and 32.15 ± 2.58% vs. 37.79 ± 3.66% on CD16+NK cell subset) (Fig. 3a, b).
Analyses of the percentage of the subsets with CD158a and CD158b receptor-expression in PBL does not show a significant change in MM patients compared to healthy controls (a); the relative percentage of CD16+ subset with CD158a and CD158b receptor expression, also, does not show a significant change in MM patients compared to healthy controls (b)
Also, regarding the density of expression of CD16 we give novel data that show a significant difference in the distribution of CD16bright and CD16dim NK cell subpopulations between MM patients and healthy controls. The percentage of CD16bright NK cells was significantly lower (Median = 66.85) in MM patients compared to controls (Median = 75.45), whereas, the percentage of CD16dim NK cells was significantly higher (Median = 41.06) in MM patients than in controls (Median = 24.18), analyzed by non-parametric Man–Whitney U test (Fig. 4a). The disturbance of the ratio of expression of CD16dim and CD16bright NK cell subpopulations is illustrated by representative flow cytometry histograms for a healthy control and MM patient (Fig. 4b and c, respectively).
(a) The expression of CD16bright NK cell subset is significantly lower, while CD16dim NK cell subset is significantly higher in MM patients compared to controls. Representative Flow cytometry histograms show a significantly lower percentage of CD16bright NK cell subset in an MM patient (c) compared to a control (b)
Obtained immunomodulation of NK cell lysis of MM patients (n = 7) with IL-2, IFNα, RA, RA and IFNα after an 18 h in vitro treatment, compared to control cultures with medium alone, was evaluated by using two different tumor target cell lines, i.e., standard K562 and a human melanoma, FemX, cell line. The obtained results show that applied immunomodulating agents, with the exception of RA, enhance NK cell cytotoxicity. The treatment-induced NK cell lysis is greater when FemX was used as tumor target cell line, in comparison to K562, for the treatments with IL-2 and IFN-α, with IL-2, of all the applied immunostimulating agents, giving the greatest enhancement of NK cell cytotoxicity (Fig. 5).
Evaluation of immunomodulating effect of IFN-α, RA, RA and IFN-α and IL-2, compared to untreated cultures, on NK cell specific lysis of MM patients after 18 h in vitro treatments of PBL shows that, by using two different tumor target cell lines, K562 and FemX, a melanoma tumor cell line, IFN-α and IL-2 give greater enhancement of NK cell lysis against FemX target cell line, in comparison to K562
The evaluation of the distribution of relative and absolute values for the CD3-CD16+CD56+ NK cell subset in peripheral blood of healthy controls and MM patients, does not show significant difference in the relative percent, however the average absolute value per liter in peripheral blood is, due to general lymphopenia in these patients, significantly lower than for healthy controls (Table 2). Although, the relative percent of CD3+CD161+ subset is significantly lower and CD3+γδ subset is higher in MM patients compared to healthy controls, the absolute numbers of these two subsets are lower in investigated patients than in healthy controls. Additional data regarding CD16dim and CD16bright subset distribution shows that in absolute numbers, CD16dim subset is somewhat smaller in MM patients than in controls, and the absolute number for the significantly smaller percent of CD16bright subset is also reflected in a smaller absolute number in peripheral blood. Although the relative numbers of populations of CD161, NKG2D, CD158a and CD158b positive cells given in Figs. 1c and 3a did not differ from controls, we show new data that the absolute numbers of these populations per liter of peripheral blood are much lower, except for CD158a, than in healthy controls (Table 2).
Discussion
It has been shown that patients with malignancies, especially in advanced clinical stage, have suppression of various types of immune response [21, 22]. Tumor-associated impairment in the function of NK cells may be the consequence of their reduced number, dysbalance in their activating and inhibitory receptor repertoire [23], as well as dysregulation of the cytotoxic machinery caused by the prevalence of immunosuppressive cytokines, IL-10 and TGFβ, which, together with numerous other inhibitory factors produced by tumor cells [24, 25], disturb their activation and function. In this sense, we show in this study that there is prior to therapy a significant impairment, compared to controls, of NK cell lysis in MM patients, whereas, NK cell lysis of patients in early, clinical stage I–III did not differ significantly from controls.
Additional evaluation of NK cell lysis of MM patients and controls against the standard NK-sensitive, K562 cell line compared to a standard NK-resistant, Daudi, cell line, shows the same trend but on a much lower level of a significantly decreased NK lysis in MM patients. As Daudi cell line is NK-resistant and LAK and ADCC susceptible, it was used as negative control in order to exclude the contribution of LAK or ADCC to the observed cytotoxicity [26].
Natural killer cell lysis of the most relevant, human melanoma, FemX, cell line shows even greater impairment of NK cell lysis than against the NK-resistant, Daudi cell line for both healthy controls and melanoma patients [27]. The resistance of this melanoma cell line may be the consequence of either a lack of expression of stress proteins characteristic for malignantly transformed cells, such as stress-inducible ligands MICA/B that activate NKG2D receptors or sustained expression of HLA class I molecules that by binding KIR, down-regulate NK cell-mediated lysis.
Additional data related to NK cell lysis of different tumor target cell lines, included HeLa, human cervical carcinoma, that, as most epithelial tumors, expresses MICA/B ligands that should up-regulate NK cell activity, and a promyelocytic leukemia, HL60 cell line that in leukemia-related subdivision to NK cell-susceptibility, can be NK-insensitive, sensitive or highly sensitive [28]. Despite these data and their unrelated origin, our data show both HeLa and HL60 cell lines the lowest susceptibility to NK lysis by either healthy controls or MM patients.
New evidence given in this study showing that the percentage of CD3-CD16+CD56+ NK cells in peripheral blood of investigated MM patients does not differ from normal controls, would suggest that the impairment in their function is not the consequence of a decrease in their number [29, 30] in peripheral blood. However, if the blood count of patients is used to calculate from the percentage of this subset the absolute number of CD3-CD16+CD56+ NK cell per liter of blood it was found that MM patients have significantly reduced number of NK cells (Table 2).
Even though it has been established that the activity of NK cells is determined by the balance of positive and negative signals that they receive through different types of receptors on their cell membrane [31], there are only few reports, up to now, that give data concerning the expression of several activating NK cell receptors. In this study we show for the first time that MM patients, prior to therapy, have a significant decrease in the expression of CD161, compared to controls. The few reports that deal with CD161 receptor expression are generally in healthy individuals or in certain hematological malignancies and give data in a very diverse manner, from the percent of CD161 receptor on the entire population of PBL, to its expression on CD3-, CD16+ or CD56+ subpopulations. To this effect, some reports, like ours, in dealing with NK cell subpopulation state in the CD3- subset the presence of CD161 on 19% of cells in healthy controls [32]. However, reports that average positivity of CD3-CD56+ NK cells for CD161 is 72% [30], is not in agreement with our extensive data for healthy controls.
Furthermore, in this study the evaluation of the expression of activating NK cell receptor of the newer generation, NKG2D, also shows, for the first time, that MM patients have a significant decrease, compared to controls, of NKG2D on CD16+ cells. As NKG2D is a relatively new NK cell receptor most of the existing reports deal with basic data pertaining to its structure and function in rodent and human NK cells and lines [4, 33], as well as with its putative ligands in animal tumors or characterized cell lines [34, 35]. However, aside from individual reports, such as the one dealing with its expression in a T cell subset [36], there are scarce reports of its expression in any innate immunity cell subpopulation in cancer patient. NKG2D activates the cytotoxic mechanism by recognition of stress proteins expressed on malignantly transformed cells, such as MHC-class-I-related molecules MICA and MICB and the ULBP1-4 proteins [37, 38]. Impairment of NK cell activity in patients with lung and colorectal cancer has been associated with down-regulation of NKG2D receptors on NK cells induced by elevated plasma levels of immunosuppressive cytokine, TGFβ [24], or by its soluble tumor-derived MIC ligands [39]. The significantly reduced expression of the analyzed receptors, CD161 and activating NKG2D, on CD16+ NK cells in investigated melanoma patients represents new data that may be associated with the shown serious impairment in the cytotoxic function of their NK cells.
In this study we also give new data for MM patients concerning NK cell expression of KIR receptors, CD158a (KIR 2DL1) and CD158b (KIR 2DL2,3), belonging to the killer cell Ig-like receptor superfamily, which upon recognition of appropriate HLA alleles (HLA-C group 1; Cw2, 4, 5, 6 and HLA-C group 2; Cw1, 3, 7, 8, respectively) on tumor cells, generally, negatively regulate NK cell activity [40]. As the reduction in KIR receptors is associated with NK cell positive regulation and proliferation [41], we report for the first time that, unlike down-regulation of NK cell activating NKG2D receptors shown in this study, there is no significant change in these patients in the percentage of KIR receptor expression, i.e., CD158a (mAb 158a recognizes inhibitory CD158a-KIR2DL1 and activating CD158h-KIR2DS1 receptors) and CD158b (mAb 158b recognizes inhibitory CD158b1/b2-KIR2DL2/3 and activating CD158j-KIR2DS2 receptors) compared to controls in either PBL or CD16+NK cells (Fig. 3). Absolute number of CD158a and CD158b positive cells in peripheral blood of MM patients shows that there is, actually, a significant decrease in CD158b positive cells in PBL of the patients (Table 2). The significance of this finding is to be elucidated, as the biology of KIR receptor expression is still being investigated and implies receptor calibration dependent on individual HLA class I repertoire expression, as well as its influence on down-regulation of activating receptor expression [42].
Our evaluation performed on a larger number of investigated healthy individuals is in agreement with previously published data of the expression of CD158a and CD158b on CD16+NK cells, although only for healthy individuals [30, 43, 44]. The few reports on KIR in melanoma investigated the influence of HLA allotypes in melanoma cell lines on inhibition of NK cell lysis and suggest that disrupting interaction of KIR with their ligands on tumor cells in vivo may enhance antitumor response mediated by both innate and adaptive immune effector cells [45].
As the prototypic antigens that define NK cell subset are CD16 and CD56, in this study we analyzed the expression of CD16, the low-affinity FcγRIII present on the surface of NK cells which binds to antibody (IgG1) coated targets and allows NK cell triggering during ADCC. CD16 is present on almost the entire subset of NK cells [8, 46]. It has been shown that CD16 is not only involved in ADCC but also in direct (non-antibody mediated) target cell recognition and killing [46, 47]. According to the relative surface density of CD16 antigen, two subpopulations have been defined, of CD16dim and CD16bright [46]. These CD16+ subsets are clearly distinct in their functional capacity in performing NK and ADCC activity. As there have been, so far, only few reports dealing with the extent and characteristics of CD16 antigen density of NK cells in humans, we show that in healthy controls, according to the density of CD16 expression, the CD16dim NK cell subpopulation represents 24.18% (median) and CD16bright subpopulation represents 75.45% (median) of NK cells, which is in accord with one former report [46]. Furthermore, we give for the first time evidence that in MM patients there is a significant shift in the ratio of these two NK cell subsets, and 41.06% (median) are CD16dim NK cells and 66.85% (median) of NK cells are CD16bright. As it has been shown by phenotypic characterization that increasing level of CD16 expression is associated with NK cell maturation, these interesting novel results may indicate a decrease in the more mature and cytolytically potent, CD16bright NK cell subset, that may contribute to the impaired NK cell cytotoxicity found in these patients. Additional data show that disturbed ratio of CD16dim and CD16bright subsets is maintained in absolute numbers in PBL of MM patients, and that owing to lymphopenia, the absolute number of the two CD16+ subsets is below that for healthy controls.
As this study, aside from pretherapy evaluation of the activity and characteristics of NK cells of MM patients, also includes investigation of predictive effect of 18 h in vitro treatments on PBL with IL-2, IFN, RA and combination of RA and IFN on NK cell lysis assayed against the standard sensitive K562, as well as a human melanoma, FemX, tumor target cell line. Obtained immunomodulation of NK cell lysis, compared to control cultures with medium alone, of MM patients shows that all applied immunomodulating agents, except RA, enhance NK cell cytotoxicity. However, IL-2 and IFN treatment-enhanced NK cell lysis show greater effect against FemX target cell line than against K562, with IL-2, of all the applied immunostimulating agents, giving the greatest enhancement of NK cell lysis [48, 49].
In this study we give extensive novel data related to the expression of NK cell activating and KIR receptors in PBL and NK cells in MM patients and healthy controls and show, for the first time, that the impairment of NK cell activity in MM is associated with decreased expression of CD161 and activating receptor NKG2D, on freshly isolated NK cells. Furthermore, we show disturbance in the ratio of CD16dim and CD16bright NK subsets in investigated MM patients that may, also, contribute to impaired NK cell tumor cytotoxicity. As immunotherapeutic agents are applied in the treatment of melanoma in order to enhance antitumor immune response, we give comparative analyses of the degree of predictive in vitro potentiation of NK cell specific lysis with IL-2, IFNα, and IFNα together with RA, and RA alone, against K562 and melanoma cell line, FemX, that can possibly be of help in the design and development of therapeutic regimens, considering that new data shows that low-dose combination of different immunomodulators represents the most promising approach in the therapy of MM.
Abbreviations
- NK:
-
Natural killer cells
- IL-2:
-
Interleukin-2
- RA:
-
13-cis retinoic acid
- FITC:
-
Fluorescein isothyocyanate
- PE:
-
Phycoerythrine
- KIR:
-
Killer immunoglobulin-like receptors
References
Kiessling R, Klein E, Wigzell H (1975) “Natural” killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol 5(2):112–117
Biron CA, Nguyen KB, Pien GC et al (1999) Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol 17:189–220
Lanier LL (1998) NK cell receptors. Annu Rev Immunol 16:359
Biassoni R, Cantoni C, Marras D et al (2003) Human natural killer cell receptors: insights into their molecular function and structure. J Cell Mol Med 7:376–387
Smyth MJ, Swann J, Kelly JM et al (2004) NKG2D recognition and perforin effector function mediate effective cytokine immunotherapy of cancer. J Exp Med 200(10):1325–1335
Rosen DB, Bettadapura J, Alsharifi M et al (2005) Cutting edge: lectin-like transcript-1 is a ligand for the inhibitory human NKR-P1A receptor. J Immunol 175(12):7796–7799
Moretta A, Bottino C, Mingari MC et al (2002) What is a natural killer cell? Nat Immunol 3:6–8
Cooper MA, Fehniger TA, Caligiuri MA (2001) The biology of human natural killer-cell subsets. Trends Immunol 22(11):633–640
Costello RT, Sivori S, Marcenaro E et al (2002) Defective expression and function of natural killer cell-triggering receptors in patients with acute myeloid leukemia. Blood 99(10):3661–3667
Konjevic G, Jurisic V, Banicevic B et al (1999) The difference in NK-cell activity between patients with non-Hodgkin’s lymphomas and Hodgkin’s disease. Br J Haematol 104(1):144–151
Konjevic G, Jovic V, Jurisic V et al (2003) IL-2-mediated augmentation of NK-cell activity and activation antigen expression on NK- and T-cell subsets in patients with metastatic melanoma treated with interferon-alpha and DTIC. Clin Exp Metastasis 20(7):647–655
Garbe C, Eigentler TK (2004) [Therapy of malignant melanoma at the stage of distant metastasis]. Hautarzt 55(2):195–213
Tarhini AA, Agarwala SS (2005) Interleukin-2 for the treatment of melanoma. Curr Opin Investig Drugs 6(12):1234–1239
Rosenthal MA, Oratz R (1998) Phase II clinical trial of recombinant alpha 2b interferon and 13 cis retinoic acid in patients with metastatic melanoma. Am J Clin Oncol 21(4):352–354
Sidell N, Famatiga E, Shau H et al (1985) Immunological aspects of retinoids in humans. III. Effects of retinoic acid on the natural killing of tumor cells. J Biol Response Mod 4(3):240–250
Labarriere N, Piau JP, Zennadi R et al (1993) Retinoic acid modulation of alpha (1,2) fucosyltransferase activity and sensitivity of tumor cells to LAK-mediated cytotoxicity. In Vitro Cell Dev Biol 29A(2):140–144
Weber RW, O’Day S, Rose M et al (2005) Low-dose outpatient chemobiotherapy with temozolomide, granulocyte-macrophage colony stimulating factor, interferon-alpha2b, and recombinant interleukin-2 for the treatment of metastatic melanoma. J Clin Oncol 23(35):8992–9000
Jackson A., Warner N (1986) Preparation, staining and analysis by flow cytometry of peripheral blood leukocytes. In: Rose N, Friedmah H, Fahey J (eds) Manual of clinical laboratory immunology, 3rd edn. American Society for Microbiology, Washington DC, pp 226–335
Konjevic G, Jovic V, Radulovic S et al (2001) Therapeutic implications of the kinetics of immunomodulation during single or combined treatment of melanoma patients with dacarbazine and interferon-alpha. Neoplasma 48(3):175–181
Brown RL, Ortaldo JR, Griffith RL et al (1985) The proliferation and function of human mononuclear leukocytes and natural killer cells in serum-free medium. J Immunol Methods 81(2):207–214
Robertson MJ, Ritz J (1990) Biology and clinical relevance of human natural killer cells. Blood 7:2421–2438
Konjevic G, Jurisic V, Spuzic I (2001) Association of NK cell dysfunction with changes in LDH characteristics of peripheral blood lymphocytes (PBL) in breast cancer patients. Breast Cancer Res Treat 66(3):255–263
Sun PD (2003) Structure and function of natural-killer-cell receptors. Immunol Res 27:539–548
Lee JC, Lee KM, Kim DW et al (2004) Elevated TGF-beta1 secretion and down-modulation of NKG2D underlies impaired NK cytotoxicity in cancer patients J Immunol 172(12):7335–7340
Konjevic G, Spuzic I (1997) Investigation of some factors that modulate the activity of NK cells. In: Lukic M, Colic M, Mostarica-Stojkovic M, Cuperlovic K (eds) Immunoregulation in health and disease. Academic Press, London pp 449–455
Seidel MG, Freissmuth M, Pehamberger H et al (1998) Stimulation of natural killer activity in peripheral blood lymphocytes of healthy donors and melanoma patients in vitro: synergism between interleukin (IL)-12 and IL-15 or IL-12 and IL-2. Naunyn Schmiedebergs Arch Pharmacol 358(3):382–389
Pende D, Parolini S, Pessino A et al (1999) Identification and molecular characterization of NKp30, a novel triggering receptor involved in natural cytotoxicity mediated by human natural killer cells. J Exp Med 190:1505–1516
Yan Y, Steinherz P, Klingemann HG et al (1998) Antileukemia activity of a natural killer cell line against human leukemias. Clin Cancer Res 4(11):2859–2868
Jovic V, Konjevic G, Radulovic S et al (2001) Impaired perforin-dependent NK cell cytotoxicity and proliferative activity of peripheral blood T cells is associated with metastatic melanoma. Tumori 87(5):324–329
Pascal V, Schleinitz N, Brunet C et al (2004) Comparative analysis of NK cell subset distribution in normal and lymphoproliferative disease of granular lymphocyte conditions. Eur J Immunol 34:2930–2940
Moretta A, Bottino C, Vitale M et al (2001) Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu Rev Immunol 19:197–223
Dunne J, Lynch S, O’Farrelly C et al (2001) Selective expansion and partial activation of human NK cells and NK receptor-positive T cells by IL-2 and IL-15. J Immunol 167:3129–3138
Andre P, Castriconi R, Espeli M et al (2004) Comparative analysis of human NK cell activation induced by NKG2D and natural cytotoxicity receptors. Eur J Immunol 34:961–971
Bauer S, Groh V, Wu J et al (1999) Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science 285:727–729
Pende D, Cantoni C, Rivera P et al (2001) Role of NKG2D in tumor cell lysis mediated by human NK cells: cooperation with natural cytotoxicity receptors and capability of recognizing tumors of nonepithelial origin. Eur J Immunol 31:1076–1086
Guerra N, Guillard M, Angevin E et al (2000) Killer inhibitory receptor (CD158b) modulates the lytic activity of tumor-specific T lymphocytes infiltrating renal cell carcinomas. Blood 95(9):2883–2889
Cosman D, Mullberg J, Sutherland CL et al (2001) ULBPs, novel MHC class I-related molecules, binds to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity 14:123–133
Sutherland CL, Chalupny NJ, Schooley K et al (2002) UL16-binding proteins, novel MHC class I-related proteins, binds to NKG2D and activates multiple signaling pathways in primary NK cells. J Immunol 168:671–679
Groh V, Wu J, Yee C et al (2002) Tumor-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature 419(6908):734–738
Gomez-Lozano N, Estefania E, Williams F et al (2005) The silent KIR3DP1 gene (CD158c) is transcribed and might encode a secreted receptor in a minority of humans, in whom the KIR3DP1, KIR2DL4 and KIR3DL1/KIR3DS1 genes are duplicated. Eur J Immunol 35:16–24
Vitale M, Della Chiesa M, Carlomango S et al (2004) The small subset of CD56bright CD16- natural killer cells is selectively responsible for both cell proliferation and interferon-γ production upon inactivation with dendritic cells. Eur J Immunol 34:1715–1722
Huard B, Karlsson L, Triebel F (2001) KIR down-regulation on NK cells is associated with down-regulation of activating receptors and NK cell inactivation. Eur J Immunol 31:1728–1735
Kogure T, Fujinaga H, Niizawa A et al (1999) Killer-cell inhibitory receptors, CD158a/b, are upregulated by interleukin-2, but not interferon-gamma or interleukin-4. Mediators Inflamm 8:313–318
Kogure T, Mantani N, Goto H et al (2002) The effect of interleukin-15 on the expression of killer-cell immunoglobulin-like receptors on peripheral natural killer cells in human. Mediators Inflamm 11:219–224
Bakker AB, Phillips JH, Figdor CG et al (1998) Killer cell inhibitory receptors for MHC class I molecules regulate lysis of melanoma cells mediated by NK cells, gamma delta T cells, and antigen-specific CTL. J Immunol 160:5239–5245
Nagler A, Lanier LL, Cwirla S et al (1989) Comparative studies of human FcRIII-positive and negative natural killer cells. J Immunol 143(10):3183–3191
Igarashi T, Wynberg J, Srinivasan R et al (2004) Enhanced cytotoxicity of allogeneic NK cells with killer immunoglobulin-like receptor ligand incompatibility against melanoma and renal cell carcinoma cells. Blood 104:170–177
Ortaldo JR, Mason A, Overton R (1986) Lymphokine-activated killer cells. Analysis of progenitors and effectors. J Exp Med 164(4):1193–1205
Kirkwood JM, Richards T, Zarour HM et al (2002) Immunomodulatory effects of high-dose and low-dose interferon alpha2b in patients with high-risk resected melanoma: the E2690 laboratory corollary of intergroup adjuvant trial E1690. Cancer 95(5):1101–1112
Acknowledgment
This work was supported by the grant of the Ministry of Science and Technology of the Republic of Serbia, number 1602. We wish to thank Mrs. Jasna Popovic Basic and Mrs. Mirjana Culafic for help and excellent technical work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Konjević, G., Mirjačić Martinović, K., Vuletić, A. et al. Low expression of CD161 and NKG2D activating NK receptor is associated with impaired NK cell cytotoxicity in metastatic melanoma patients. Clin Exp Metastasis 24, 1–11 (2007). https://doi.org/10.1007/s10585-006-9043-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-006-9043-9