Abstract
Inland Antarctic nunataks typically have simple physically weathered soils and limited ecosystem complexity. In this paper we present quantitative measurements of soil physical and chemical properties at one Antarctic nunatak. We measured pH, grain size, field capacity, soil organic carbon, phosphate, nitrate, ammonium and the cations magnesium, calcium and potassium along two transects. The data obtained indicated that very low levels of nutrients were present/available to biota, and that liquid water was absent, at least from the surface depths of soil, except during periods of active snow melt. Consequently, biological activity is severely limited. We conclude that, due to the climatic and microclimatic conditions at this location, the development of biological communities and soils is maintained in an extremely simple but still apparently stable ‘quasi climax’ state. Increased soil development and biological complexity can be expected if the contemporary rapid regional warming in the Antarctic Peninsula region continues.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Terrestrial, ice-free habitats in Antarctica are restricted to less than 0.35% of the entire Antarctic continent, with the remainder permanently covered by ice (British Antarctic Survey 2004). Over most terrestrial ice free habitats precipitation rates are very low, and many are classified as frigid deserts (Walton 1984; Sømme 1995). Desiccation stress is considered to be at least as great a constraint on biological activity as are the more obvious low temperatures (Kennedy 1993). Within Antarctica, terrestrial biologists conventionally recognise three distinct biogeographical zones: the continental, the maritime and the sub-Antarctic (Smith 1984).
Research on Antarctic soils has focused on the continental cold desert soils of the Dry Valleys in Victoria Land, on coastal areas of Wilkes Land, and on soils of the maritime Antarctic. The latter are generally described as sub-Antarctic brown soils or protorankers (Campbell and Claridge 1987; Bockheim and Ugolini 1990). Histosols also have been described from the maritime Antarctic (Fabiszewski and Wojtun 1993). Leptosols, regosols and podzols are typical soil formations of the coastal continental Antarctic (Blume and Bölter 1993, 1996). In contrast, soils of the frigid desert Victoria Land Dry Valleys are characterised by physical weathering only. Accumulation of carbonates and salts frequently occurs (Campbell and Claridge 1987, Bockheim and Ugolini 1990, Beyer et al. 1999).
Among the spectrum of abiotic factors involved in the relatively simple Antarctic ecosystems, soils and their properties are an important element (Holdgate 1970). Antarctic soils are frequently exposed to climatic freeze—thaw events that lead to disturbance of substrata by cryoturbation (Chambers 1967; Hall and Walton 1992), leading to the formation of frost-sorted soil polygons, circles, nets, steps or stripes. These soil formations are often described as “patterned ground” (Washburn 1956). Their mode of formation has been modelled by Kessler and Werner (2003). Briefly, as ice forms on the soil surface, water is drawn up from the underlying soil by capillary action and soil particles are uplifted to take its place. As a result of this upwards frost heave process, loose stones lying on the surface are displaced, eventually collecting in a frost-sorted pattern around each small surface rise. More complicated patterns, such as polygons, arise when a second mechanism interacts with frost heave. The stone piles are compressed when the soil around them freezes and expands. If this compression is sufficiently intense, avalanches of stones form lines and polygons.
Detailed studies have been carried out on polygon soil nutrients and biological colonisation at Signy Island (South Orkney Islands, northern maritime Antarctic, 60°S). Here, nitrate has been found to be a limiting nutrient for soil biota (Arnold et al. 2003), supporting the earlier studies of Davey and Rothery (1992) on factors limiting the growth of terrestrial microalgae on this island. Low levels of nitrogen, rather than the physical environmental factors of low temperatures and freeze-thaw events, lead to the limitation of microalgae in late summer. Arnold et al. (2003) found no significant variations in inorganic nutrients either within or between polygons, while Davey and Rothery (1992) reported clear intra-polygonal differences in physical and chemical properties but very limited inter-polygonal differences. Based on striking differences in microalgal community composition between otherwise similar soil polygons, Davey and Rothery (1992) hypothesised that colonisation of fellfield polygons is a stochastic rather than a nutrient limited event.
In Antarctic soils cryoturbation may be a limiting factor for the establishment and survival of biota (Smith 1993; Wynn-Williams 1993) and, hence, the development of ecosystems. Cryptogamic communities composed of lichens and bryophytes, which lack the root systems of higher plants, as well as soil inhabiting microbiota, are potentially well-equipped to tolerate such conditions, and ice free areas of the continental and maritime Antarctic are predominantly colonised by these organisms (Smith 1984; Kanda et al. 2002; Olech 2002; Seppelt 2002). However, to date research on soils and their developing communities in the maritime Antarctic has been restricted to the relatively well developed and colonised soils of coastal locations of the northern and central maritime Antarctic, especially those of Signy Island (ca. 60ºS) in the South Orkney Islands, the South Shetland Islands (62–63°S) and Adelaide Island (ca. 68ºS). However, at the southern limit of the maritime Antarctic (70–72°S), ice-free ground is found at locations remote from maritime influence, particularly in the ablation areas of the Fossil Bluff Formation exposed on south-eastern Alexander Island (Convey and Smith 1997). These areas are visibly subject to considerable cryoturbation, as well as containing biological communities that are apparently at an earlier stage of development and with much lower complexity than those of more northern localitions. Little effort has yet been made to describe the properties of polygon soils at these inland nunataks, or consider how they influence the development of biological communities.
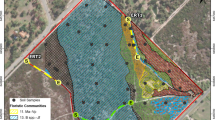
In this study we examined soil properties at Coal Nunatak (72°4′S 68°31′W) (Fig. 1) and their implications for ecosystem development. Coal Nunatak is located on south-eastern Alexander Island off the west coast of the Antarctic Peninsula. It is protected from the direct influence of the open sea, over 200 km due west in summer, by the high landmass of Alexander Island and the ice shelves that fringe its west coast, and by the permanent ice shelf that occupies George VI Sound to the east (6 km from the study site) and south (20 km from the study site). Lying at the extreme southern limit of the maritime Antarctic, this region’s climate is considered to be intermediate between that of the moist maritime region and the colder and drier continental zone (Smith 1988).
Localities: a Alexander Island, the arrow marks the location of Coal Nunatak; b Coal Nunatak, the circle marks the location of the research area at the north eastern extent of the Nunatak; c The research area on Coal Nunatak, position of transect 1 (T1) and transect 2 (T2), the numbers 1–6 mark the positions of the polygons 1–6
Materials and methods
Research site
Coal Nunatak is located in the south-eastern part of Alexander Island (Fig. 1). It is one of a number of nunataka at the southern end of a mountain ridge, dissected by glaciers, oriented north-south along the east coast of Alexander Island. Geologically, Alexander Island consists of thick fluviatile, deltaic and submarine fan sedimentary rocks forming the Fossil Bluff Group. This was deposited unconformably during the Cretaceous on the older deformed metasediments of the LeMay Group, the oldest rocks to outcrop on Alexander Island, formed during the middle Jurassic, prior to the break-up of Gondwana. Coal Nunatak is a member of the Triton Point formation. Its upper 150 m is composed of very fine to very coarse sandstones and mudstones with interbedded palaeosols and occasional beds of vitric tuff representing fluvial channel floodplain deposits with meandering facies including notable exposures of fossil forests (Nichols and Cantrill 2002; Howe and Francis 2005 and references therein). Terrestrial habitats of Coal Nunatak include areas of frost-sorted polygon sandstone and mudstone dominated cryosol soils formed from the weathering of local sedimentary rocks, and exposed bedrock formations present along the relatively broad and flat summit ridge of the nunatak (380–424 m asl). A very limited lichen and bryophyte flora is present, restricted to rock surfaces and crevices and to the margins of soil polygons. Air temperatures range between extremes of −45°C in the winter months and +7°C in summer. The mean annual temperature is about −10°C. The mean annual humidity has not been measured. Surfaces of rocks exposed to direct insolation can reach temperatures up to +26°C in summer.
Two research sites were selected at Coal Nunatak (Fig. 1). Transect 1, (T1) was 240 m long at an altitude of ca. 380 m asl, sloping ca. 5°WNW. It consisted of polygons of approximately 2.5 m diameter. Transect 2 (T2) was 70 m long at an altitude of ca. 420 m asl. It included smaller polygons of approximately 1 m diameter, and sloped ca. 10°S to end above the margin of a glacier. Transect T1 was covered by snow until beginning of January, then remained snow free until early March, while T2 was covered by snow until mid-December and then remained snow free until early March. Along both transects three soil polygons were selected for more detailed studies of soil properties. In T1 they are numbered polygon 1, 2 and 3 and in T2 polygon 4, 5 and 6 (Fig. 1c). Soil samples were taken from each polygon at three different sampling points. One sample was taken from the polygon centre and two samples form opposite positions of the polygon margins. Therefore each transect includes nine sampling points. During the summer study period no permafrost was present at the study sites up to a depth of 50 cm.
Soil sampling
Along each transect samples of soil fines were collected from each of the three selected polygons. Samples were taken from three different micro sites across each polygon, one from each margin (collecting the fines from <5 cm from the margin) and one from the polygon centre. Sampling depth was 0–7 cm. This sampling depth was chosen to correlate the results parallel to investigations on microbial diversity (sampling depth 0–5 cm). Quantities sampled were limited by the requirement not to damage the habitat, and targeted at the biological/chemical analyses. The samples (ca. 30 g) were collected using acid washed polyethylene cylinders (diameter 45 mm, length 70 mm), and were stored and transported with care so as to avoid disturbance and mixing. They were transported to the Rothera Research Station, after which they were stored and transported to Germany at −20°C.
A broad range of standard soil analytical methods was used to provide detailed characterisation of the soils at Coal Nunatak. For each set of three soil samples from each polygon, these analyses included the following: grain size distribution, total organic carbon (TOC), phosphorus, nitrogen, nutrient cations (K+, Ca2+, Mg2+), cation exchange capacity (CEC), soil pH, field capacity (a measure of the percentage of water remaining in the soil 2–3 days after it has been saturated and free drainage has ceased) and soil water content. For all analyses except pH, field capacity and water content, dried samples of fine soil (grain size < 2 mm) were used. For TOC analysis 2 g of fine soil was used. Investigations on pH, field capacity and water content were carried out using intact soil cores. Combustion of the samples was carried out at 480°C to determine organic carbon. 5 g of fine soil was required for analyses of the content of phosphorus and the soil pH. For nitrogen analysis 15 g (5 g for ammonium and 10 g for nitrate) and for cations 5 g of fine soil was required. The grain size distribution was determined from a sample of 30 g of fine soil. For measurement of field capacity and water content entire soil cores were used.
Grain size distribution of silt, clay and sand was obtained using the pipette analysis of Köhn (1928) for fractions <0.063 mm, and the sand fraction (0.063–2 mm) was quantified by sieve analysis (Leser 1977). TOC was quantified using a carbon analyser DIMA-1000 (Dimatec). Available phosphate was quantified using the CAL-method (Schüller 1969; Hoffmann 1991). Ammonium and nitrate were quantified by a photometric method using the commercially available kits Spectroquant 14752 and 14733, respectively (E. Merck, Darmstadt). Cations (K+, Ca2+, Mg2+) were determined by atomic absorption spectrometry (AAS) (Varian SpectrAA 100). For AAS-analyses, cations were initially extracted from soil samples into an ammonium-acetate extraction solution (pH 6.8–7.2) of 1 mol l−1 (Scheffer and Schachtschabel 2002). CEC is calculated by adding up the milli-equivalents of acidity, calcium, magnesium and potassium. For determination of soil pH, 5 g sub-samples of soil were eluted with distilled water (10 ml) and 10 mM CaCl2. The pH of the resulting suspension was measured using a commercial glass-electrode and a pH meter (Jenway pH-Meter 3310). Soil water content and field capacity were measured using a gravimetric method.
Results
Transect 1 (T1)
There were few systematic differences between the three polygons along this transect, although the field capacity was lower at polygon 3 (Fig. 2). Soil water content was greatest at polygon 2 (Fig. 3), located in a slight depression where snow and meltwater can accumulate, but water content at all three polygons was clearly lower than the field capacity. Two polygons shared an almost homogenous distribution of grain size fractions, while the coarse sand fraction was dominant at one polygon (Fig. 4). Soil pH was close to neutral and consistent across all three polygons (Fig. 5). Total organic carbon (TOC) was present at low levels, with the minimum being seen in polygon 3 (Fig. 6).
Soil nutrient levels were very low across T1 (Fig. 7). Nitrogen levels (both ammonium and nitrate) were below detection limits (nitrate < 1.00 ppm, ammonium < 0.03 ppm). Cation composition was dominated by Ca2+, with both Mg 2+ and K+ detected at low levels (Fig. 8). Cation exchange capacity (CEC) varied little across T1 (Fig. 9).
Transect 2 (T2)
Along T2 field capacity (Fig. 2) and soil water content (Fig. 3) were comparable at each polygon, with soil water content again being lower than the field capacity. Grain size distribution in all three polygons was dominated by the sand fraction (Fig. 4). Soil pH was again close to neutral, although there was a trend towards increasing acidity down the slight slope from polygon 4 to 6 (Fig. 5). TOC also increased between each polygon down this slope, with the greatest levels of TOC present in polygons 5 and 6 (Fig. 6). Phosphate concentrations in polygon 5 and 6 were clearly higher than that of polygon 4 (Fig. 7). Nitrogen levels (ammonium and nitrate) were again below detection limits. Cation content was again dominated by Ca2+ (Fig. 8), with highest cation concentrations and CEC found in polygons 5 and 6 (Fig. 9).
Comparisons between T1 and T2
Notwithstanding the overall similarity in the measured soil parameters, subsamples obtained across each polygon were generally different, such that only those from polygons 5 and 6 (T2) showed no intrapolygonal variation. Field capacity (Fig. 2) differed little between the two transects, varying between 17.9 and 23.7% dry weight. Soil water contents were generally somewhat lower in T2 (Fig. 3). Grain size of soils across T2 was dominated by the sand fraction, while most of T1 showed more homogenous grain size distributions (Fig. 4). Soil pH differed little between the two transects (Fig. 5). The mean content across T2 was higher than that of T1. The lowest TOC levels were found in polygon 3 (T1; 2.58 mg kg−1), and the greatest in polygon 6 (T2; 12.5 mg kg−1 soil) (Fig. 6). Concentrations of the nutrient phosphate ion were also distinctly higher across T2, and ranged between 23.7 mg kg−1 soil (polygon 1) and 104.03 mg kg−1 (polygon 2) (Fig. 7). Low nitrogen levels at both transects indicate that these soils are extremely deficient in available nitrogen. Cation concentrations and CEC were mostly greater in T2 than T1 (Figs. 8, 9), Ca2+ consistently being present at the highest level in all polygons and K+ at the lowest. Ca2+ had consistently the greatest influence on CEC, followed by Mg 2+, K+ and H+.
Discussion
The extremely simple terrestrial ecosystem present on Coal Nunatak appears to be held at a very early developmental stage in comparison with better known and more-developed ecosystems from more northern, generally low altitude coastal, locations elsewhere in the maritime Antarctic or even those at lower altitude on Alexander Island (Smith 1984, 1988; Convey and Smith 1997). This could be considered to mirror the early stages of ecosystem development after glacial retreat, with the severe environmental conditions at locations such as Coal Nunatak serving to restrict further development and succession.
The soil parameters obtained in this study demonstrate some heterogeneity between polygons along the two transects consistent with the particle size sorting processes of cryoturbation and solifluction (Blume et al. 2002). The source of finer material is likely to result from the weathering of local bedrock with some proportion redistributed by wind. One of the most significant factors influencing both the physical processes in pedogenesis and the biological processes of colonisation and establishment is the availability of soil water. This is considered as the primary limiting factor for many soils in Antarctica, particularly those classified as frigid deserts (Kennedy 1993; Ellis-Evens 1997; Wynn-Williams et al. 1997; Campbell et al. 1998; Freckman and Virginia 1998). Although, during the Antarctic summer, water may become available locally through snow and glacial melt, the ability of soil to retain this is fundamental, and this is influenced primarily by grain size and organic content. The water contents of the soils investigated here ranged between 4.2 and 7% (mean 4.9%), comparable to values reported from coastal ecosystems of McMurdo Sound (Campbell et al. 1997, 1998). More extreme conditions are typical in the soils of the neighbouring Victoria Land Dry Valleys, averaging 0.45% in the soil surface layer up to 4.5 cm depth. The polygons present along the two study transects on Coal Nunatak differ in their developmental stage, with those of T2 being at an earlier stage, as indicated by being less well sorted and the presence of more coarse grained material.
Soil pH across the study site was close to neutral, indicative of soils in their earliest stage of development, as seen in the soils of extremely young moraines (<100 years) of the Windmill Islands (continental Antarctic coast) (Blume et al. 2002). However, within the data obtained some trends are apparent. The downslope decrease in soil pH along T2 may be linked with the higher TOC values also obtained along this transect, as soil acidification is often an effect of soil biological activity. The TOC levels found in Coal Nunatak soils, although very low in absolute terms, are likely to be derived from the activity of soil microbiota including bacteria, cyanobacteria (Brinkmann et al. 2007), algae and fungi (Brinkmann et al., unpublished data). Ahumic soils are defined as containing <1% dry mass of organic material (Bölter et al. 2002), and clearly include most of the soils examined in the present study. The soils of Coal Nunatak are generally intermediate between most of the soils of the Antarctic Peninsula and soils of continental Antarctica, such as the McMurdo Dry Valley.
The soils of polygons 5 and 6 (T2) can be classified as being primitive humic soils, slightly exceeding (1.14 and 1.25% TOC, respectively) the 1% threshold (Bölter et al. 2002). These values are very low even in comparison with other Antarctic soils, although comparable low levels have been reported by Blume et al. (2002) in cryosols of King George Island (South Shetland Islands) characterised by very low vegetation cover. In comparison, vegetated podzolised soils of the Windmill Islands contain 0.55–4.0% TOC, and guano enriched soils from recent Adélie Penguin rookeries in the same area reach 16–30% TOC (Blume et al. 2002). Lawley et al. (2004) measured organic contents ranging between 5.94% in soils of Rothera Point, Adelaide Island (68°S, western Antarctic Peninsula), 2.56% at Mars Oasis (72°S, Alexander Island, a low altitude site adjacent to the George VI Sound ice shelf, ca. 20 km north of Coal Nunatak), 2.3% at Signy Island (60°S, northern maritime Antarctic), 1.83% at Sky Hi Nunataks (75°S, Ellsworth Land) and 0.42% at the LaGorce Mountains (87°S, Transantarctic Mountains). In comparison with the current study at Coal Nunatak, these organic matter levels are all at least 2–5 times greater, with the exception of the extreme southern location of the La Gorce Mountains. However, Lawley et al. (2004) also reported higher TOC levels than the current study (4.34%) from different soil polygons elsewhere on Coal Nunatak, highlighting the possibility of considerable inter-site differences.
The very low nutrient levels found across all the study polygons do not permit informative statistical analyses. As with organic content, several nutrient measures (phosphorus, nutrient cations (Ca2+, Mg2+) and CEC) tended to be higher along T2. The content of K+ did not differ between transects, while nitrogen (nitrate or ammonium) levels were uniformly below detection limits. The slightly greater nutrient availability in soils of T2 may again be linked with the slightly greater levels of TOC and biological activity. The phosphorus contents of Coal Nunatak soils are comparable to the mineral soils of the Windmill Islands (Beyer et al. 2002), and more generally are slightly lower than values obtained from sandy soils, typically between 200 and 800 mg kg−1 at temperate latitudes (Scheffer and Schachtschabel 2002).
While water is acknowledged as one of most important and limiting factors for colonisation processes in Antarctic habitats, at least equally important is the availability of micro-niches where such water as is present can be retained. Such conditions are present along the margins of some well developed soil polygons in T1. The polygon margins have a more complex three dimensional structure than the majority of the polygon soil surface, and may experience decreased evaporation rates, thereby becoming more suitable for colonisation of macro-organisms such as lichens.
Soil movement may inhibit soil maturation and, therefore, colonisation by slow growing macro-organisms as lichens. This is despite the fact that soil under active cryoturbation can be effectively colonised by micro-organisms including algae and cyanobacteria (Bölter et al. 2002). Seen in this context, the fact that lichen and bryophyte colonisation is seen only along the margins of certain polygons in T1 is likely to be linked to reduced soil movement relative to the polygon centre. Increased water availability by snow accumulation may also be of importance. Romeike (2002) proposed a simple model for the colonisation by lichens of soils at polygon margins on Coal Nunatak. This model involved the initial fixation of unstable soil by fungal hyphae and cleistothecia as a prerequisite for colonisation by the lichen Psoroma cf. tenue. This species is the only lichen found at Coal Nunatak that includes both green algae and cyanobacteria as photobionts, and hence has nitrogen fixing capacity. Subsequently, community complexity increases as Lepraria sp. and Candelariella flava become established on and overgrow P. cf. tenue. Lichens of the genus Candelariella are known to be nitrophilic species which nevertheless are able to colonise nitrogen poor habitats if nitrogen fixing cyanobacteria are present (Poelt and Mayrhofer 1988).
It is clear from this study that these terrestrial ecosystems of Coal Nunatak include some of the most nutrient-poor soils yet to be examined from the Antarctic. Very low TOC values and an almost neutral soil pH indicate only very low levels of soil biological activity. These very basic soil ecosystems represent a very early stage in soil ecosystem physical and biological development, which nevertheless appears to be held at this relatively stable ‘quasi-climax’ level due to the prevailing extreme environmental conditions. In the context of the rapid regional warming that is being measured along the Antarctic Peninsula, and by comparison with neighbouring low-altitude sites at Mars Oasis and more distant locations throughout the maritime Antarctic, these soil ecosystems provide a valuable baseline for monitoring the consequences of rapid environmental change and for identifying when ‘quasi-climax’ conditions are replaced by accelerated biological successional processes relevant to both polar regions.
References
Arnold RJ, Convey P, Hughes KA, Wynn-Williams DD (2003) Seasonal periodicity of physical factors, inorganic nutrients and microalgae in Antarctic fellfields. Polar Biol 26:396–404
Beyer L, Blume HP, Bölter M, Kappen L, Kuhn D, Seppelt RD (2002) Soil ecology in relation to plant patterns. In: Beyer L, Bölter M (eds) Geoecology of Antarctic ice-free coastal landscapes. Ecological studies, vol 154. Springer, Berlin, pp 375–391
Beyer L, Bockheim JG, Campbell IB, Claridge GG (1999) Genesis, properties and sensitivity of Antarctic gelisols. Antarct Sci 11:387–398
Blume HP, Bölter M (1993) Soils of Casey Station, Antarctica. In: Gilichinski D (ed) Proceedings of 1st international symposium on cryopedol, Pushchino, Inst t Soil Sci Photosynth, Pushchino, pp 96–103
Blume HP, Bölter M (1996) Wechselwirkungen zwischen Boden- und Vegetationsentwicklung in der Kontinentalen Antarktis. Verh Ges Ökol 25:25–34
Blume HP, Kuhn D, Bölter M (2002) Soils and soilscapes. In: Beyer L, Bölter M (eds) Geoecology of Antarctic ice-free coastal landscapes. Ecological studies, vol 154. Springer, Berlin, pp 91–113
Bockheim JG, Ugolini FC (1990) A review of pedogenetic zonation in well-drained soils of the southern circumpolar region. Quad Res 34:47–66
Bölter M, Beyer L, Stonehouse B (2002) Antarctic coastal landscapes: characteristics, ecology and research. In: Beyer L, Bölter M (eds) Geoecology of Antarctic ice-free coastal landscapes. Ecological studies, vol 154. Springer, Berlin, pp 5–15
Brinkmann M, Pearce D, Convey P, Ott S (2007) The cyanobacterial community of polygon soils at an inland Antarctic nunatak. Polar Biol 30:1505–1511
British Antarctic Survey (2004) Antarctica, 1:10 000 000 scale map. BAS (Misc) 11. British Antarctic Survey, Cambridge
Campbell IB, Claridge GG (1987) Antarctica: soils. Weathering processes and environments. Elsevier, New York
Campbell IB, Claridge GG, Balks MR, Campbell DI (1997) Moisture content in the soils of Mc Murdo Sound and Dry Valley region of Antarctica. In: Lyons WB, Howard-Williams C, Hawes I (eds) Ecosystem processes in Antarctic ice-free landscapes. Blakema, Rotterdam, pp 61–76
Campbell IB, Claridge GG, Balks MR, Campbell DI (1998) The soil environment of the McMurdo Dry Valleys, Antarctica. In: Priscu J (ed.) Ecosystem Dynamics in a Polar Desert: the Mc Murdo Dry Valleys. American Geophysical Union Antarctic Research Series, vol 72. pp 297–332
Chambers MG (1967) Investigations of patterned ground at Signy Island, South Orkney Islands: III. Miniature patterns, frost heaving and general conclusions. Brit Antact Surv B 12:1–22
Convey P, Smith RIL (1997) The terrestrial arthropod fauna and its habitats in northern Marguerite Bay and Alexander Island, maritime Antarctic. Antarct Sci 9:12–26
Davey MC, Rothery P (1992) Factors causing the limitation of growth of terrestrial algae in maritime Antarctica during later summer. Polar Biol 12:595–602
Ellis-Evens JC (1997) Micro-scale distribution of photoautotropic micro-organismsin relation to light, temperature and moisture in Antarctic lithosols. In: Lyons WB, Howard-Williams C, Hawes I (eds) Ecosystem processes in Antarctic ice-free landscapes. Blakema, Rotterdam, pp 89–102
Fabiszewski J, Wojtun B (1993) Peat-forming vegetation. In: Rakusa-Suszczzewski S (ed) The maritime Antarctic caostal ecosystem of Admirality Bay. Polish Academy of Science, Warsaw, pp 189–195
Freckman DW, Virginia RA (1998) Soil biodiversity and community structure in the Mc Murdo Dry Valleys, Antarctica. In: Priscu J (ed) Ecosystem dynamics in a Polar Desert: the Mc Murdo dry Valleys. American Geophysical Union Antarctic Research Series, vol 72, pp 323–335
Hall KJ, Walton DWH (1992) Rock weathering, soil development and colonisation under a changing climate. Philos Trans R Soc London Ser B 338:269–277
Hoffmann G (1991) Die Untersuchung der Böden. VDLUFA-Methodenbuch Band I, VDLUFA-Verlag, Darmstadt
Holdgate MW (1970) Introduction to Part VII. Vegetation. In: Holdgate MW (ed) Antarctic ecology. Academic Press, London, pp 729–732
Howe J, Francis JE (2005) Metamorphosed palaeosols associated with Cretaceous fossil forests, Alexander Island, Antarctica. J Geol Soc 162:951–957
Kanda H, Ohtani S, Imura S (2002) Plant communities at Dronning Maud Land. In: Beyer L, Bölter M (eds) Geoecology of Antarctic ice-free coastal landscapes. Ecological studies, vol 154. Springer, Berlin, pp 249–264
Kennedy AD (1993) Water as a limiting factor in the Antarctic terrestrial environment: a biogeographical synthesis. Arctic Alpine Res 25:308–315
Kessler MA, Werner BT (2003) Self-organisation of sorted patterned ground. Science 299:380–383
Köhn M (1928) Korngrößenbestimmung vermittels Pipettanalyse. Tonindustrie-Zeitung 53:729–731
Lawley B, Ripley S, Bridge P, Convey P (2004) Molecular analysis of geographic patterns of eukaryotic diversity in Antarctic soils. Appl Environ Microb 5963–5972
Leser H (1977) Feld- und Labormethoden der Geomorphologie. de Gruyter, Berlin
Nichols GJ, Cantrill DJ (2002) Tectonic and climatic controls on a Mezozoic forearc basin succession, Alexander Island, Antarctica. Geol Mag 139:313–330
Olech M (2002) Plant Communities on King George Island. In: Beyer L, Bölter M (eds) Geoecology of Antarctic ice-free coastal landscapes. Ecological studies, vol 154. Springer, Berlin, pp 215–231
Poelt J, Mayrhofer H (1988) Über Cyanotrophie bei Flechten. Pl Syst Evol 158:265–281
Romeike J (2002) Mikroklima und Musterbildungsprozesse—Komplexe Interaktionen in der Flechtenvegetation entlang eines Transekts der maritimen Antarktis. PhD Thesis. Heinrich-Heine-Universität, Mathematisch-Naturwissenschaftliche Fakultät, Düsseldorf
Scheffer F, Schachtschabel P (2002) Lehrbuch der Bodenkunde. Springer, Berlin Heidelberg
Schüller H (1969) Die CAL-Methode, eine neue Methode zur Bestimmung des pflanzenverfügbaren Phosphats in Böden. Pflanzenernährung und Bodenkunde 123:48–63
Seppelt R (2002) Plant communities at Wilkes Land. In: Beyer L, Bölter M (eds) Geoecology of Antarctic ice-free coastal landscapes. Ecological studies, vol 154. Springer, Berlin, pp 233–248
Smith RIL (1984) Terrestrial plant biology of the sub-Antarctic and Antarctic. In: Laws RM (ed) Antarctic ecology. Academic Press, London, pp 61–162
Smith RIL (1988) Bryophyte oases in ablation valleys on Alexander Island, Antarctica. Bryologist 91:45–50
Smith RIL (1993) Dry coastal ecosystems of Antarctica. In: van der Maarle E (ed) Ecosystems of the world 2A, dry coastal ecosystems. Polar regions and Europe. Elsevier, Amsterdam, pp 51–71
Sømme L (1995) Invertebrates in hot and cold arid environments. Springer, Berlin Heidelberg
Walton DWH (1984) The terrestrial environment. In: Laws RM (ed) Antarctic ecology. Academic Press, London, pp 1–60
Washburn AL (1956) Classification of patterned ground and review of suggested origins. GSA Bull 67:823–866
Wynn-Williams DD (1993) Microbial processes and the initial stabilisation of fellfield soil. In: Miles J, Walton DWH (eds) Primary Succession on Land. Blackwell, Oxford, pp 17–32
Wynn-Williams DD, Russel NC, Edwards HGM (1997) Moisture and habitat structure as regulators for microalgalcolonists in diverse Antarctic terrestrial habitats. In: Lyons WB, Howard-Williams C, Hawes I (eds) Ecosystem processes in Antarctic ice-free landscapes. Blakema, Rotterdam, pp 77–88
Acknowledgments
We thank the BAS field assistants Neil Stevenson and Robin Jarvis for their invaluable technical support in the field. The British Antarctic Survey and its staff at Rothera Research Station are thanked for their support. This study was financially supported by the Deutsche Forschungsgemeinschaft (Ot 96/10–1/2) and the Düsseldorf Entrepreneurs Foundation, and also forms an output of the BAS BIOFLAME, CACHE and SCAR EBA scientific programmes. The results are included in the doctoral thesis of A.E.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Engelen, A., Convey, P., Hodgson, D.A. et al. Soil properties of an Antarctic inland site: implications for ecosystem development. Polar Biol 31, 1453–1460 (2008). https://doi.org/10.1007/s00300-008-0486-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-008-0486-0