Abstract
The Antarctic Peninsula is one of three regions of the planet that have experienced the highest rates of climate warming over recent decades. Based on a comprehensive large-scale resurvey, allowing comparison of new (2009) and historical data (1960s), we show that the two native Antarctic vascular plant species have exhibited significant increases in number of occupied sites and percent cover since the 1960s: Deschampsia antarctica increasing in coverage by 191 % and in number of sites by 104 %. Colobanthus quitensis increasing in coverage by 208 % and number of sites by 35 %. These changes likely occurred in response to increases of 1.2 °C in summer air temperature over the same time period. Both species exhibited changes with elevation due to the interaction of multiple drivers (climatic factors and animal disturbance), producing heterogeneity of responses across an elevation gradient. Below an elevation of 20 m fur seal activity exerted negative impacts. Between 20 and 60 m, both plant species underwent considerable increases in the number of sites and percent cover, likely influenced by both climate warming and nutrient input from seals. Above an elevation threshold of 60 m the maximum elevation of the sites occupied decreased for both species, perhaps as a consequence of physical disturbance at higher elevations due to the permafrost conditions and/or the snow cover thickness and persistence. Understanding the role of disturbance drivers for vegetation change in cold regions may become a research priority to enable improved forecasting of biological responses and feedbacks of climate warming on ecosystems in these globally influential regions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Climate change has been particularly evident in recent decades along the Antarctic Peninsula, one of three regions of the planet recording the most rapid atmospheric warming over the last 50 years (Turner et al. 2009). Impacts are clearly evident on both abiotic (e.g. glacier retreat) and biotic components of ecosystems (Convey and Smith 2006; Convey 2011). Among the globally-recognized effects of rapid atmospheric warming on angiosperms are accelerated growth and biomass allocation (Elmendorf et al. 2012), improved metabolic performance and changes in phenology, range shifts and upwards distributional migration (Walther et al. 2002).
Studies in the Arctic have highlighted that recent vegetation changes over multiple decades may include apparently contrasting responses (increase, decrease, stability). These result from the impacts and interactions of multiple drivers (climate, permafrost, biotic interactions), complex processes (competition, facilitation) and, in some instances, spatial heterogeneity of species responses (Callaghan et al. 2013). In the Antarctic, observed changes have been primarily interpreted as climate warming resulting in a vegetation increase (Fowbert and Smith 1994; Convey 1996a; le Roux and McGeoch 2008; Parnikoza et al. 2009; Torres Mellado et al. 2011), or to the generally negative impacts of recent rapid recovery of Antarctic fur seal populations following historical over-exploitation (Smith 1988, 2003; Favero-Longo et al. 2011), with only one case of facilitation reported only for selected exotic plant species in the sub-Antarctic (Haussmann et al. 2013).
In the maritime Antarctic, the two species of native vascular plant (Deschampsia antarctica Desv. and Colobanthus quitensis (Kunth) Bartl.) have exhibited significant expansions in local range and population numbers over up to the last 50 years at several locations (Fowbert and Smith 1994; Convey 1996a; Parnikoza et al. 2009; Torres Mellado et al. 2011).
Both species are pioneer colonists with wide ecological amplitude, occurring in habitats ranging from mineral to organic soils, although neither colonizes active patterned ground and unstable surfaces (Edwards 1972; Smith 2003). D. antarctica occasionally forms extensive stands within its Antarctic distribution. Environmental manipulation experiments have shown that both species respond positively to warming, with increased above-ground biomass, growth rate, water use efficiency, flower and seed production (Day et al. 2008; Ruhland and Krna 2010), but that water or nutrient additions had few detectable effects (Day et al. 2008). Hill et al. (2011), however, demonstrated that D. antarctica is a particularly effective competitor for available nitrogen in the soil, and its efficient acquisition of the N released in decomposition of soil organic matter may give it an advantage over competing mosses.
Signy Island (South Orkney Islands) lies within the Antarctic Peninsula region that has experienced the most rapid atmospheric warming over the past 50 years (Smith 1990; Royles et al. 2012). It is also one of the few high latitude locations in the Southern Hemisphere where detailed and extensive vegetation surveys were made around 50 years ago, with the spatial distribution and abundance of D. antarctica and C. quitensis carefully documented across the island during the 1960s (1961–1970; Edwards 1972).
Here we assess: 1) whether these native vascular plant species have undergone any change in number of sites occupied and percent cover since the 1960s, 2) how different environmental drivers (climate warming, animal and human disturbance) have affected them, producing heterogeneity of responses across an elevational gradient.
2 Materials and methods
2.1 Study area
Signy Island (60°43′S, 45°38′W) is located in the South Orkney Islands (Maritime Antarctic). It is characterised by a cold oceanic climate, with mean annual air temperature of −3.5 °C and annual precipitation of 400 mm, primarily as summer rain (Smith 1990; Royles et al. 2012). An ice cap covers about half of the island’s area, although this is currently shrinking rapidly (Favero-Longo et al. 2012). Ice-free ground is underlain by continuous permafrost, with an active layer depth ranging between 40 cm and 2 m (Cannone et al. 2006; Guglielmin et al. 2008, 2012). Soils are mainly Gelisols and Fibristels (Guglielmin et al. 2012).
Two major vegetation formations are dominant, the Antarctic herb tundra formation (characterized by the two native vascular plants D. antarctica and C. quitensis) and the more widespread Antarctic non-vascular cryptogam tundra formation (Smith 1972). Most of the ice-free area of the island is covered by cryptogamic vegetation.
A notable environmental change on the island since the late 1970s has been the large increase in the numbers of resting and moulting Antarctic fur seals (Arctocephalus gazella Peters 1875) present during the summer months (Waluda et al. 2010) due to recent rapid recovery following historical over-exploitation. This species’ breeding populations are centred primarily on South Georgia, and non-breeding fur seals were first seen on Signy Island in the late 1970s, with numbers then increasing rapidly to 10–20,000 individuals being present during the 1990s, and this number remaining relatively stable to the present day. Fur seal activity has drastically impacted vegetation on the island, both through trampling/crushing (Smith 1988), and polluting levels of nitrogen release in faeces/urine (Favero-Longo et al. 2011).
2.2 Methods
A detailed survey of D. antarctica and C. quitensis across Signy Island was carried out during the 1960s (Edwards 1972). All sites colonized by these two species were mapped with a resolution of 20–25 m, with the size of the population at each site also being documented. Population sizes were classified as follows (Edwards 1972):
-
a)
D. antarctica: populations of discrete plants containing 1 (s1 - smallest), 2–20 (s2 - small), or >20 discrete plants or few clumped plants (s3 - intermediate); plants forming coalesced swards <10m2 (s4 – large) or >10m2 in area (s5 – largest);
-
b)
C. quitensis: populations of discrete plant sites containing 1 (s1 - smallest), 2–10 (s2 - small), 10–30 (s3 - intermediate), 30–50 (s4 – large), or >50 cushions (s5 – largest).
During January and February 2009, we carried out a field survey of both species across Signy Island following the criteria adopted by Edwards (1972) for comparison with the previous detailed surveys carried out in the 1960s.
The maps provided by Edwards (1972) were geo-referenced, included in a GIS system and re-drawn using ArcGIS 9.2. The data recorded in 2009 were mapped using the same software. Each colonized site was characterized in terms of elevation (m a.s.l.), slope (°) and aspect (divided into 8 sectors: N; NE; E; SE; S; SW; W; NW), using the most recent digital elevation model of Signy Island. For each site occupied by either species, distance (m) from trails used by humans and proximity to penguin colonies were also estimated. For each plant occurrence site identified during the field survey we assessed the occurrence/absence of fur seal disturbance on the basis of the visible health and indication of impacts on the surrounding bryophyte communities (e.g. crushing/flattening, colour changes due to urine deposition, etc). Both the new and previously-published survey data were spatially referenced to topographic features (elevation, slope, aspect), anthropogenic influence (trails) and biotic (fur seal, penguin) disturbance.
To assess any changes of the two species with respect to elevation, we performed non parametric statistics (maximum and minimum elevations; median, 25 % and 75 % quartiles, providing the core of the species distribution) (Maggini et al. 2011). We compared the elevation of the sites occupied in the 1960s and 2009 data. These analyses were applied to the entire dataset as well as to each population size class separately (s1-s5, from smallest to largest) of each species. Differences with respect to elevation, slope and aspect were tested using the Wilcoxon test. These analyses were performed using Statistica®.
Relationships between the occupied sites topography, climate and disturbance were analyzed using multivariate approaches. Canonical Correspondence Analyses (CCA, using biplot scaling for inter-species distances, Hill’s scaling for inter-sample distances; choosing the forward selection of variables option; performing the Monte Carlo permutation test on the first and all ordination axes) were performed using CANOCO 4.5 (Ter Braak and Verdonschot 1995) to analyse the patterns present in the different population size classes of both plant species and to evaluate all the environmental factors affecting them in the 1960s vs. 2009 and in 2009. Comparing the two surveys, as increasing precipitation acted as an inflation factor autocorrelated with air temperature warming, we deleted it from the analysis. The factor “fur seal disturbance” was converted in a dummy variable (1 = presence, 0 = absence) (Lepš and Smilauer 2003).
There are no specific long-term climatic data currently being collected on Signy Island. We therefore quantified the rate of climate change in this region of maritime Antarctica by analyzing the trends in climate using the century-long data record provided from neighbouring Orcadas AWS (Orcadas Station, Laurie Island, c. 50 km from Signy Island). This is the nearest WMO (World Meteorological Observation) long-term monitoring station to our study area, and its temperature record is very closely correlated (p < 0.01, r2 = 98 %, as tested by linear regression) to the 47 year (1948–1995) record available from Signy Island (Royles et al. 2012). We computed the mean annual and seasonal (spring = September, October, November; summer = December, January, February; autumn = March, April, May; winter = June, July, August) air temperatures (°C) and precipitation (mm) over the period 1960–2009. Air temperature and precipitation trends over time were obtained by linear regression using Statistica®.
3 Results
3.1 Climate
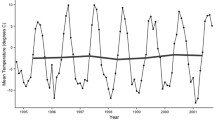
Over the period 1960–2009, atmospheric temperature showed an increasing trend of +0.9 °C in mean annual air temperature (p < 0.05) and in the seasonal data, especially in summer which showed an increase of +1.2 °C (p < 0.01) (Fig. 1). Analyzing the changes of seasonal and mean annual air temperature at the decadal scale, while the 1970s were a cold period (except for summer), since the early 1980s there has been an almost continuous warming trend (with the exception of winter in the last decade). Summer and autumn exhibited the largest air temperature changes (Table 1 Supplementary Materials), with the summer increase commencing in the 1970s, and that in autumn only in the last decade.
Since 1960 total annual precipitation has also increased by c. 72 mm (Royles et al. 2012). The strongest precipitation increase has occurred since 1993, with the recent trend being almost double that between 1960 and 1993 (+28 mm/y vs. +14.5 mm/y) (Fig. 1). At the decadal scale precipitation exhibited a decrease during the 1970s while, since the 1990s, both seasonal and mean annual values continued to increase (as some precipitation data were absent between 1983 and 1992, changes in means of seasonal and annual precipitation from the 1980s were not statistically significant) (Table 1 Supplementary Materials). As with air temperature, the largest precipitation increases were observed in summer (Table 1 Supplementary Materials), and there was also increased occurrence (more than twofold) of intense precipitation events (>30 mm water equivalent per day). Summer precipitation also now falls mainly as rain and is therefore immediately available to terrestrial organisms.
3.2 Deschampsia antarctica
Fifty years after Edward’s (1972) survey, D. antarctica and C. quitensis have both undergone large increases in number of sites occupied, and percent cover (Table 1). The total number of sites occupied by D. antarctica increased by 104 %, and many areas previously occupied by scattered populations are now characterized by patches of continuous sward. Newly colonized sites were predominantly adjacent to those which hosted the grass in the 1960s. A simple estimate of total percent cover suggests a 191 % increase between the two surveys (Table 1). There was no evidence for any upwards migration at higher elevations (Fig. 2a) and, indeed, the highest elevation recorded decreased from 137 to 91 m (Fig. 2a). Almost 95 % of D. antarctica records were located below 60 m both in the 1960s and 2009 (Fig. 2a), this elevation acting apparently as a threshold.
Percentage of population (columns) and number of sites (squares) occurring at different ranges of elevation (0–20; 21–40; 61–80; 81–100; 101–120; 121–140 m a.s.l.) and aspect (sectors, see Section 2) for Deschampsia antarctica (a elevation, b aspect) and Colobanthus quitensis (c elevation, d aspect). Legend: * = statistically significant differences between 1960s and 2009 (p < 0.05); + = occurrence at one site at an elevation between 121 and 140 m in 1960
Considering all data, there was a maximum elevation decrease (‘All’ in Fig. 3a) of D. antarctica, although this integrated different patterns apparent in the separate population size classes (Fig. 3a). There was a maximum elevation decrease in the small (S2) and intermediate (S3) populations, and the opposite pattern for the smallest (S1), large (S4) and largest (S5) populations. Only the largest population (S5) exhibited an increase of +10 % over the 50 year period above 60 m. The differences in elevation of sites occupied were statistically significant (Wilcoxon test) in the entire dataset (All) as well as in separate population size classes other than S2 (Fig. 3a).
Relation of the occupied sites with elevation (m) of a D. antarctica and b C. quitensis, for the entire populations (All) and the single population size classes (S1-S5, from smallest to largest) with changes of minimum, median, 25° and 75° percentile and maximum elevation. Legend: statistically significant differences between 1960s and 2009 (according to the Wilcoxon test) * = p < 0.05; ** = p < 0.01
The changes with slope were not statistically significant (p > 0.05, Wilcoxon test) (data not shown).
There were statistically significant changes with respect to aspect for the entire dataset (p < 0.01, Wilcoxon test) (Fig. 2b), with a decrease of the proportion of the overall population occurring in north-exposed sites in favour of the west, south-east and south-west sectors. However, among the different population sizes, only the small (S2) exhibited statistically significant differences (p = 0.01, Wilcoxon test) with respect to aspect (data not shown).
Fur seal damage (expressed as the percent of D. antarctica sites measured that had fur seal damage) was mainly observed below 60 m, decreased with increasing elevation, and was highest from sea level up to 20 m (0–20 m = 64.6 %; 21–40 m = 49.3 %; 41–60 m = 22.2 %; above 61 m < 20 %).
The multivariate analyses (CCA) showed that the different population size classes (s1-s5, smallest to largest) underwent changes between the 1960s and 2009 (Fig. 4a). The most important environmental factor responsible for these changes was air warming (p < 0.01, F = 53.8), affecting all the size classes. Topographic parameters acted as secondary factors (slope: p < 0.01, F = 3.2; elevation: p < 0.01, F = 2.9; aspect: p < 0.01, F = 2.7). The biotic disturbance factors exerted a limited influence (F < 0.6) when compared to that of air warming. Among them, fur seal disturbance (F = 0.57) exhibited a direct relationship with the dominant climate factor and was correlated to the observed population changes since the 1960s, but with low conditional effects (Table 2 Supplementary Materials) and, among the 2009 populations, a tighter association to the smallest and small populations (S1, S2).
Biplots of the canonical correspondence analysis (CCA) showing the position of the different sized populations (s1-s5, smallest to largest, represented by quadrats of increasing size) of D. antarctica with respect to the environmental factors (represented by vectors) in: a 1960s vs. 2009 (cumulative percentage of variance of species-environmental relation: Axis 1 = 84.0 %; Axis 2 = 11.2 %); b 2009 (cumulative percentage of variance of species-environmental relation: Axis 1 = 72.7 %; Axis 2 = 15.9 %). Each biplot shows the correlation between quadrats and vectors. The product of the vector length and the cosine of its angle with each biplot axis (axis1, axis2) is proportional to the correlation of each vector with each axis (e.g. in 1960s vs. 2009 ‘air warming’ is the most important factor correlated with axis 1 while ‘aspect’ and ‘slope’ correlate with axis 2; in 2009 ‘fur seals’ is the most important factor correlated with axis 1, while ‘elevation’ and ‘slope’ correlate with axis 2). Ranking of each quadrat along a vector is inferred by projecting the quadrat onto the vector (e.g. in 1960s vs. 2009, the 1960s populations show negative correlations with air warming, while the 2009 populations show positive correlations with this factor; in 2009 the smallest and small populations - S1, S2 - are more strongly correlated to fur seals than the large and largest - S4, S5 - populations). In both analyses, the direction of the vector labelled as “aspect” indicated south. Scores of the CCA are reported in Supplementary Materials Table 2
With reference to the contemporary influence of biotic and anthropogenic disturbance factors on D. antarctica (2009 data, Fig. 4b), of the potential biotic and anthropogenic disturbance factors, evidence for fur seal disturbance was apparent in the smallest, small and intermediate (S1, S2, S3) rather than the large and largest (S4, S5) population size classes (p < 0.01, F = 7.47) (Fig. 4b). Penguins (F = 0.33) and trails (F = 0.94) exerted an extremely limited influence.
In both surveys, C. quitensis was considerably less common than D. antarctica, but it again showed a clear increase of 35 % in the number of sites occupied across the island (Table 1). New colonization again occurred adjacent to sites which were already occupied in the 1960s. Total estimated percent cover increased by 208 % between the two surveys (Table 1). There was again no evidence of upwards migration, with a decrease of the maximum elevation of sites occupied recorded from 116 to 88 m, and areas of local decrease generally at altitudes below 20 m (see bars in Fig. 2c). The maximum elevation decrease was attributable to the smallest (S1) and intermediate (S3) population size classes (which decreased the maximum elevation of their sites of 75 m and c. 60 m, respectively) (Fig. 3b), while the small (S2), large (S4) and largest (S5) classes increased their maximum elevation by between c. 20 and 65 m. Overall, the core distribution of the entire dataset of C. quitensis suffered a range contraction over the last 50 years (Fig. 3b). Changes were also detected with respect to aspect (Fig. 2d), with increases in the north- and north-west facing sites and decreases in the other aspects, contrasting with the decrease in north-facing slopes documented for D. antarctica. However, the changes with slope (data not shown) and aspect (Fig. 2d) were not statistically significant (p > 0.05, Wilcoxon test).
Fur seal damage (expressed as the percent of sites measured that had fur seal damage) on C. quitensis was observed only below 60 m (0–20 m = 21.4 %; 21–40 m = 21.4 %; 41–60 m = 37.5 %).
The multivariate analysis (CCA) did not provide statistically significant results for C. quitensis, probably due to the smaller number of records available.
4 Discussion
4.1 Number of sites colonized and percent cover changes
Our data show that both species exhibited large increases in the number of sites occupied and percent cover, across Signy Island in the last 50 years. These results confirm the trends of these two species reported at some more restricted locations in the maritime Antarctic. According to Smith (1994) the large rates of increase recorded here for these two species (Table 1) are greater than would be expected either in a state of climatic equilibrium (where establishment of new plants should compensate loss of old plants) or during the colonization of recently deglaciated sites (where the increase rate is relatively low). In the context of other recent studies (Smith 1994; Fowbert and Smith 1994; Parnikoza et al. 2009; Torres Mellado et al. 2011; Vera 2011), it is likely that the changes observed in D. antarctica at Signy Island have been promoted by recent climate warming (Fig. 4a). The available studies have covered different time spans, smaller physical areas and overall population sizes than those described here: 1960s–1990s by Fowbert and Smith (1994) and subsequently to 2007/08 by Parnikoza et al. (2009) in the Argentine Islands; 1980s–2009 by Torres Mellado et al. (2011) for areas of King George and Robert Islands. Furthermore, some of these studies have indicated that trends may not be continuous (Parnikoza et al. 2009), or spatially uniform, with some locations showing contrasting patterns (Torres Mellado et al. 2011).
Of the studies available, our data are most comparable with those of Parnikoza et al. (2009), with both studies covering a similar time period (1960s-2008/9). However, the extremely small population of C. quitensis in the Argentine Islands means that comparisons can only be made for D. antarctica. Over this period, in both studies this species showed similar overall increases in the number of occupied sites (+136 % in Parnikoza et al. (2009), compared with +104 % in this study), even though the rate of summer warming was greater at Signy Island (β = 0.0224, p < 0.01) than at the Argentine Islands (β = 0.0128, p < 0.01) (as tested by linear regression).
4.2 Environmental drivers and mechanisms of change
Among the factors likely to underlie the increase in populations, environmental manipulation experiments have suggested that atmospheric warming is more influential than water or nutrient addition (Day et al. 2008). However, air warming could also improve nutrient availability due to the potential increase in the rate of soil organic matter decomposition (Mack et al. 2004). The spring and autumn warming experienced at the Argentine Islands may also have extended the growing season length and improved seed maturation, germination and establishment (Convey 1996a), thereby enhancing the reproductive success and recruitment of D. antarctica. As well as air warming, the strong increase in summer precipitation at Signy Island (Fig. 1, Table 1 Supplementary Materials) is likely to have enhanced water availability.
Relating to the processes of spatial colonization and development, both species adopted similar strategies through recruitment primarily taking place from neighbouring pre-existing populations and the formation of coalesced aggregates of individuals, as new colonization for both species predominantly occurred adjacent or very close to sites which were already occupied in the 1960s. This has been noted elsewhere for D. antarctica in Antarctica (Vera et al. 2013), and for other species in the colonization of open and disturbed alpine and polar environments, such as at the treeline (Gehrig-Fasel et al. 2007) and in shrub expansion and the re-colonization of active layer detachment slides (Cannone et al. 2010).
Warming temperatures have led ecologists to predict that vegetation gradients will “march up the hill” as climate envelopes shift with elevation (Walther et al. 2002; Breshears et al. 2008). Such shifts in elevation can be understood as the result of enhanced growth and new establishment at higher elevations. However, our data are not consistent with this general prediction, with both species showing a decrease of their maximum elevation below the 1960s values. Above the 60 m elevation threshold, despite a small increase in the number of occupied sites, both species suffered a range contraction, as their maximum elevation decreased from that recorded in the 1960s’, although this integrated different patterns in the separate population size classes (Figs. 2a, c, 3a, b).
Further, more than 90 % of the individuals of both species were located below an elevation threshold of 60 m (Figs. 2a, c, 3a, b). Notably, this threshold coincides with the upper boundary of fur seal occurrence confirmed by direct observations of the occurrence of seal trampling/crushing of the surrounding bryophyte communities. The island’s annual fur seal census also covers those parts of the island from sea level up to 60 m (Favero-Longo, personal communication), with the most intense fur seal impacts occurring between sea level and 20 m, then decreasing progressively with elevation.
D. antarctica and C. quitensis are relatively tolerant (the former more so) to physical impacts caused by trampling and compaction by the increased fur seal numbers now present in summer at many maritime Antarctic sites (Smith 2003). Through its roots D. antarctica is able to acquire N as short peptides (i.e. at an early stage of protein decomposition) faster than amino acids, nitrate or ammonium, giving a significant advantage over competing mosses (Hill et al. 2011). Coastal bird and seal colonies are known to influence vegetation in their vicinity through increased nitrogen deposition (Lindeboom 1984). At Signy Island, fur seal occurrence is associated with a large increase in soil N stock; in sites influenced by fur seals C:N ratio ranges between 7.5 and 9.9, while in areas where fur seals are absent the ratio is much higher (13.1) (Favero-Longo et al. 2011).
We therefore hypothesize that the large increases in fur seals numbers recorded at Signy Island since the 1970s, may have induced a facilitation process at elevations between 20 and 60 m, which has promoted the increase of both D. antarctica (the number of sites where D. antarctica occurs increased, see squares in Fig. 2a) and of C. quitensis (the % of population and the number of occupied sites increased, see bars and squares in Fig. 2c), at least at locations where this effect was not outweighed by the damage of physical trampling.
Between 20 and 60 m, it is likely that the combination of climate warming and fur seal nutrient input interacted to produce convergent positive impacts. Indeed, the large/largest (S4, S5) populations of both species (and the intermediate S3 of C. quitensis) increased the elevation of their core distribution (75 % increased for all, the median increased only for S3 and S4 of C. quitensis, Fig. 3a, b), although remaining well below the maximum elevation recorded in the 1960s dataset.
The presence of seabirds and marine mammals provides effective predictors for the distribution of D. antarctica in the South Shetland Islands (Park et al. 2012), due to the preference of this species for fertilized soils, although soil fertility alone is not enough to promote success, which depends on a combination of soil fertility, drainage and snow patterns (Park et al. 2012). A manipulation experiment involving fertilization carried out on D. antarctica over a decade at Signy Island (Smith 1994) demonstrated that the success of fertilization depends on soil chemical characteristics: at a high elevation site deficient in soil nitrogen D. antarctica responded positively to nutrient addition, while there was no significant change at a low elevation site with optimal soil nutrient status.
Facilitation of plant establishment by fur seals has been reported recently for some exotic species occurring on Marion Island (Haussmann et al. 2013). In this instance their physical trampling damaged the native vegetation and provided new niches for the exotic species, as trampling had greater impact on less resilient native species, and the soil nutrient enrichment gave them a competitive advantage (Haussmann et al. 2013).
Climate warming is expected to interact with the environmental envelopes of terrestrial plants and animals, leading to alterations in ranges upwards or towards higher latitudes mainly in response to increases in air temperature (Walther et al. 2002). At sub-Antarctic Marion Island, vascular plant distributions have increased their maximum elevation by around 70 ± 30 m over a 40 y period (1966–2006) in response to a warming of +1.2 °C (le Roux and McGeoch 2008). Most of the species analyzed on Marion Island were habitat generalists, as are D. antarctica and C. quitensis in the current study. Therefore, the heterogeneity of responses with elevation observed at Signy Island may depend on the effect of other environmental drivers.
Disturbance can affect ecosystems and physical disturbance has been recognized to be a potentially effective factor in shaping species distributions (le Roux et al. 2013). At high latitudes and elevations disturbance related to geomorphological processes (frost creep, frost heave, cryoturbation, landslides, solifluction, gelifluction, erosion, etc.) is frequent, widespread and often intense, and affects the fine-scale distribution and dynamics of many biological communities (Convey 1996b; Cannone and Gerdol 2003; Walker et al. 2004; Engelen et al. 2008; Lantz et al. 2009; Cannone et al. 2010; Virtanen et al. 2010). The effect of such physical disturbance may explain some idiosyncratic and unexpected species responses to climate warming, such as instances of downslope migration or lack of upward migration, that have been described worldwide in high elevation and high latitude sites (Walther et al. 2005; Cannone et al. 2007). At Signy Island the observed decreases in maximum elevation since the 1960ss may be related to changes in the permafrost conditions on the island, and/or disturbance related to the active layer thickness and dynamics, frost heave and freeze-thaw cycles. At higher elevations the depth of frost action coincides with the root depth zone of vascular plants (c. 10–20 cm, direct field observation) and this could limit plant colonization and persistence through gelifluction, cryoturbation and/or ice segregation in the soil (Guglielmin et al. 2012). Manipulation experiments performed at Signy Island demonstrated that at a high elevation site (Jane Col, 140 m), the survival of D. antarctica was reduced due to the exposure to low temperatures combined with freeze-thaw cycles and the uprooting effect on plants of needle ice formation (occurring in the upper 1–2 cm of the soil), while the same type of manipulation did not exert negative impacts on the survival of this species at a low elevation site (Factory Cove, 5 m) (Smith 1994), where these physical disturbances did not occur.
Snow cover thickness and persistence may also be a potential driver: snow cover is likely to be thicker and spring melt occur later at higher elevations, hence reducing the growing season length. An indirect confirmation of this hypothesis is provided by the distribution patterns of D. antarctica and C. quitensis at Livingston Island, where at the highest altitudes both species only occur at restricted sites that are frequently snow-free in the early austral summer (Vera 2011). Park et al. (2013) reported that heavy snowfall may affect the survival of D. antarctica and that the amount of snowfall could be an important factor limiting the species’ distribution, its density being higher where snow melts earlier.
Therefore, the recent increase in number of occupied sites and percent cover of D. antarctica and C. quintensis at Signy Island is likely to be underlain by the interaction of multiple drivers. These changes also indicate that the ecological processes occurring in maritime Antarctica are similar to those in the Arctic (Callaghan et al. 2013). Understanding the role of disturbance drivers for vegetation change in cold regions may become a research priority to enable improved forecasting of biological responses and feedbacks between climate warming and ecosystems in these globally influential regions.
References
Breshears DD, Huxman TE, Adams HD, et al. (2008) Vegetation synchronously leans upslope as climate warms. PNAS 105:11591–11592
Callaghan TV, Jonasson C, Thierfelder T, et al. (2013) Ecosystem change and stability over multiple decades in the Swedish subarctic: complex processes and multiple drivers. Phil Trans R Soc B 368:20120488
Cannone N, Gerdol R (2003) Vegetation as an ecological indicator of surface instability in rock glaciers. Arct Antarct Alp Res 35:384–390
Cannone N, Ellis Evans JC, Strachan R, Guglielmin M (2006) Interactions between climate, vegetation and active layer in Maritime Antarctica. Antarct Sci 18(3):323–333
Cannone N, Sgorbati S, Guglielmin M (2007) Unexpected impacts of climate change on alpine vegetation. Front Ecol Environ 5(7):360–365
Cannone N, Lewkowicz AG, Guglielmin M (2010) Vegetation colonization of permafrost-related landslides, Ellesmere Island, Canadian High Arctic. J Geophys Res 115:G04020
Convey P (1996a) Reproduction of Antarctic flowering plants. Antarct Sci 8:127–134
Convey P (1996b) The influence of environmental characteristics on life history attributes of Antarctic terrestrial biota. Biol Rev Cambr Phil Soc 71:191–225
Convey P (2011) Antarctic terrestrial biodiversity in a changing world. Pol Biol 34(11):1713–1725
Convey P, Smith RIL (2006) Responses of terrestrial Antarctic ecosystems to climate change. Plant Ecol 182:1–10
Day TA, Ruhland C, Xiong FS (2008) Warming increases aboveground plant biomass and C stocks in vascular-plant-dominated Antarctic tundra. Glob Chang Biol 14:1827–1843
Edwards JA (1972) Studies in Colobanthus quitensis (Kunth) Bartl. and Deschampsia antarctica Desv.: distribution, ecology and vegetative performance on Signy Island. Br Antarct Surv Bull 28:11–28
Elmendorf SC, Henry GHR, Hollister RD, et al. (2012) Plot-scale evidence of tundra vegetation change and links to recent summer warming. Nat Clim Chang 2:453–457
Engelen A, Convey P, Hodgson DA, et al. (2008) Soil properties of an Antarctic inland site: implications for ecosystem development. Pol Biol 12:1453–1460
Favero-Longo SE, Cannone N, Worland MR, et al. (2011) Changes in lichen diversity and community structure with fur seal population increase on Signy Island, South Orkney Islands. Antarct Sci 23:65–77
Favero-Longo SE, Worland MR, Convey P, et al. (2012) Primary succession of lichen and bryophyte communities following glacial recession on Signy Island, South Orkney Islands, Maritime Antarctic. Antarct Sci 24(4):323–336
Fowbert JA, Smith RIL (1994) Rapid population increases in native vascular plants in the Argentine Islands, Antarctic Peninsula. Arct Alp Res 26:290–296
Gehrig-Fasel J, Guisan A, Zimmermann NE (2007) Tree line shifts in the Swiss alps: climate change or land abandonment? J Veg Sci 18:571–582
Guglielmin M, Ellis Evans CJ, Cannone N (2008) Active layer thermal regime under different vegetation conditions in permafrost areas. A case study at Signy Island (Maritime Antarctica). Geoderma 144:73–85
Guglielmin M, Worland MR, Cannone N (2012) Spatial and temporal variability of ground surface temperature and active layer thickness at the margin of maritime Antarctica, Signy Island. Geomorphology 155:20–33
Haussmann NS, Rudolph EM, Kalwij JM, et al. (2013) Fur seal populations facilitate establishment of exotic vascular plants. Biol Conserv 162:33–40
Hill PW, Farrar J, Roberts P, et al. (2011) Vascular plant success in a warming Antarctic may be due to efficient nitrogen acquisition. Nat Clim Chang 1:50–53
Lantz TC, Kokelj SV, Gergel SE, et al. (2009) Relative impacts of disturbance and temperature: persistent changes in microenvironment and vegetation in retrogressive thaw slumps. Glob Chang Biol 15(7):1664–1675
le Roux PC, McGeoch M (2008) Rapid range expansion and community reorganization in response to warming. Glob Chang Biol 14:2950–2962
le Roux PC, Virtanen L, Luoto M (2013) Geomorphological disturbance is necessary for predicting fine-scale species distributions. Ecography 36:800–808
Lepš J, Smilauer P (2003) Multivariate analysis of ecological data using CANOCO. Cambridge University Press, Cambridge, 269pp
Lindeboom HJ (1984) The nitrogen pathway in a penguin rookery. Ecology 65:269–277
Mack MC, Schuur EAG, Bret-Harte MS, et al. (2004) Ecosystem carbon storage in Arctic tundra reduced by long-term nutrient fertilization. Nature 431:440–443
Maggini R, Lehmann A, Kéry M, et al. (2011) Are Swiss birds tracking climate change? Detecting elevational shifts using response curve shapes. Ecol Model 222:21–32
Park JS, Ahn I-Y, Lee EJ (2012) Influence of soil properties on the distribution of Deschampsia antarctica on King George Island, Maritime Antarctica. Polar Biol 35:1703–1711
Park JS, Ahn I-Y, Lee EJ (2013) Spatial distribution of the Antarctic hair grass Deschampsia antarctica in relation to environmental variables on Barton Peninsula, King George Island. Arct Antarct Alp Res 45(4):563–574
Parnikoza I, Convey P, Dykyy I, et al. (2009) Current status of the Antarctic herb tundra formation in the central Argentine Islands. Glob Chang Biol 15:1685–1693
Royles J, Ogée J, Wingate L, et al. (2012) Carbon isotope evidence for recent climate-related enhancement of CO2 assimilation and peat accumulation rates in Antarctica. Glob Chang Biol 18(10):3112–3124
Ruhland CT, Krna MA (2010) Effects of salinity and temperature on Deschampsia antarctica. Pol Biol 33:1007–1012
Smith RIL (1972) Vegetation of the south Orkney islands with particular reference to Signy Island. Br Antarct Surv Sci Rep 68:124 pp
Smith RIL (1988) Destruction of Antarctic terrestrial ecosystems by a rapidly increasing fur seal population. Biol Conserv 45:55–72
Smith RIL (1990) Signy Island as a paradigm of biological and environmental change in Antarctic terrestrial ecosystems. In: Kerry KR, Hempel G (eds) Antarctic ecosystems: ecological change and conservation. Springer, Berlin, pp. 32–50
Smith RIL (1994) Vascular plants as bioindicators of regional warming in Antarctica. Oecologia 99:322–328
Smith RIL (2003) The enigma of Colobanthus quitensis and Deschampsia antarctica in Antarctica. In: Huiskes AHL et al. (eds) Antarctic biology in a global context. Backhuys Publishers, Leiden, pp. 234–239
Ter Braak CJF, Verdonschot PFM (1995) Canonical correspondence analysis and related multivariate methods in aquatic ecology. Aquat Sci 57:255–264
Torres Mellado GA, Jaña J, Casanova-Katny MA (2011) Antarctic hairgrass expansion in the South Shetland archipelago and Antarctic Peninsula revisited. Pol Biol 34:1679–1688
Turner J, Bindschadler R A, Convey P, et al (eds) (2009) Antarctic climate change and the environment. Scientific Committee on Antarctic Research, Cambridge, 526pp
Vera ML (2011) Colonization and demographic structure of Deschampsia antarctica and colobanthus quitensis along an altitudinal gradient on Livingston Island, South Shetland Islands, Antarctica. Pol Res 30:7146. doi:10.3402/polar.v30i0.7146
Vera LM, Fernandez-Teruel T, Quesada A (2013) Distribution and reproductive capacity pf Deschampsia antarctica and Colobanthus quitensis on Bryers Peninsula, Livingston Island, South Shetland Islands, Antarctica. Antarct Sci 25(2):292–302
Virtanen R, Luoto M, Rämä T, et al. (2010) Recent vegetation changes at the high latitude tree line ecotone are controlled by geomorphological disturbance, productivity and diversity. Glob Ecol Biogeogr 19:810–821
Walker DA, Epstein HE, Gould WA, et al. (2004) Frost-boil ecosystems: complex interactions between landforms, soils, vegetation and climate. Perm Periglac Proc 15:171–188
Walther G, Post E, Convey P, et al. (2002) Ecological responses to recent climate change. Nature 416:389–395
Walther GR, Beißner S, Burga CA (2005) Trends in the upward shift of alpine plants. J Veg Sci 16:541–548
Waluda CM, Gregory S, Dunn MJ (2010) Long-term variability in the abundance of Antarctic fur seals Arctocephalus gazella at Signy Island, South Orkneys. Pol Biol 33:305–312
Acknowledgments
The authors thank PNRA (Progetto Nazionale di Ricerca in Antartide, Project 2013/C1.01; Project PDR2009/A2.12) for providing funding and BAS (British Antarctic Survey) for logistical support. The study contributes to the BAS ‘Polar Science for Planet Earth’ and SCAR ‘State of the Antarctic Ecosystem’ and ‘Antarctic Thresholds - Ecosystem Resilience and Adaptation’ (AnT-ERA) research programmes. Climatic data were provided by the Servicio Meteorologico Nacional, Centro de Informacion Meteorologica de Buenos Aires (Argentina), and we thank Lic. Ana Teresa Gomez. We thank three anonymous reviewers and the Editor for their comments and suggestions, allowing significant improvement of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cannone, N., Guglielmin, M., Convey, P. et al. Vascular plant changes in extreme environments: effects of multiple drivers. Climatic Change 134, 651–665 (2016). https://doi.org/10.1007/s10584-015-1551-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10584-015-1551-7







