Abstract
Background and aims
Determining which strategies confer a competitive advantage in variable environments with strong short-term resource fluctuations (i.e., seasonal drought and flooding) is critical for understanding ecosystem structure and function, and predicting how ecosystems will respond to global change. Here, we ask how functional traits of plant species vary in response to drought and flooding and whether trait plasticity is a better predictor of plants’ cover than average functional trait values.
Methods
We transplanted vegetative recruits (similar size and age) of eight common species from a moderately grazed alpine wetland in the Eastern Tibetan Plateau to a common garden where we evaluated the trait responses of these species to drought and flooding. We also measured the coverage, biomass, and height of these plant species in the field communities.
Results
The leaf morphological and physiological traits of these eight species generally showed different responses to drought and flood conditions. Variation (plasticity) of leaf morphological or physiological traits rather than average values of these traits were better predictors of cover and biomass in the field community. Species with lower plasticity in leaf morphological traits had higher plasticity in leaf physiology and the higher coverage and biomass in the field community.
Conclusions
Our results suggest that the ability to quickly adjust leaf physiology to changing environmental conditions provides an advantage in this ecosystem, where intra-annual rainfall variability is high and thus resource fluctuations are pulsed in time. In contrast, greater functional trait plasticity in leaf morphology was a disadvantage, possibly because the benefits of these adjustments did not outweigh their initial costs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plant functional traits, such as leaf morphology and leaf-level gas exchange, influence species’ survival, growth, reproduction (Sultan et al. 1998; Valladares et al. 2000; Wright et al. 2004, 2005) and fitness (Ackerly 2003; Poorter et al. 2008), as well as ecosystem functioning (Lavorel and Garnier 2002; Chapin 2003; Dìaz et al. 2004). For example, studies that investigated a range of tree species found that the functional traits varied among tree species and could largely explain the variation of growth and survival of these species (Poorter et al. 2008). Furthermore, leaf morphological and gas exchange traits are widely used to measure the response of plants to different environmental conditions and can be indicative of growth strategy, carbon economy, resource exploitation and conservation (Reich et al. 1998a; Reich et al. 1998b; Westoby et al. 2002; Pywell et al. 2003; Wright et al. 2004; Poorter and Bongers 2006; Violle et al. 2007; Pierce et al. 2013; Lopez-Iglesias et al. 2014). Many of these studies, however, have focused on average trait values (Reich et al. 1998b; Pywell et al. 2003; Poorter and Bongers 2006; Lopez-Iglesias et al. 2014), rather than “trait plasticity”, even though plasticity is argued to be an important component of plant strategies (Grime and Mackey 2002). In this study, we determined the relative influence of both average trait values and their plasticity in predicting plant cover and biomass.
Many individuals and communities face variable environmental conditions with disturbances and resource fluctuations (Jonas et al. 2008). Therefore, the survival and competitive ability of plants (e.g. achieving a larger population size, cover, or biomass) is often influenced by their capacity to cope with fluctuating environmental conditions (Martina and von Ende 2012). Plasticity in morphology and physiology is a common strategy for maintaining growth despite environmental variation, such as temporal variability in resource availability or stress throughout a plants life cycle (Sultan 2003; Richards et al. 2006; Valladares et al. 2007; Nicotra et al. 2010). Responding to changing environments by altering morphology and physiology may improve the plant performance and even contribute to plant invasion of new environments (e.g., Grime and Mackey 2002; Nicotra et al. 2010; Warren and Lake 2012), although other studies have found that trait plasticity is not necessarily related to plant performance (e.g., Davidson et al. 2011).
The variation of plant functional traits in response to different environmental conditions has long been considered to be an important part of plant ecology (Funk et al. 2007). Despite this, quantitative evaluation of the relationship between variation of functional traits and the community level cover or biomass of certain species is still rare. Some studies have compared the variation of traits between different species (Reich et al. 1991), between different treatments (Nicotra et al. 2007), even between species from different habitats (Kudo and Hirao 2006), but a better understanding of the linkages between leaf-level traits and patterns at the plant, community, and ecosystem scales is still needed (Lavorel and Garnier 2002; Suding et al. 2008). A common method to link species’ traits with community performance is to use the measured characteristics of plants under different controlled conditions to relate their abundance in naturally occurring plant communities (Grime and Hunt 1975).
This study was conducted on the Tibetan Plateau, a region that functions as the “water tower of Asia” and greatly affects the hydrological cycling and climate dynamics of southern and eastern Asia (Hao 2008). The wetlands of the Tibetan Plateau play an important role in maintaining biodiversity, ecosystem productivity, and water storage/quality (Nie and Li 2011). These wetlands are now threatened by climate change, overgrazing, agricultural expansion, and other local impacts from a growing population (Du et al. 2004; Kang et al. 2010; Chen et al. 2013). For example, many wetlands have ¾come drier with seasonal drought between rain events, although they are still occasionally flooded by the heavy rainfall events (Xiang et al. 2009; Ma et al. 2011). Like many other grasslands, threats to this ecosystem may become amplified under forecasted climate changes, with greater risks of both drought and floods (e.g. Knapp et al. 2002, 2008). Droughts and floods could filter species by the average traits and/or trait plasticity. To our knowledge, no studies have examined the plant community responses to varying water availability in these wetlands in eastern Tibetan Plateau, despite their social and ecological importance.
Using a range of species from moderately degraded wetlands in eastern Tibetan Plateau, we quantified species’ cover and biomass at the community level with functional trait means and plasticity in response to treatments of drought and flooding. This experimental framework allowed us to: 1) determine the response of species’ functional traits to drought and flooding conditions; 2) evaluate the extent to which the values of functional traits and their plasticity explain the plants’ cover and biomass at the community level. Based on results from these sections, we propose that plant physiological plasticity improves plants cover and biomass in this ecosystem with high intra-annual rainfall variability, whereas morphological plasticity may actually be a disadvantage under these same conditions.
Materials and methods
Study site and species
The study site was located at the Alpine Meadow and Wetland Ecosystems Research Station of Lanzhou University in the eastern Tibetan Plateau (33°39′N, 101°53′E, elevation 3660 m asl.), China. The mean annual temperature is 1.2 °C and the mean annual precipitation (calculated using annual data from 1975 to 2010) was 620 mm, falling mostly during the short and cool summer (from June to August) (Niu et al. 2014). Intra-annual rainfall variability at this site is relatively large (Fig. 1). Using monthly precipitation data from 2002 to 2015 (the best data available for this site), the standard deviation of precipitation during the growing season is 50 mm. Over the 14-years of data, it was common for a year to have at least one month with <30 mm of precipitation and 1–2 months where rainfall was at least twice the rainfall in other months. The soil texture is sandy loam and classified as alpine meadow soil (Gong 1999). The plant community type is mainly meadow and is affected by potential degradation by intense grazing in recent decades (Yu et al. 2012).
A typical moderately degraded alpine wetland was selected (20 m × 20 m), where the condition of flooding and drought alternatively occurred during the growing season. Eight common species from different genera and five families were selected from this wetland (Table 1). All species are perennial herbaceous plants with only the belowground gemmae surviving through the winter. We selected these species because each has a relative coverage of >2 % in the community and could be found in all quadrats during our field surveys. Furthermore, the study species vary in their performance (i.e, relative biomass, relative coverage and mean of height). Together, these selected species account for a large portion of total biomass (83.6 %) and coverage (80.0 %) (see Table 1 for details).
Experiment design
In early May (from 1st to 6th) 2012, individuals of the eight selected species of similar size and age (2–4 years old, determined by age tags), with only one or two small leaves had emerged, were transplanted from the wetland to a common garden at the Alpine Meadow and Wetland Ecosystems Research Station. These individual plants were then repotted to plastic pots (diameter of 20 cm at top and 18 cm at the bottom, height of 14 cm). 40 plants (one per pot) were transplanted for each species. The pots were then filled with sieved and mixed soil from the same site and placed under a plastic-covered shelter with metal supports to prevent the pots from receiving natural rainfall. The shelter allowed adequate solar radiation for growth and good air circulation from the outer environment; measurements of air temperature, relative humility, and irradiance indicate ambient environmental conditions for plant growth in the region (Appendix Table 3). A plastic sheet below prevented the plants from accessing moisture from the ground.
Two weeks after transplanting, the plants were assigned to two water treatments: drought and flooding. The four least healthy plants per species were excluded and the remaining 36 pots (18 pots per treatment, per species) were randomly allocated to each treatment in three blocks with a randomized block design. Pots were placed on plastic sheets, which were laid on the ground with the edges fixed just above ground level. The flooding treatment was irrigated every third day with adequate water to keep the water level at about 8–10 cm height around the pots, keeping the soil always saturated. The drought treatment was irrigated with less water (300 ml per pot) every third day, resulting in relatively dry soil (Appendix Table 4). Measurements of plant photosynthesis indicate that plants in the natural community experience moderate water stress periodically throughout the growing season.
Gas exchange measurements
Leaf gas exchange for each species was measured for one fully expanded healthy leaf in each of ten plants (randomly selected) for each species under each treatment. The measurements were conducted between 10:00 am and 12:30 pm during sunny clear days from July 18th to 21st (at the peak of growing season and about eight weeks after water treatments began) using a GSF-3000 portable photosynthesis system (GFS-3000, Heinz Walz, Germany). The measurements were taken at a constant leaf temperature of 23 °C and PAR (Photosynthetic active radiation) of 1800 μmol m−2 s−1, which is equal to the average light intensity of sunny days during the measurement. The CO2 concentration (about 340 ppm) was the same as the ambient concentration, and relative humidity was between 50 % and 65 % (see Appendix Table 3). The measured data were logged when the values of net photosynthesis rate became stable, which typically took about three minutes after the leaf was put into the leaf chamber.
Leaf morphology
After gas exchange measurements, another fully expanded leaf was randomly selected from ten plants per species per treatment at noon on sunny days and stored in sealed plastic bags with moist paper towel inside for less than three hours before measurements. We watered plants every three days in both the drought and flooding treatments, and selected leaves for leaf morphology measurements 1.5 days after watering to avoid temporary effects of recent watering. The fresh leaf was weighed, scanned (300dpi, EPSON 1670, Seiko Epson Corporation, Japan), then dried at 80 °C for 48 h and reweighed. The area of each leaf was calculated using Image J (Abràmoff et al. 2004). For each leaf, we calculated the specific leaf area (SLA = area/dry weight, m2 g−1), leaf thickness (measured using a digital caliper to the nearest, mm), leaf area (cm2), and relative leaf water content (RLWC = (fresh weight-dry weight) /fresh weight, %).
Vegetation survey in field communities
In late August, we established thirty-two randomly located quadrates (0.5 m × 0.5 m) in an area of 400 m × 400 m near the site where the propagules for the potted plants originated. Quadrates placement followed a systematic sampling design along two transects (25 m apart from each transects and at least 10 m apart from each quadrate). The height and coverage of the eight studied species in each quadrat was recorded. All aboveground green biomass was clipped down to the ground, sorted by species, and then oven dried at 80 °C for 48 h and weighed. The relative biomass and coverage were calculated based on the mean values per species across the quadrates (i. e. the mean biomass of a species divided by the average total biomass per plot). Variation in biomass and coverage between the different quadrats are presented in Appendix Table 5.
Data analysis
The effects of treatments and species and their interaction on the traits were analyzed by two-way ANOVA with block as random factor. The difference between the treatments for given species was compared using ANOVA (at P = 0.05). The coefficient of variation (CV, standard deviation/mean) of each trait across the treatments was calculated for each species as a proxy of the phenotypic plasticity (Nicotra et al. 2007). CV was calculated using all replicates from both treatments. Correlations between the relative biomass or coverage of the eight species and the mean values of trait in each treatment and across treatments as well as the CV of these trait in response to water availability was calculated using regression analysis. Mean and CV of each trait were ln-transformed prior to the correlation analysis. Because relative coverage and relative biomass are strongly related (r2 = 0.91, P < 0.0001), the results for the case of relative coverage are reported in the main text while the results for the case of relative biomass are reported in the appendix (see Appendix Figs. 5 and 6). All statistics were performed in SPSS 16.0 (SPSS, Chicago, Illinois, USA).
Results
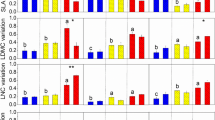
The interaction of water treatments and species was significant for all functional traits except leaf thickness (Table 2), suggesting that functional trait responses varied between species (Fig. 2). For example, the SLA of Kobresia capilifolia was significantly higher, while that of Caltha palustris, Trollius farreri was significantly lower, in drought than the flooding treatment (P < 0.05, Fig. 2a); but the relative leaf water content (RLWC) of these three species was significantly decreased from flooding to drought treatment (Fig. 2b). There was a significant decrease in leaf area for C. palustris from flooding to drought treatment (P < 0.05), while slight increases occurred in Potentilla anserina, T. farreri and S. filiformis (Fig. 2c). The leaf thickness of C. palustris, and T. farreri was higher in the drought treatment, but lower for Sanguisorba filiformis and Carex kansuensis (Fig. 2d). The net photosynthesis rate under saturating light (Amax), stomatal conductance (gs), and transpiration rate (E) were lower for most species in the drought treatment, with significant decreases of gs for P. anserina, C. kansuensis, Nardostachys chinensis (Fig. 2e, f, g). This pattern varied for other species, i.e. Elymus nutans and T. farreri had relative higher Amax, and T. farreri had higher gs and E under drought condition (Fig. 2e, f, g).
Values (mean ± 1SE) of traits for eight common species under flood and drought treatments: (a) SLA (K capilifolia: F = 13.49, P = 0.022, C palustris: F = 15.63, P = 0.002, T farreri: F = 5.76, P = 0.035); (b) LWC (K capilifolia: F = 7.49, P = 0.021, C palustris: F = 12.08, P = 0.008, T farreri: F = 5.76, P = 0.035); (c) leaf area (C palustris: F = 14.27, P = 0.005); (d) leaf thickness (C palustris: F = 9.37, P = 0.028; T farreri: F = 7.25, P = 0.029; S filiformis: F = 7.34, P = 0.031 and C kansuensis: F = 2.34, P = 0.042); (e) Amax and (f) gs (P anserine: F = 8.35, P = 0.044; C kansuensis: F = 22.02, P = 0.018; N chinensis: F = 28.52, P = 0.006;); and (g) E (P anserine: F = 6.35, P = 0.044; C kansuensis: F = 18.02, P = 0.021; N chinensis: F = 2752, P = 0.026;). See Table 1 for the abbreviations of traits
No significant correlation was present between average values of the leaf traits and the community level cover and biomass for all the eight perennial herbaceous species under either flooding or drought condition and across the two treatments (Fig. 3; Appendix Fig. 5). Instead, the coefficient of variation (CV) of each trait (an indicator of plasticity of functional traits) across the treatment was a better predictor of species cover/biomass. Interestingly, some of the relationships between leaf morphological plasticity and relative cover/biomass were negative and none were significantly positive (Fig. 4; Appendix Fig. 6). For example, the plasticity of SLA was negatively related with both relative coverage (r2 = 0.54, P = 0.021, Fig. 4a) and biomass (r2 = 0.56, P = 0.019, Appendix Fig. 6a). Plasticity (CV) of the leaf thickness was also negatively correlated with relative coverage (r2 = 0.63, P = 0.012, Fig. 4b) and biomass (r2 = 0.62, P = 0.012, Appendix Fig. 6b). In contrast to leaf morphological plasticity, plasticity (CV) of gs had a positive relationship with relative coverage (r2 = 0.50, P = 0.029, Fig. 4c) and biomass (r2 = 0.55, P = 0.021, Appendix Fig. 6c); plasticity (CV) of transpiration (E) also had a positive relationship with relative coverage (r2 = 0.47, P = 0.039, Fig.4d) and biomass (r2 = 0.44, P = 0.046, Appendix Fig. 6d).
Correlation between the trait mean and relative coverage under drought (red circles) and flooding (black circles) conditions. There were no significant correlations between the traits mean and relative coverage (The P-value of regression was >0.05). Nc: N chinensis; Pa: P anserine; Ck: C kansuensis; Sf: S filiformis; En: E nutans; Kc: K capilifolia; Cp: C palustris; Tf: T farreri. See Table 1 for the abbreviations of traits
Bivariate plots of relative coverage against (a) variation in SLA (r2 = 0.54, P = 0.021), (b) variation in leaf thickness (r2 = 0.63, P = 0.012), (c) variation in gs (r2 = 0.50, P = 0.029), and (d) variation in E (r2 = 0.47, P = 0.039). The CV of traits was log transformed (ln). See Table 1 for the abbreviations of traits
Correlation between the trait mean and relative biomass under drought (red circles) and flooding (black circles) conditions. There were no significant correlations between the traits mean and relative coverage (The P-value of regression was >0.05). See Table 1 for the abbreviations of traits
Regression plots of relative biomass against (a) variation in SLA (r2 = 0.56, P = 0.019), (b) variation in leaf thickness (r2 = 0.62, P = 0.012), (c) variation in gs (r2 = 0.48, P = 0.038), and (d) variation in E (r2 = 0.44, P = 0.046). The CV of any given trait was then the natural logarithm (ln) transformed. Nc: N chinensis; Pa: P anserine; Ck: C kansuensis; Sf: S filiformis; En: E nutans; Kc: K capilifolia; Cp: C palustris; Tf: T farreri. See Table 1 for the abbreviations of traits
Discussion
Resource availability and disturbance impacts plant performance across varying scales in space and time, and it is clear that multiple strategies can confer an advantage for a given combination of average resource availability and resource variability (Lavorel and Garnier 2002). Some studies have shown an important role of average leaf trait values in predicting plant performance in response to changing environments at the community level (Poorter et al. 2008; Cornwell and Ackerly 2010), while other have stressed the adaptive response of altering leaf morphology and physiology (Nicotra et al. 2010; Warren and Lake 2012). Our study provides experimental evidence that when an herbaceous plant community faced resource fluctuations in the form of high intra-annual rainfall variability, a strategy that includes reduced plasticity in leaf morphology and greater plasticity of leaf physiology was correlated with greater species-level dominance.
We found that overall the three graminoid species in the study (one Poacea and the two Cyperaceae) had the higher SLA and lower gs values, while the forbs (e.g. T. farreri, P. anserina and N. chinensis) had the lower SLA and higher gs (Fig. 2). SLA is an important trait affecting light interception, photosynthesis and plant growth (Niinemets 2001; Poorter et al. 2009); With the shade effects of grasses on forbs, forbs have lower SLA and have higher gs to regulate plant water relations (Farquhar and Sharkey 1982). The difference in SLA and gs reflect the varied strategies of plant species in coping with competition and/or environmental changes (Farquhar and Sharkey 1982; Poorter et al. 2009).
Given the low temperatures, frequent droughts, and often anoxic conditions in alpine meadows, we expected that species with certain traits would be favored by filtering processes, depending on the relative effects of drought versus flooding (Weiher and Keddy 2001; Cornwell et al. 2006). With equalizing mechanisms, the habitat usually selects a set of species that are somewhat functionally equivalent in some regard (Chesson 2000). Similarly, functional traits are thought to be key filters in community assembly models (Woodward and Diament 1991; Keddy 1992; Woodward and Cramer 1996). In this study, watering treatment effects were only significant for four out of seven traits, whereas species by treatment interactions were significant for six out of seven traits. These species-specific responses of functional traits to different water treatments in our study were largely in contrast to the equalizing mechanism, indicating large differentiation between species. Although some species responded in the same direction, the response generally has a different magnitude of trait variation, especially for gs and E (Fig. 2). For example, the response of SLA to water treatments varied among species, suggesting a lack of equalizing mechanisms (Fig. 21a, Table 2). These results differ from most past studies, where increases in nitrogen and water availability generally led to an increased SLA, while increases in light availability strongly decreased SLA (Cunningham et al. 1999; Knops and Reinhart 2000; Merilo et al. 2006; Poorter et al. 2009). We also found different responses in physiological traits among the species in our study to changing water conditions, which furthermore demonstrated the large inter-species differences. Considering that the response of plant species to environmental conditions is an important determinant of the capacity to persist in a given habitat (Baraloto et al. 2005; Baraloto et al. 2006; Sterck et al. 2006; Poorter and Markesteijn 2008), the varying responses of these species to soil water conditions indicate a range of strategies to cope with fluctuating water environments (Grime 2006). These changes in plant functional traits, in turn, could potentially alter ecosystem functioning and services (Suding et al. 2008).
Our study also provides insights on whether average values of the leaf traits explains plant cover and biomass in our field communities, which are subjected to fluctuating water availability and other environmental variability. We found no evidence for relationships between average values of the leaf functional traits or leaf gas exchange and the community level cover and biomass for the eight study species. Again, this result is in contrast to previous research, which typically reports that being at one extreme of a functional axis leads to dominance (a linear relationship between trait values and species dominance) or that species with “average” traits are dominant (a significant quadratic relationship between trait values and dominance) (Cornwell and Ackerly 2010).
Instead, plasticity of plant traits, not their average, may serve as better predictors of species cover and biomass within communities (Grime and Mackey 2002; Nicotra et al. 2010; Warren and Lake 2012). Species with higher CV (the plasticity of traits) for the leaf gas exchange traits of gs and E had higher relative biomass and coverage in this degraded alpine wetland (Fig. 3, Appendix Fig. 6). This strategy can allow species to cope with rapid and/or short-term variability in water availability, with potential implications for species’ strategies to withstand future environmental changes (Cochrane et al. 2015). In the specific case of alpine wetlands, having plastic gs (as we observed) might be especially critical, because it allows individual plants to transpire excessive soil moisture, thereby avoiding anoxia (Bailey-Serres and Voesenek 2008). However, this system also faces drought conditions for a substantial portion of most growing seasons. In response to drought, many plants employ a quick reduction in gs to reduce water loss through transpiration and avoid severe water stress and/or death (Hetherington and Woodward 2003). The null relationship between the CV of Amax and cover, juxtaposed with the positive correlation between the CV of gs and cover supports the idea that stomatal regulation has key impacts on plant water balance beyond the limitation of photosynthesis (Farquhar and Sharkey 1982; Chaves et al. 2002). This could partly explain the lack of synchronization between changes in net photosynthetic rate and gs between water treatments in our study (Fig. 2 e, f). Overall, our results support recent conjectures that plasticity of certain traits plays an important role in explaining plant performance in communities under climate changes (Nicotra et al. 2010; Lin et al. 2015). We also note that the species having higher plasticity in gas exchange and lower plasticity in leaf morphological traits share many similar characteristics (i.e., elongated leaves, plant stature in middle of the vertical structure of community).
Interestingly, higher plasticity in morphology, especially in SLA and leaf thickness, was correlated with lower abundance in the communities. This result varied from our original hypotheses and from other studies where more plastic species were able to compete over a wider range of conditions than less plastic species (Richards et al. 2006). For instance, SLA is considered a key plant trait and often serves as a predictor of many aspects of plant life-history (Givnish 1987; Reich et al. 1997; Cunningham et al. 1999; Poorter and De Jong 1999; McDonald et al. 2003) and therefore, we would expect that being able to tailor SLA to current conditions would confer a competitive advantage. For example, moderate variability in SLA has shown to be beneficial for plant functioning under water stress (e.g., Poorter et al. 2009). Leaf thickness is closely related with SLA, thus leading to similar relationships between water availability and leaf thickness and tissue density (Niinemets 2001, Wilson et al. 1999). However, some studies have also indicated that plastic morphology and physiology are not necessarily related to plant performance (e.g., Davidson et al. 2011). Consistent with our results, Valladares et al. (2000) found that for Mediterranean oaks, maintaining a more constant morphology sometimes improved plant dominance.
Based on our results and other work, we propose that the benefits species gain from plasticity in morphology and physiology may depend largely on the frequency and intensity of resource fluctuations over time. Altering morphology by changing existing tissues or building new ones has initial energy and material costs, but can allow plants to potentially achieve greater long-term photosynthetic rates than those that adjust solely through changes in physiology (Schwinning and Sala 2004). Therefore, altering, growing, or shedding tissues in response to environmental variation might become an advantage when a change in resource availability is maintained for months, at which point the benefits of morphological plasticity supersede their initial costs (e.g. Schwinning and Sala 2004). For instance, plants with higher SLA have been shown to have higher survival during floods (Mommer et al. 2006; Jung et al. 2010), but higher SLA is a disadvantage during drought (Wright et al. 2004). Therefore, if flood and drought conditions are separated in time it might be beneficial to have greater morphological plasticity. However, in environments with frequent or intense intra-annual resource fluctuations, changes in morphology can be a net cost, potentially reducing whole-plant growth and competitive ability (Fig. 4a b, DeWitt et al. 1998; Kishida and Nishimura 2006). Moreover, tissues adjusted to decreases in resource availability or increase in anoxia stress should perform poorly when resource availability returns to long-term mean conditions (e.g. Westoby et al. 2002). Compared to altering morphology, altering physiological plasticity occurs quickly and has a lower energetic cost (Schwinning and Sala 2004). Therefore, physiological plasticity may be a beneficial strategy in ecosystems with high intra-annual resource variability (days to weeks), allowing the plant to adjust its gas exchange to frequent changes in resource variability, and in the case of alpine grasslands, anoxia stress (Fig. 4c d; Cowan and Farquhar 1977, Mommer et al. 2006; Nippert et al. 2007). Looking at long-term climate data at our site, the intra-annual variability is relatively large (Fig. 1). In most of the years for which we had climate data, precipitation often varied 3-fold within a single growing season, with several months of ≤50 mm of precipitation at the beginning and end of the growing season, but one or two months in the middle of the growing season with ≥150 mm of precipitation. Overall, these climate conditions and our study results are consistent with the case where an ecosystem has high intra-annual variability (Fig 1), with filtering in favor of physiological plasticity and against morphological plasticity (Fig. 4).
Conclusion
Our study investigated morphological and physiological responses of common alpine plants to drought and flooding conditions. The results show largely divergent species responses to the flooding and drought, and could have implications for species co-existence in other grazed meadows and wetlands, especially those associated with historically high grazing rates. We also find a contrasting association of morphological and physiological plasticity with plant cover and biomass. In this environment with high intra-annual rainfall variability, herbaceous plants with reduced plasticity in leaf morphology and greater plasticity of leaf physiology achieved greater specific-level dominance. Our study helps clarify the role of morphological and physiological plasticity of plant traits for predicting plant cover and biomass in an alpine community and suggests that the ability of plants to track rapid changes through physiological adjustments may be a key regulator of species responses to future environmental change.
References
Abràmoff MD, Magalhães PJ, Ram SJ (2004) Image processing with ImageJ. Biophoton Int 11:36–43
Ackerly DD (2003) Community assembly, niche conservatism, and adaptive evolution in changing environments. Int J Plant Sci 164:165–184
Bailey-Serres J, Voesenek LACJ (2008) Flooding stress: acclimations and genetic diversity. Annu. Rev. Plant Biol 59:313–339
Baraloto C, Goldberg DE, Bonal D (2005) Performance trade-offs among tropical tree seedlings in contrasting microhabitats. Ecology 86:2461–2472
Baraloto C, Bonal D, Goldberg DE (2006) Differential seedling growth response to soil resource availability among nine neotropical tree species. J Trop Ecol 22:487–497
Chapin FS (2003) Effects of plant traits on ecosystem and regional processes: a conceptual framework for predicting the consequences of global change. Ann Bot 91:455–463
Chaves MM, Pereira JS, Maroco J, Rodrigues ML, Ricardo CPP, Osório ML, et al. (2002) How plants cope with water stress in the field? Photosynthesis and growth. Ann Bot 89:907–916
Chen H, Zhu Q, Peng C, Wu N, Wang Y, Fang et al (2013) The impacts of climate change and human activities on biogeochemical cycles on the Qinghai-Tibetan plateau. Glob Chang Biol 19:2940–2955.
Chesson P (2000) Mechanisms of maintenance of species diversity. Annu Rev Ecol Syst 31:343–366
Cochrane A, Hoyle GL, Yates CJ, Wood J, Nicotra AB (2015) The phenotypic response of co-occurring Banksia species to warming and drying. Plant Ecol 216:27–39
Cornwell WK, Ackerly DD (2010) A link between plant traits and abundance: evidence from coastal California woody plants. J Ecol 98:814–821
Cornwell WK, Schwilk DW, Ackerly DD (2006) A trait-based test for habitat filtering: convex Hull volume. Ecology 87:1465–1471
Cowan IR, Farquhar GD (1977) Stomatal function in relation to leaf metabolism and environment. Symp Soc Exp Biol 31:471–505
Cunningham SA, Summerhayes B, Westoby M (1999) Evolutionary divergences in leaf structure and chemistry, comparing rainfall and soil nutrient gradients. Ecol Monogr 69:569–588
Davidson AM, Jennions M, Nicotra AB (2011) Do invasive species show higher phenotypic plasticity than native species and, if so, is it adaptive? A meta-analysis. Ecol Lett 14:419–431
DeWitt TJ, Sih A, Wilson DS (1998) Costs and limits of phenotypic plasticity. Trends Ecol Evol 13:77–81
Dìaz S, Hodgson JG, Thompson K, Cabido M, Cornelissen JHC, Jalili A, et al. (2004) The plant traits that drive ecosystems: evidence from three continents. J Veg Sci 15:295–304
Du M, Kawashima S, Yonemura S, Zhang X, Chen S (2004) Mutual influence between human activities and climate change in the Tibetan plateau during recent years. Glob Planet Chang 41:241–249
Farquhar GD, Sharkey TD (1982) Stomatal conductance and photosynthesis. Annu Rev Plant Physiol 33:317–345
Funk JL, Jones CG, Lerdau MT (2007) Leaf-and shoot-level plasticity in response to different nutrient and water availabilities. Tree Physiol 27:1731–1739
Givnish TJ (1987) Comparative studies of leaf form: assessing the relative roles of selective pressures and phylogenetic constraints. New Phytol 106:131–160
Gong ZT (1999) Chinese Soil Taxonomy: Theories Methods and Applications. Science Press, Beijing
Grime JP (2006) Plant strategies, vegetation processes, and ecosystem properties. John Wiley & Sons, New York
Grime JP, Hunt R (1975) Relative growth-rate: its range and adaptive significance in a local flora. J Ecol 63:393–422
Grime JP, Mackey JML (2002) The role of plasticity in resource capture by plants. Evol Ecol 16:299–307
Hao X (2008) A green fervor sweeps the Qinghai-Tibetan plateau. Science 321:633–635
Hetherington AM, Woodward FI (2003) The role of stomata in sensing and driving environmental change. Nature 424:901–908
Jonas T, Rixen C, Sturm M, Stoeckli V (2008) How alpine plant growth is linked to snow cover and climate variability. J Geophys Res Biogeosci 113:G030103
Jung V, Violle C, Mondy C, Hoffmann L, Muller S (2010) Intraspecific variability and trait-based community assembly. J Ecol 98:1134–1140
Kang S, Xu Y, You Q, Flügel WA, Pepin N, Yao T (2010) Review of climate and cryospheric change in the Tibetan plateau. Environ Res Lett 5:015101
Keddy PA (1992) Assembly and response rules: two goals for predictive community ecology. J Veg Sci 3:157–164
Kishida O, Nishimura K (2006) Flexible architecture of inducible morphological plasticity. J Anim Ecol 75:705–712
Knapp AK, Fay PA, Blair JM, Collins SL, Smith MD, Carlisle JD, et al. (2002) Rainfall variability, carbon cycling, and plant species diversity in a mesic grassland. Science 298:2202–2205
Knapp AK, Beier C, Briske DD, Classen AT, Luo Y, Reichstein M, et al. (2008) Consequences of more extreme precipitation regimes for terrestrial ecosystems. Bioscience 58:811–821
Knops JM, Reinhart K (2000) Specific leaf area along a nitrogen fertilization gradient. Am Midl Nat 144:265–272
Kudo G, Hirao AS (2006) Habitat-specific responses in the flowering phenology and seed set of alpine plants to climate variation: implications for global-change impacts. Popul Ecol 48:49–58
Lavorel S, Garnier E (2002) Predicting changes in community composition and ecosystem functioning from plant traits: revisiting the holy grail. Funct Ecol 16:545–556
Lin YS, Medlyn BE, Duursma RA, Prentice IC, Wang H, Baig S, et al. (2015) Optimal stomatal behaviour around the world. Nat Clim Chang 5:459–464
Lopez-Iglesias B, Villar R, Poorter L (2014) Functional traits predict drought performance and distribution of Mediterranean woody species. Acta Oecol 56:10–18
Ma M, Zhou X, Du G (2011) Soil seed bank dynamics in alpine wetland succession on the Tibetan plateau. Plant Soil 346:19–28
Martina JP, von Ende CN (2012) Highly plastic response in morphological and physiological traits to light, soil-N and moisture in the model invasive plant Phalaris arundinacea. Environ Exp Bot 82:43–53
McDonald PG, Fonseca CR, Overton J, Westoby M (2003) Leaf-size divergence along rainfall and soil-nutrient gradients: is the method of size reduction common among clades? Funct Ecol 17:50–57
Merilo E, Heinsoo K, Kull O, Söderbergh I, Lundmark T, Koppel A (2006) Leaf photosynthetic properties in a willow (Salix viminalis and Salix dasyclados) plantation in response to fertilization. Eur J For Res 125:93–100
Mommer L, Lenssen JPM, Huber H, Visser EJW, de Kroon H (2006) Ecophysiological determinants of plant performance under flooding: a comparative study among seven plant families. J Ecol 94:1117–1129
Nicotra AB, Hermes JP, Jones CS, Schlichting CD (2007) Geographic variation and plasticity to water and nutrients in pelargonium australe. New Phytol 176:136–149
Nicotra AB, Atkin OK, Bonser SP, Davidson AM, Finnegan EJ, Mathesius U, et al. (2010) Plant phenotypic plasticity in a changing climate. Trends Plant Sci 15:684–692
Nie Y, Li A (2011) Assessment of alpine wetland dynamics from 1976 to 2006 in the vicinity of Mount Everest. Wetlands 31:875–884
Niinemets Ü (2001) Global-scale climatic controls of leaf dry mass per area, density, and thickness in trees and shrubs. Ecology 82:453–469
Nippert JB, Fay PA, Knapp AK (2007) Photosynthetic traits in C3 and C4 grassland species in mesocosm and field environments. Environ Exp Bot 60:412–420
Niu K, Choler P, de Bello F, Mirotchnick N, Du G, Sun S (2014) Fertilization decreases species diversity but increases functional diversity: a three-year experiment in a Tibetan alpine meadow. Agric Ecosyst Environ 182:106–112
Pierce S, Brusa G, Vagge I, Cerabolini BE (2013) Allocating CSR plant functional types: the use of leaf economics and size traits to classify woody and herbaceous vascular plants. Funct Ecol 27:1002–1010
Poorter L, Bongers F (2006) Leaf traits are good predictors of plant performance across 53 rain forest species. Ecology 87:1733–1743
Poorter H, De Jong ROB (1999) A comparison of specific leaf area, chemical composition and leaf construction costs of field plants from 15 habitats differing in productivity. New Phytol 143:163–176
Poorter L, Markesteijn L (2008) Seedling traits determine drought tolerance of tropical tree species. Biotropica 40:321–331
Poorter L, Wright SJ, Paz H, Ackerly DD, Condit R, Ibarra-Manríquez G, et al. (2008) Are functional traits good predictors of demographic rates? Evidence from five neotropical forests. Ecology 89:1908–1920
Poorter H, Niinemets Ü, Poorter L, Wright IJ, Villar R (2009) Causes and consequences of variation in leaf mass per area (LMA): a meta-analysis. New Phytologist 182:565–588
Pywell RF, Bullock JM, Roy DB, Warman LIZ, Walker KJ, Rothery P (2003) Plant traits as predictors of performance in ecological restoration. J Appl Ecol 40:65–77
Reich PB, Uhl C, Walters MB, Ellsworth DS (1991) Leaf lifespan as a determinant of leaf structure and function among 23 Amazonian tree species. Oecologia 86:16–24
Reich PB, Walters MB, Ellsworth DS (1997) From tropics to tundra: global convergence in plant functioning. Proc Natl Acad Sci 94:13730–13734
Reich PB, Ellsworth DS, Walters MB (1998a) Leaf structure (specific leaf area) modulates photosynthesis–nitrogen relations: evidence from within and across species and functional groups. Funct Ecol 12:948–958
Reich PB, Walters MB, Tjoelker MG, Vanderklein D, Buschena C (1998b) Photosynthesis and respiration rates depend on leaf and root morphology and nitrogen concentration in nine boreal tree species differing in relative growth rate. Funct Ecol 12:395–405
Richards CL, Bossdorf O, Muth NZ, Gurevitch J, Pigliucci M (2006) Jack of all trades, master of some? On the role of phenotypic plasticity in plant invasions. Ecol Lett 9:981–993
Schwinning S, Sala OE (2004) Hierarchy of responses to resource pulses in arid and semi-arid ecosystems. Oecologia 141:211--220.
Sterck FJ, Poorter L, Schieving F (2006) Leaf traits determine the growth-survival trade-off across rain forest tree species. Am Nat 167:758–765
Suding KN, Lavorel S, Chapin FS, Cornelissen JH, Díaz S, Garnier E, et al. (2008) Scaling environmental change through the community-level: a trait-based response-and-effect framework for plants. Glob Chang Biol 14:1125–1140
Sultan SE (2003) Phenotypic plasticity in plants: a case study in ecological development. Evol Dev 5:25–33
Sultan SE, Wilczek AM, Bell DL, Hand G (1998) Physiological response to complex environments in annual Polygonum species of contrasting ecological breadth. Oecologia 115:564–578
Valladares F, Martinez-Ferri E, Balaguer L, Perez-Corona E, Manrique E (2000) Low leaf-level response to light and nutrients in Mediterranean evergreen oaks: a conservative resource-use strategy? New Phytol 148:79–91
Valladares F, Gianoli E, Gómez JM (2007) Ecological limits to plant phenotypic plasticity. New Phytol 176:749–763
Violle C, Navas ML, Vile D, Kazakou E, Fortunel C, Hummel I, Garnier E (2007) Let the concept of trait be functional! Oikos 116:882–892
Warren RJ, Lake JK (2012) Trait plasticity, not values, best corresponds with woodland plant success in novel and manipulated habitats. J Plant Ecol rts035
Weiher E, Keddy P (2001) Assembly rules as general constraints on community composition. Ecological assembly rules: perspectives, advances, retreats. Cambridge University Press, Cambridge, pp. 251–271
Westoby M, Falster DS, Moles AT, Vesk PA, Wright IJ (2002) Plant ecological strategies: some leading dimensions of variation between species. Annu Rev Ecol Syst 33:125–159
Wilson PJ, Thompson KEN, Hodgson JG (1999) Specific leaf area and leaf dry matter content as alternative predictors of plant strategies. New Phytol 143:155–162
Woodward FI, Cramer W (1996) Plant functional types and climatic change: introduction. J Veg Sci 7:306–308
Woodward FI, Diament AD (1991) Functional approaches to predicting the ecological effects of global change. Funct Ecol 5:202–212
Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, et al. (2004) The worldwide leaf economics spectrum. Nature 428:821–827
Wright IJ, Reich PB, Cornelissen JH, Falster DS, Garnier E, Hikosaka K, et al. (2005) Assessing the generality of global leaf trait relationships. New Phytol 166:485–496
Xiang S, Guo R, Wu N, Sun S (2009) Current status and future prospects of Zoige Marsh in Eastern Qinghai-Tibet plateau. Ecol Eng 35:553–562
Yu K, Pypker TG, Keim RF, Chen N, Yang Y, Guo S, et al. (2012) Canopy rainfall storage capacity as affected by sub-alpine grassland degradation in the Qinghai–Tibetan plateau, China. Hydrol Process 26:3114–3123
Acknowledgments
The authors thank Dr. Adrienne Nicotra and the members of Nicotra Lab at the Australian National University and Dr. Wei Li and Xianhui Zhou for providing rainfall data, and Lanping Li for providing phenology data. This work was supported by National Natural Science Foundation of China (grant No. 41171214). ZR was supported by NSF DBI 1402033.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Susan Schwinning.
Honglin Li and Kailiang Yu contributed equally to this study
Appendices
Appendix 1
Appendix 2
Appendix 3
Appendix 4
Appendix 5
Rights and permissions
About this article
Cite this article
Li, H., Yu, K., Ratajczak, Z. et al. When variability outperforms the mean: trait plasticity predicts plant cover and biomass in an alpine wetland. Plant Soil 407, 401–415 (2016). https://doi.org/10.1007/s11104-016-2898-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-016-2898-x