Abstract
Selfish genetic elements (SGE) get a transmission advantage (drive) thanks to their non-Mendelian inheritance. Here I identify eight steps during the reproductive cycle that can be subverted by SGEs to thrive in natural populations. Even though only three steps occur during meiosis, most cases of segregation distortion are considered “meiotic drive sensu lato.” As this is a source of unnecessary contradictions, I suggest always using the term “transmission ratio distortion” (TRD). Chromosomal SGEs (e.g., B chromosomes) exhibit almost all types of TRD. In plants, the best-studied type of TRD for B chromosomes occurs post-meiotically during male gametophyte maturation. However, in animals, the two main types are pre-meiotic and meiotic TRDs, in all cases associated with gonotaxis (i.e., a preference of B chromosomes for germ cells). Frequently, TRD drivers in genic SGEs (e.g., t-alleles and segregation distorters in Drosophila) are paralogous copies of genes from the standard genome, whereas their targets can be other genes or satellite DNA (satDNA). As B chromosomes are often rich in satDNA and contain paralogous copies of A chromosome genes, perhaps their drive mechanisms are similar to those of genic SGEs. So far, the only association between a B chromosome gene and TRD is the gene haplodizer in Nasonia vitripennis. The discovery of B-genes controlling B-drive in other species does not appear to be far off, but experimental crosses will be needed to simultaneously test the TRD of a given B chromosome and the expression of its genes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Dissecting Mendelian segregation
Mendelian segregation predicts that a heterozygote yields half of its gametes carrying one or the other allele. Mendel (1865) inferred his law of segregation analyzing the progeny of controlled crosses, without knowing the existence of either chromosomes or meiosis. He compared parents with offspring in controlled crosses and recognized the importance of both the production of gamete classes at an equal frequency and their random fertilization, in order that his predictions could be made on the basis of probability laws (Mendel 1865).
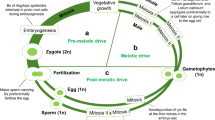
Mendelian inheritance is achieved when all variants in every locus have the same chance to be present in the next generation. However, reproduction is a complex process with numerous putative vulnerabilities that SGEs can exploit to gain a transmission advantage (named “drive”) (Fig. 1). Notably, only three of these steps (3–5) show a relationship with the mechanics of meiosis, in spite of which it is extremely widespread the use of the term “meiotic drive” for every kind of segregation distortion, even though Sandler and Novitski (1957), who first introduced the term, explicitly said that “where such a force, potentially capable of altering gene frequencies, is a consequence of the mechanics of the meiotic divisions, we suggest that the name meiotic drive be applied.” Few years later, Lewontin (1967), Wright (1968) and Sandler himself (Zimmering et al. 1970) noticed that the term meiotic drive was being used for cases in which meiotic disturbance was not apparent.
Opportunities for TRD of selfish genetic elements (SGE) during reproductive cycle in plants (a) and animals (b). The numbers within circles indicate (1) differential viability of SGEs during embryo cleavage mitoses; (2) preference of SGEs for flowers (in plants) or for germline cells during germ-soma differentiation (in animals); this is termed gonotaxis (see Burt and Trivers 2006); (3) absence of pairing during first meiotic prophase; (4) anomalous segregation at first meiotic anaphase; (5) non-disjunction during the equational division at second meiotic anaphase; (6) mitotic non-disjunction and preferential destiny to the generative nucleus (i.e., gonotaxis) during pollen grain maturation (in plants) or sperm killing during spermiogenesis (in animals); (7) mitotic non-disjunction and gonotaxis towards the egg nucleus during embryo sac maturation (in plants), or meiotic preferential segregation at anaphase I or II (depending on species) once female meiosis is reactivated after fertilization; and (8) preferential fertilization, especially in plants, as pollen phenotype largely depends on gene expression (Wendel et al. 1987). Note that only steps 3–5 occur during meiosis. In plants, meiosis is completed prior to gamete maturation and fertilization, whereas, in animals, fertilization triggers the completion of female meiosis
Although it appears to be only a semantic problem, using the term meiotic drive for every kind of segregation distortion, instead of only when steps 3–5 are involved, may give the impression that Mendelian segregation is equal to meiotic segregation. For instance, Cockburn (1991, page 72) defined segregation distortion as “the non–random partitioning of the chromosomal complement at meiosis.” Recent literature is not free from similar problems. For instance, it is confusing to read that “These results are consistent with the TRD mechanism being deviation from Mendelian inheritance rather than meiotic drive or segregation distortion” (Eversley et al. 2010), as if the two latter would not be cases of non-Mendelian inheritance. Or else “such conflict plays out in the arenas of meiosis and gametogenesis, and results in meiotic drive” (Lindholm et al. 2016), as if meiosis were not part of gametogenesis, a problem that is also apparent in “the classic meiotic drive systems in animals, including t-haplotype in mouse and Segregation Distorter (Sd) in Drosophila, are actually gamete killers that act during spermatogenesis rather than meiosis” (Fishman and Mcintosh 2019).
Even in the case that post-meiotical phenotypes (e.g., reduced sperm competitive ability or spore killing) were gestated during meiosis, e.g., through meiotic silencing of unpaired DNA (see Hammond et al. 2012; Svedberg et al. 2021), I suggest limiting definitions to phenotypic manifestation, as the ultimate causes still can change with research progress whereas phenotypes will remain the same. For instance, think about illnesses (e.g., dementia) manifested when we are old that were gestated during previous years and we still consider them to be elderly diseases.
I realize that using meiotic drive sensu lato (Tao et al. 2007) is not a problem for specialists but, in my opinion, it is an excessive simplification causing harm to teaching and science communication. Recently, Ren et al. (2021) defined “transmission ratio distortion” (TRD) as “the significant deviation from the expected ratio under Mendelian inheritance theory, which may be resulted from multiple disrupted biological processes, including germline selection, meiotic drive, gametic competition, imprint error, and embryo lethality.” On this same vein, I suggest using TRD for any kind of segregation distortion, as first done by Lyon (1984). TRD can manifest as “drive” or “drag,” when the transmission ratio is higher or lower than 0.5, respectively, and, when the exact nature of TRD is known, we can use “genic drive,” “centromeric drive,” “chromosome drive,” “meiotic drive,” “gamete or spore killing,” etc. Even though Sandler and Novitski (1957) illustrated their meiotic drive proposal with some examples of segregation distortions that were clearly post-meiotic, it is understandable that the unknowns from so many years ago justified this inconsistency. However, to give them due recognition, we should use “meiotic drive” only when TRD occurs during meiosis.
Next, I will review the known cases of TRD for B chromosomes in different organisms, as well as the putative genetical basis for them in several species where gene and repetitive DNA content have been analyzed, and also their similarities with the main features found for TRD in genic SGEs.
Non-Mendelian transmission of B chromosomes
Among the SGEs being able to subvert reproduction in its favor, supernumerary (B) chromosomes display examples of TRD for most of the weaknesses summarized in Fig. 1. The very nature of B chromosomes dictates their variation in number within populations, because they do not always go in pairs and cannot fulfill step 3 in Fig. 1. If B bivalents persist until meiotic metaphase I, they segregate to opposite poles and have no chance for meiotic drive or loss; however, B univalents can divide equationally in the first meiotic division and can sometimes lag and, perhaps, be lost (Fig. 2). The presence of B chromosome univalents is promoted by B mitotic instability, which is common in many species, as this yields intra-individual variation in the number of B chromosomes. This adds sophistication to B chromosome polymorphisms, as they can be restricted to the germline thus being less harmful on carrier fitness and being better tolerated by carriers (Camacho et al. 2000; Camacho 2005).
Examples of meiotic pairing between B chromosomes in the grasshoppers Eyprepocnemis plorans (a–b) and Locusta migratoria (c–d). The C-banded metaphase I cells in a and b show the heterochromatic nature of the two B chromosomes carried by an E. plorans male, as two univalents (B) in a and as a B-bivalent in b (BB). c and d show anaphase I and telophase I cells, respectively, which were submitted to FISH for LmiSat02-176, a satDNA which represents 55% of the B chromosome DNA in L. migratoria (Ruiz-Ruano et al. 2018). Both cells contain three B chromosomes, but the anaphase I (c) shows two Bs properly integrated with a given pole (B) whereas the other B is divided equationally into its two chromatids (b) which are in the same pole. In the telophase I cell (d); however, the two B chromatids (b) of the equationally divided B chromosome are lagging
A summary of the known cases of TRD for B chromosomes demonstrates that they entangle a variety of mechanisms for advantageous transmission illustrating most steps mentioned above. A general feature of B chromosome TRD is gonotaxis, i.e., their tendency to preferentially move to the germline during mitosis or meiosis (Burt and Trivers 2006). To remark the differentiation between meiosis and the whole Mendelian reproductive cycle, I will review the known cases of non-Mendelian inheritance of B chromosomes grouped as pre-meiotic, meiotic, and post-meiotic TRD.
Pre-meiotic TRD (steps 1 and 2)
Prior to meiosis, germline cells can undergo processes that may bias Mendelian inheritance. Male germ cells multiply through many rounds of spermatogonial mitoses which are the basis for the extremely high numbers of spermatozoa produced in the male sex. Oogonia undergo a much more limited number of pre-meiotic mitoses, and they begin meiosis much sooner than spermatogonia, so that, at birth, have their oocytes stopped in the middle of meiosis.
The paternal sex ratio chromosome (PSR) is a B chromosome found in some populations of the parasitoid wasp Nasonia vitripennis (Werren 1991). This B chromosome was first described as a sex ratio factor producing all-male broods (Werren et al. 1981) and then as a supernumerary (B) chromosome (Nur et al. 1988). The presence of this B chromosome in the sperm makes that, upon fertilization, the diploid zygotes (destined to be females) are converted into haploid ones (yielding PSR-carrying males) through the destruction of all paternal A chromosome set (Werren et al. 1987). This operates during the first mitotic division of the zygote (step 1) by the heterochromatinization of the paternal A chromosomes, whereas the B itself is unaffected. This reminds the poison-antidote mechanism of gamete killers (step 6 in Fig. 1b) but with its effect retarded after fertilization. PSR is thus an extremely parasitic B chromosome that reduces to zero the fitness of every A chromosome set that enters into contact with it (Beukeboom and Werren, 2000). In fact, as noted by Burt and Trivers (2006), its mode of action restricts PSR presence to males thus losing the chance to be also transmitted through females, like other B chromosomes. Therefore, although the transmission ratio through males is very high (> 0.9), the null transmission through females yields, as a whole, an average near-Mendelian ratio slightly lower than 0.5. This explains the low frequency of PSR in natural populations, e.g., 11.8% in Utah (Skinner 1983). Therefore, PSR is extremely parasitic but poorly efficient as SGE.
A special pathway for B chromosome survival is leakage during asexual reproduction of gynogenetic parthenogenesis in flatworms and fish. Polycelis nigra is a simultaneously hermaphroditic flatworm that can reproduce asexually through pseudogamous parthenogenesis, so that the sperm is only used for egg activation but does not contribute genetically to the progeny (Beukeboom et al. 1996). However, these authors demonstrated that B chromosomes in this species sometimes escape from expulsion and display 6–36% of paternal inheritance. Similar leakage was observed in the Amazon molly, the fish Poecilia formosa, an all-female species reproducing by gynogenesis whose unreduced diploid eggs undergo embryogenesis after activation by sperm from males of closely related species (for review, see Lamatsch et al. 2004). Supernumerary chromosomes in this species probably arose by escape from the elimination of sperm chromosomes and, at least in the Río Purificación basin, they constituted a widespread polymorphism (Lamatsch et al., 2004).
Mitotic instability and gonotaxis can lead to B chromosome drive when Bs reach a higher frequency in the germline than in the somatic line. This pre-meiotic TRD has been reported in grasshopper species such as Camnula pellucida (Carroll 1920; Nur 1969), Neopodismopsis abdominalis (Rothfels 1950), Calliptamus palaestinensis (Nur 1963), and Locusta migratoria (Nur 1969). The demonstration of a higher number of Bs in spermatocytes than in somatic cells (gastric caeca) from the same individuals was due to Kayano (1971) and Viseras et al. (1990) in L. migratoria, and the non-disjunction of this B chromosome was visualized in 5-day-old embryos by Pardo et al. (1995). In the plant Crepis capillaris, the frequency of B chromosomes is higher in flowers than in roots (Rutishauser and Röthlisberger 1966), in resemblance to pre-meiotic drive in grasshoppers. Anyway, the final demonstration of gonotaxis during embryo mitotic divisions is still waiting in all cases.
Meiotic TRD (steps 3–5)
True meiotic drive rests on some kind of functional asymmetry of meiocytes. In female meiosis, asymmetry results from the production of only one viable gamete out of the four meiotic products. A molecular explanation has been found for this asymmetry, mediated by CDC42 signaling and microtubule tyrosination (Akera et al. 2017). Some clear-cut examples of non-Mendelian segregation during female meiosis are, for instance, the preferential segregation to the functional megaspore of the knobbed chromosome 10 in maize (Rhoades & Dempsey 1966) or of the B chromosomes to the ovum in the grasshoppers Melanoplus femur-rubrum (Lucov & Nur 1973), Myrmeleotettix maculatus (Hewitt 1973, 1976), Heteracris littoralis (Cano & Santos 1989), Locusta migratoria (Pardo et al. 1994), and Eyprepocnemis plorans (Zurita et al. 1998), to mention only a few cases (for additional information, see Jones and Rees 1982; Jones 1991, 2018; Camacho 2005; Houben 2017).
On the male side, however, meiotic drive sensu stricto is more problematic, as all four genomic sets of spermatogonia are usually present in gametes. An exception is the lecanoid system in some hemipteran insects, where the paternal set of chromosomes is heterochromatinized during the blastula stage (Schrader 1921; Brown and Nelson-Rees 1961) and its inverted spermatogenesis consists of two highly modified divisions, the first being equational and the second reductional. In the second division, the heterochromatic and euchromatic sets are segregated to opposite poles, yielding two heterochromatic and two euchromatic products, with only the latter producing sperm (Hughes-Schrader 1948). This functional asymmetry of spermatogenesis in the lecanoid system is exploited by the B chromosome of the mealybug Pseudococcus affinis (formerly P. obscurus), which begins meiosis being heterochromatic during prophase I (i.e., positively heteropycnotic), it is euchromatic at metaphase I (i.e., negatively heteropycnotic as the euchromatic set of A chromosomes), and it finally segregates with the euchromatic set at anaphase II (Nur 1962). It is unknown how B chromosomes manage to go preferentially with the euchromatic set of A chromosomes, but it is a highly interesting question to address with next-generation sequencing (NGS) tools. Anyway, this case shows a certain resemblance with PSR in N. vitripennis, as both imply the destruction of the paternal set of chromosomes.
Post-meiotic TRD (steps 6–8)
Maturation of male and female gametophytes in plants implies the occurrence of several mitoses, during which B chromosome non-disjunction and gonotaxis can operate to provide post-meiotic TRD by preferential migration to the generative nucleus in the pollen grain or to the egg in the embryo sac (steps 6 and 7, respectively). Examples of male post-meiotic TRD have been reported, for instance, in Secale cereale (Hasegawa 1934), Festuca pratensis (Bösemark 1954), Aegilops speltoides (Mendelson and Zohari 1972), Hypochoeris maculata (Parker 1976), and Prospero autumnale (Lanzas et al. 2018). In rye, experimental crosses by Müntzing (1945) demonstrated that post-meiotic TRD during gametophyte maturation takes place also through the female side (step 7), thus making rye B chromosomes one of the few examples, along with the migratory locust (Pardo et al. 1994), where B chromosomes exhibit drive through both sexes. It is not by chance that both B chromosome polymorphisms show worldwide distribution. In maize, however, B chromosome non-disjunction takes place only in the male gametophyte and acts during the second pollen grain mitosis. This yields one sperm nucleus with 2B and the other with 0B. If they would fertilize at random, no drive would result. However, there is preferential fertilization by B-carrying sperm nuclei (Roman 1948; Carlson 1969) resulting in the only known case of TRD during step 8.
In contrast to plants, no B chromosomes are known in animals with post-meiotic TRD in any sex (steps 6–7). In females, there is no chance for this kind of TRD as meiosis finishes after fertilization, so that step 8 precedes the end of steps 4 or 5. In males, however, sperm maturation (spermiogenesis) is long and complex and, although it does not require any additional mitosis, it would give a chance to sperm killing, but no case has yet been reported.
Genetic basis for B chromosome TRD
Research on genic SGEs, mainly t-alleles in mice, segregation distorters in Drosophila, and spore killers in fungi, has revealed interesting evolutionary pathways for TRD, by uncovering the involvement of specific gene duplications and other genomic elements, mainly satDNA and the RNA interference pathway (Wu et al. 1988; Lyttle 1991; Herrmann et al. 1999; Merrill et al. 1999; Bauer et al. 2005, 2007; Muirhead and Presgraves 2021; and see this special issue). As shown below, the scarce data on B chromosome TRD are remarkably similar.
NGS has brought about an exponential increase in publications on the DNA content of B chromosomes, both on protein-coding genes and repetitive DNA. This has given interesting insights for non-Mendelian inheritance only in those B chromosome systems where the possible existence of drive had already been investigated, and they are mentioned below.
In plants, three B chromosome systems fulfill the former condition, namely those in rye (Secale cereale), maize (Zea mays), and the goatgrass (Aegilops speltoides). Rye B chromosomes show post-meiotical TRD during the maturation of both gamethopytes (steps 6 and 7). The first indication for a genetic control of B drive in rye was put forward by Lima-De-Faria (1962), who suggested the existence of a controlling element located in the heterochromatic block of the distal region of the long arm of the standard B chromosome (corroborated by Puertas et al. 1998). This control was then associated with the B-specific E3900 and D1100 satDNA families (Langdon et al., 2000), both of which are transcribed producing a heterogeneous collection of non-coding RNAs (Carchilan et al., 2007). Later, Banaei-Moghaddam et al. (2012) found that non-disjunction of Bs is accompanied by centromere activity and is likely caused by extended cohesion of the B sister chromatids and concluded that B drive in rye results from a combination of non-disjunction and asymmetric spindle formation at first pollen mitosis leading to the accumulation of Bs in the generative nucleus (step 6). Up to this moment, no genes have been shown to participate in the control of B drive in rye, but the finding of many protein-coding genes by Martis et al. (2012) opened this possibility.
As reviewed by Jones et al. (2008), B chromosomes in maize show three events influencing its overall non-Mendelian transmission: (i) non-disjunction during the second pollen grain mitosis (step 6), (ii) preferential fertilization of the sperm nuclei carrying the two B chromatids (step 8), and (iii) suppression of meiotic loss of unpaired Bs (step 3), with a complex control by the A and B chromosomes. Interestingly, recent NGS sequencing by Blavet et al. (2021) has found 758 genic sequences residing in the B chromosome, many of which show putative functions dealing with non-disjunction, preferential fertilization, and univalent stabilization, which might be useful to help B transmission drive. This opens new exciting avenues of research to uncover the molecular details of the complex cross-talk between A and B chromosomes at the gene expression level, triggered by the arms race between them.
Goatgrass plants may carry B chromosomes in the aerial parts but not in the roots, and these Bs show directed non-disjunction at anaphase of the first pollen mitosis (Mendelson and Zohari 1972). Most recently, Ruban et al. (2020) demonstrated that the elimination of B chromosomes in the roots is a strictly controlled process where B chromosomes exhibit non-disjunction and chromatid lagging during mitotic anaphases, leading to the formation of micronuclei containing the B chromosome, whose degradation is the final step of B chromosome elimination from roots. In addition, NGS analysis allowed these authors to find 229 genes presumably residing in the B chromosome, which opens interesting questions for future research to ascertain if some of the B genes play an essential role in B drive during the first pollen grain mitosis or B elimination in roots.
In animals, the species where drive and DNA content of B chromosomes have been analyzed are the grasshopper Eyprepocnemis plorans, the locust Locusta migratoria, the jewel wasp Nasonia vitripennis, and the cavefish Astyanax mexicanus. In addition, B chromosomes in Pseudococcus affinis are highly interesting, due to their exceptional drive during male meiosis and the clear evidence for drive suppression reported by Nur and Brett (1985, 1987). This B chromosome system is thus waiting for NGS research to identify possible genes and repetitive DNAs that may be involved in the control of B drive.
In the grasshopper E. plorans, Navarro-Domínguez et al. (2017) found ten protein-coding genes residing in the B chromosome, five of which were transcribed from the B chromosome and coded for functions related to cell division. In the same vein, Ruiz-Ruano et al. (2019) found 25 protein-coding genes in L. migratoria, one of which (apc1) codes for the large subunit of the anaphase-promoting complex or cyclosome (APC/C), an E3 ubiquitin ligase involved in the metaphase-anaphase transition. The possibility that a putatively higher amount of APC1 protein in B-carrying cells might favor metaphase-anaphase transition in spite of the orientation of the two B chromatids towards the same pole, demands future research.
The molecular details about non-Mendelian inheritance have experienced much less progress for B chromosomes than for other types of SGEs. This may be due to the scarcity of B-carrying species with high-quality genome sequencing, the exceptions being D. melanogaster, N. vitripennis, and A. mexicanus.
In D. melanogaster, Bauerly et al. (2014) found mitotically unstable B chromosomes in a laboratory stock and showed that they are transmitted through both sexes, with no apparent drive. The genetic analysis performed by Bauerly et al. (2014) suggested that B chromosomes in D. melanogaster most likely devoid functional or nonfunctional genic sequences, and subsequent high-throughput sequencing by Hanlon et al. (2018) revealed that this B chromosome does not contain known protein-coding genes, apart from several highly repetitive elements, one of which (the AAGAT satellite) is specific to chromosome 4, as expected if the B chromosome would have arisen from this autosome. The possibility exists that the mitotic instability of this B chromosome might be associated with subsequent gonotaxis, as in grasshoppers (Kayano 1971; Viseras et al. 1990), and it could be investigated by comparing the number of B chromosomes in germ and somatic cells from the same individuals.
In N. vitripennis, Benetta et al. (2020) analyzed, by NGS, the fine-scale sequence composition and expression of PSR. They found 44 genes in the B chromosome and used RNA interference to demonstrate that PSR action is mediated through testis-specific expression of a PSR-linked gene named haploidizer, which encodes a putative protein with a DNA binding domain. This is the best available evidence for a functional link between a B gene and a B chromosome drive.
Recently, through high-quality genome assembly, Imarazene et al. (2021) have identified 63 genes in the B chromosome of the Pachón cavefish (A. mexicanus), one of which was the master sex-determining gene growth differentiation factor 6b (gdf6b). The high sequence similarity between the A and B chromosome paralogous copies impeded ascertaining whether the B chromosome actually plays a role in sex determination, but this is an interesting prospect for future investigations.
Therefore, although the gene content of B chromosomes has grown exponentially during the last years, the research on the meaning and putative function of these genes is still in diapers, compared to the details unveiled on the genetic basis for TRD in genic SGEs (see this special issue).
TRD similarities between B chromosomes and other SGEs
The cases of TRD for B chromosomes reviewed here, and their possible genetic basis, display a high resemblance with those described for genic SGEs, which can be summarized into three main aspects:
-
1)
TRD is manifested in inter-population or interspecific crosses but suppressed at an intra-population level as part of the coadaptation process. For instance, the B chromosome polymorphism in the grasshopper E. plorans is the paradigm of the near-neutral model of B chromosome evolution, which is a variant of the parasitic model that includes drive suppression (Camacho et al. 1997). Remarkably, drive for E. plorans Bs has been observed only in inter-population crosses (Herrera et al. 1996) (Fig. 3) and during an ongoing B invasion (Zurita et al. 1999). In the first case, nine 1B females from the Salobreña population were mated by Herrera et al. (1996) with two different 0B males, one from the same population and the other from a different population, and it yielded 10 intra- and 12 inter-population estimates of B transmission ratio (kB). As Fig. 3 shows, the comparison of these two types of kB with the Mendelian ratio (0.5) revealed that inter-population kBs were higher than Mendelian expectations, with an effect size (measured by the paired mean difference) equal to 0.159 (95% CI: 0.0857, 0.217), whereas the intra-population kBs did not differ, on average, from 0.5 (effect size = 0.0224, 95% CI: − 0.0926, 0.106).
By analogy with other cases where TRD manifests only in interspecific crosses, such as the Winters Sex Ratio trait in D. simulans (Muirhead and Presgraves 2021), differential survival in Mus musculus-spretus crosses (Eversley et al. 2010), and centromeric drive in Mimulus guttatus (Finseth et al. 2021), it appears that SGE success can be paralleled by coadaptation breakdown, as also illustrated by the emergence of a new supernumerary chromosome during interspecific crosses in Nasonia (Perfectti and Werren 2001). Coadaptation thus implies drive suppression to subdue SGEs to the rules of the parliament of genes (Leigh 1971).
-
2)
TRD is mediated by genes in most genic SGEs, the exceptions probably being due to information missing. In the case of B chromosomes, the presence of active protein-coding genes has just beginning to be uncovered. It is remarkable that the only gene functionally related with B chromosome TRD is haplodizer in N. vitripennis, and it showed no homology with the known genes of the standard genome but appeared to be derived from an insect bacterial symbiont (Benetta et al. 2020) in consistency with the interspecific origin of this B chromosome (McAllister and Werren 1997). It is presumable that the next years are going to witness an extraordinary advance in the knowledge of how B chromosome drive depends on genes residing on B chromosomes.
Fig. 3 TRD for B chromosomes in the grasshopper E. plorans is manifested in inter-population crosses. These Gardner-Altman graphs display B transmission ratios (kB) obtained by Herrera et al. (1996) for nine 1B females from the Salobreña population, after double mating with a male from the same population and another male from a different population. The comparisons were made as suggested by Ho et al. (2019) and the graphs were built using https://www.estimationstats.com. The upper graphs display the comparison of the observed kB with Mendelian expectation (kB = 0.5) for intra- and inter-population crosses. Note that only three intra-population crosses showed kB > 0.6, whereas most inter-population crosses (except two) showed kB > 0.6. The lower graph displays the distribution of kB values in the two types of crosses, revealing that the paired mean difference (i.e., the effect size) between the observed kB and the Mendelian ratio (0.5) was close to zero (on average) for intra-population crosses, and about 0.15 for inter-population ones
-
3)
The target of drivers can be other genes or satDNA, and both are abundant on B chromosomes. The raw material for TRD is actually present on B chromosomes, as they contain a multitude of paralogous copies of A chromosome genes, some of which might yield functional proteins (Ma et al. 2016) and others can be pseudogenical and source of endogenous siRNAs that may influence gene expression (Banaei-Moghaddam et al. 2013). Perhaps the RNAi pathway could explain the rapid suppression of drive in E. plorans (Perfectti et al. 2004). In addition, B chromosomes are enriched in satDNA (Camacho et al. 2021), which has shown to play a crucial role in rye B drive and it is the target for Sd in D. melanogaster. However, most observations on B chromosomes are still incidental and demand future research which, inspired on genic SGE’s research, may uncover the transcriptomic and proteomic cross-talk between the DNA content of A and B chromosomes characterizing their co-evolutionary arms race.
Abbreviations
- APC/C:
-
Anaphase promoting complex or cyclosome
- NGS:
-
Next-generation sequencing
- PSR:
-
Paternal sex ratio
- RNAi:
-
RNA interference pathway
- satDNA:
-
Satellite DNA
- Sd:
-
Segregation distorter
- SGE:
-
Selfish genetic element
- TRD:
-
Transmission ratio distortion
References
Akera T, Chmátal L, Trimm E, Yang K, Aonbangkhen C, Chenoweth DM et al (2017) Spindle asymmetry drives non-Mendelian chromosome segregation. Science 358:668–672. https://doi.org/10.1126/science.aan0092
Banaei-Moghaddam AM, Schubert V, Kumke K, Weiβ O, Klemme S, Nagaki K et al (2012) Nondisjunction in favor of a chromosome: the mechanism of rye B chromosome drive during pollen mitosis. Plant Cell 24:4124–4134
Banaei-Moghaddam AM, Meier K, Karimi-Ashtiyani R, Houben A (2013) Formation and expression of pseudogenes on the B chromosome of rye. Plant Cell 25:2536–2544. https://doi.org/10.1105/tpc.113.111856
Bauer H, Willert J, Koschorz B, Herrmann BG (2005) The t complex-encoded GTPase-activating protein Tagap1 acts as a transmission ratio distorter in mice. Nat Genet 37:969–973. https://doi.org/10.1038/ng1617
Bauer H, Véron N, Willert J, Herrmann BG (2007) The t-complex-encoded guanine nucleotide exchange factor Fgd2 reveals that two opposing signaling pathways promote transmission ratio distortion in the mouse. Genes Dev 21:143–147. https://doi.org/10.1101/gad.414807
Bauerly E, Hughes SE, Vietti DR, Miller DE, McDowell W, Scott Hawley R (2014) Discovery of supernumerary B chromosomes in Drosophila melanogaster. Genetics 196:1007–1016. https://doi.org/10.1534/genetics.113.160556
Benetta ED, Antoshechkin I, Yang T, Nguyen HQM, Ferree PM, Akbari OS (2020) Genome elimination mediated by gene expression from a selfish chromosome. Sci Adv 6:eaaz9808. https://doi.org/10.1126/sciadv.aaz9808
Beukeboom LW, Seif M, Mettenmeyer T, Plowman AB, Michiels NK (1996) Paternal inheritance of B chromosomes in a parthenogenetic hermaphrodite. Heredity 77:646–654. https://doi.org/10.1038/hdy.1996.192
Beukeboom LW, Werren JH (2000) The paternal-sex-ratio (PSR) chromosome in natural populations of Nasonia (Hymenoptera: Chalcidoidea). J Evol Biol 13:967–975. https://doi.org/10.1046/j.1420-9101.2000.00231.x
Blavet N, Yang H, Su H, Solanský P, Douglas RN, Karafiátová M et al (2021) Sequence of the supernumerary B chromosome of maize provides insight into its drive mechanism and evolution. Proc Nat Acad Sci USA 118:e2104254118. https://doi.org/10.1073/PNAS.2104254118
Bösemark NO (1954) On accessory chromosomes in Festuca pratensis. II. Inheritance of the standard type of accessory chromosomes. Hereditas 49:425–437. https://doi.org/10.1111/j.1601-5223.1954.tb02981.x
Brown SW, Nelson-Rees WA (1961) Radiation analysis of a lecanoid genetic system. Genetics 46:983–1007. https://doi.org/10.1093/genetics/46.8.983
Burt A, Trivers R (2006) Genes in conflict: the biology of selfish genetic elements. The Belknap Press of Harvard University Press, Cambridge
Camacho JPM (2005) B chromosomes. In: Gregory TR (ed) The evolution of the genome. Elsevier Academic Press, San Diego, pp 223–286
Camacho JPM, Sharbel TF, Beukeboom LW (2000) B-chromosome evolution. Phil Trans Roy Soc Lond B 355:163–178. https://doi.org/10.1098/rstb.2000.0556
Camacho JPM, Shaw MW, López-León MD, Pardo MC, Cabrero J (1997) Population dynamics of a selfish B chromosome neutralized by the standard genome in the grasshopper Eyprepocnemis plorans. Am Nat 149:1030–1050
Camacho JPM, Ruiz-Ruano FJ, López-León MD, Cabrero J (2021) Satellite DNA is an inseparable fellow traveller of B chromosomes. In: Ugarković Ð. (eds) Satellite DNAs in physiology and evolution. Progress in Molecular and Subcellular Biology, vol 60. Springer, Cham. https://doi.org/10.1007/978-3-030-74889-0_4
Cano MI, Santos JL (1989) Cytological basis of the B chromosome accumulation mechanism in the grasshopper Heteracris litoralis (Ramb). Heredity 62:91–95. https://doi.org/10.1038/hdy.1989.12
Carchilan M, Delgado M, Ribeiro T, Costa-Nunes P, Caperta A et al (2007) Transcriptionally active heterochromatin in rye B chromosomes. Plant Cell 19:1738–1749. https://doi.org/10.1105/tpc.106.046946
Carlson WR (1969) Factors affecting preferential fertilization in maize. Genetics 62:543–554. https://doi.org/10.1093/genetics/62.3.543
Carroll M (1920) An extra dyad and an extra tetrad in the spermatogenesis of Camnula pellucida (Orthoptera): numerical variation in the chromosome complex within the individual. J Morph 34:375–455
Cockburn A (1991) An introduction to evolutionary ecology. Blackwell Scientific Publications, Oxford
Eversley CD, Clark T, Xie Y, Steigerwalt J, Bell TA, de Villena FPM et al (2010) Genetic mapping and developmental timing of transmission ratio distortion in a mouse interspecific backcross. BMC Genet 11:98. https://doi.org/10.1186/1471-2156-11-98
Finseth FR, Nelson TC, Fishman L (2021) Selfish chromosomal drive shapes recent centromeric histone evolution in monkeyflowers. PLoS Genet 17(4):e1009418. https://doi.org/10.1371/journal.pgen.1009418
Fishman L, Mcintosh M (2019) Standard deviations: the biological bases of transmission ratio distortion. Ann Rev Genet 53:347–372. https://doi.org/10.1146/annurev-genet-112618-043905
Hammond TM, Rehard DG, Xiao H, Shiu PKT (2012) Molecular dissection of Neurospora Spore Killer meiotic drive elements. Proc Nat Acad Sci USA 109(30):12093–12098. https://doi.org/10.1073/pnas.1203267109
Hanlon S, Miller DE, Eche S, Hawley RS (2018) Origin, composition and structure of the supernumerary B chromosome of Drosophila. Genetics 210:1197–1212. https://doi.org/10.1534/genetics.118.300904
Hasegawa N (1934) A cytological study on 8-chromosome rye. Cytologia 6:68–77
Herrera J, López-León MD, Cabrero J, Shaw MW, Camacho JPM (1996) Evidence for B chromosome drive suppression in the grasshopper Eyprepocnemis plorans. Heredity 76(6):633–639. https://doi.org/10.1038/hdy.1996.90
Herrmann BG, Koschorz B, Wertz K, McLaughlin KJ, Kispert A (1999) A protein kinase encoded by the t complex responder gene causes non-Mendelian inheritance. Nature 402:141–146. https://doi.org/10.1038/45970
Hewitt G (1973) Variable transmission rates of a B-chromosome in Myrmeleotettix maculatus (Thunb) (Acrididae:Orthoptera). Chromosoma 40:83–106. https://doi.org/10.1007/BF00319837
Hewitt GM (1976) Meiotic drive for B-chromosomes in the primary oocytes of Myrmeleotettix maculatus (Orthoptera: Acrididae). Chromosoma 56:381–391. https://doi.org/10.1007/BF00292957
Ho J, Tumkaya T, Aryal S, Choi H, Claridge-Chang A (2019) Moving beyond P values: data analysis with estimation graphics. Nat Methods 16(7):565–566. https://doi.org/10.1038/s41592-019-0470-3
Houben A (2017) B Chromosomes - a matter of chromosome drive. Front Plant Sci 8:210. https://doi.org/10.3389/fpls.2017.00210
Hughes-Schrader S (1948) Cytology of coccids (Coccoidea-Homoptera). Advanc Genet 2:127–203
Imarazene B, Du K, Beille S, Jouanno E, Feron R, Pan Q et al (2021) A supernumerary “B-sex” chromosome drives male sex determination in the Pachón cavefish, Astyanax mexicanus. Curr Biol 31:4800-4809.e9. https://doi.org/10.1016/j.cub.2021.08.030
Jones RN (1991) B-chromosome drive. Am Nat 137:430–442
Jones RN (2018) Transmission and drive involving parasitic B chromosomes. Genes 9(8):388. https://doi.org/10.3390/genes9080388
Jones RN, González-Sánchez M, González-García M, Vega JM, Puertas MJ (2008) Chromosomes with a life of their own. Cytogenet Genome Res 120:265–280. https://doi.org/10.1159/000121076
Jones RN, Rees H (1982) B Chromosomes. Academic Press, New York
Kayano H (1971) Accumulation of B chromosomes in the germ line of Locusta migratoria. Heredity 27:119–123. https://doi.org/10.1038/hdy.1971.76
Lamatsch DK, Nanda I, Schlupp I, Epplen JT, Schmid M, Schartl M (2004) Distribution and stability of supernumerary microchromosomes in natural populations of the Amazon molly, Poecilia formosa. Cytogenet Genome Res 106:189–194. https://doi.org/10.1159/000079286
Langdon T, Seago C, Jones RN, Ougham H, Thomas H, Forster JW et al (2000) De novo evolution of satellite DNA on the rye B chromosome. Genetics 154:869–884. https://doi.org/10.1093/genetics/154.2.869
Lanzas P, Perfectti F, Garrido-Ramos MA, Ruíz-Rejón C, González-Sánchez M, Puertas M et al (2018) Long-term monitoring of B-chromosome invasion and neutralization in a population of Prospero autumnale (Asparagaceae). Evolution 72:1216–1224. https://doi.org/10.1111/evo.13501
Leigh EG (1971) Adaptation and Diversity. Freeman Cooper and Co, San Francisco
Lewontin RC (1967) Population Genetics Ann Rev Genet 1:37–70
Lima-de-Faria A (1962) Genetic interaction in rye expressed at the chromosome phenotype. Genetics 47:1455–1462
Lindholm AK, Dyer KA, Firman RC, Fishman L, Forstmeier W, Holman L et al (2016) The ecology and evolutionary dynamics of meiotic drive. Trends Ecol Evol 31:315–326. https://doi.org/10.1016/j.tree.2016.02.001
Lucov Z, Nur U (1973) Accumulation of B-chromosomes by preferential segregation in females of the grasshopper Melanoplus femur-rubrum. Chromosoma 42:289–306. https://doi.org/10.1007/BF00284776
Lyon MF (1984) Transmission ratio distortion in mouse t-haplotypes is due to multiple distorter genes acting on a responder locus. Cell 37:621–628. https://doi.org/10.1016/0092-8674(84)90393-3
Lyttle TW (1991) Segregation distorters. Annu Rev Genet 25:511–557. https://doi.org/10.1146/annurev.ge.25.120191.002455
Ma W, Gabriel TS, Martis MM, Gursinsky T, Schubert V, Vrána J et al (2016) Rye B chromosomes encode a functional Argonaute-like protein with in vitro slicer activities similar to its A chromosome paralog. New Phytol 213:916–928. https://doi.org/10.1111/nph.14110
Martis MM, Klemme S, Banaei-Moghaddam AM, Blattner FR, Macas J, Schmutzer T et al (2012) Selfish supernumerary chromosome reveals its origin as a mosaic of host genome and organellar sequences. Proc Nat Acad Sci USA 109:13343–13346. https://doi.org/10.1073/pnas.1204237109
McAllister BF, Werren JH (1997) Hybrid origin of a B chromosome (PSR) in the parasitic wasp Nasonia vitripennis. Chromosoma 106:243–253. https://doi.org/10.1007/s004120050245
Mendel G (1865) Versuche über Pflanzen-Hybriden. Verh Des Naturf Vereines in Brünn (abhandlungen) 4:3–47
Mendelson D, Zohari D (1972) Behaviour and transmission of supernumerary chromosomes in Aegilops speltoides. Heredity 29:329–339. https://doi.org/10.1038/hdy.1972.97
Merrill C, Bayraktaroglu L, Kusano A, Ganetzky B (1999) Truncated RanGAP encoded by the segregation distorter locus of Drosophila. Science 283(5408):1742–1745. https://doi.org/10.1126/science.283.5408.1742
Muirhead CA, Presgraves DC (2021) Satellite DNA-mediated diversification of a sex-ratio meiotic drive gene family in Drosophila. Nat Ecol Evol 5:1604–1612. https://doi.org/10.1038/s41559-021-01543-8
Müntzing A (1945) Cytological studies of extra fragment chromosomes in rye. II. Transmission and multiplication of standard fragments and iso-fragments. Hereditas 31:457–477
Navarro-Domínguez B, Ruiz-Ruano FJ, Cabrero J, Corral JM, López-León MD, Sharbel TF et al (2017) Protein-coding genes in B chromosomes of the grasshopper Eyprepocnemis plorans. Sci Rep 7:45200. https://doi.org/10.1038/srep45200
Nur U (1962) A supernumerary chromosome with an accumulation mechanism in the lecanoid genetic system. Chromosoma 13:249–271. https://doi.org/10.1007/BF00577042
Nur U (1963) A mitotically unstable supernumerary chromosome with an accumulation mechanism in a grasshopper. Chromosoma 14:407–422. https://doi.org/10.1007/BF00326786
Nur U (1969) Mitotic instability leading to an accumulation of B-chromosomes in grasshoppers. Chromosoma 27:1–19. https://doi.org/10.1007/BF00326108
Nur U, Brett BLH (1985) Genotypes suppressing meiotic drive of a B chromosome in the mealybug, Pseudococcus obscurus. Genetics 110:73–92. https://doi.org/10.1093/genetics/110.1.73
Nur U, Brett BL (1987) Control of meiotic drive of B chromosomes in the mealybug, Pseudococcus affinis (obscurus). Genetics 115:499–510. https://doi.org/10.1093/genetics/115.3.499
Nur U, Werren JH, Eickbush DG, Burke WD, Eickbush TH (1988) A “selfish” B chromosome that enhances its transmission by eliminating the paternal genome. Science 240(4851):512–514. https://doi.org/10.1126/science.3358129
Pardo MC, López-León MD, Cabrero J, Camacho JPM (1994) Transmission analysis of mitotically unstable B chromosomes in Locusta migratoria. Genome 37:1027–1034. https://doi.org/10.1139/g94-146
Pardo MC, López-León MD, Viseras E, Cabrero J, Camacho JPM (1995) Mitotic instability of B chromosomes during embryo development in Locusta migratoria. Heredity 74:164–169. https://doi.org/10.1038/hdy.1995.24
Parker JS (1976) The B-chromosome system of Hypochoeris maculata 1. B-distribution, meiotic behaviour and inheritance. Chromosoma 59:167–177. https://doi.org/10.1007/BF00328485
Perfectti F, Werren JH (2001) The interspecific origin of B chromosomes: Experimental evidence. Evolution 55:1069–1073. https://doi.org/10.1554/0014-3820(2001)055[1069:tioobc]2.0.co;2
Perfectti F, Corral JM, Mesa JA, Cabrero J, Bakkali M, López-León MD et al (2004) Rapid suppression of drive for a parasitic B chromosome. Cytogenet Genome Res 106:338–343. https://doi.org/10.1159/000079309
Puertas MJ, González-Sánchez M, Manzanero S, Romera F, Jiménez MM (1998) Genetic control of the rate of transmission of rye B chromosomes. IV. Localization of the genes controlling B transmission rate. Heredity 80:209–213. https://doi.org/10.1038/sj.hdy.6882930
Ren P, Deng F, Chen S, Ran J, Li J, Yin L et al (2021) Whole-genome resequencing reveals loci with allelic transmission ratio distortion in F1 chicken population. Mol Genet Genom 296:331–339. https://doi.org/10.1007/s00438-020-01744-z
Rhoades MM, Dempsey E (1966) The effect of abnormal chromosome 10 on preferential segregation and crossing over in maize. Genetics 53:989–1020. https://doi.org/10.1093/genetics/53.5.989
Roman H (1948) Directed fertilization in maize. Proc Natl Acad Sci USA 34:36–42. https://doi.org/10.1073/pnas.34.2.36
Rothfels KH (1950) Chromosome complement, polyploidy and supernumeraries in Neopodismopsis abdominalis (Acrididae). J Morph 87:287–316
Ruban A, Schmutzer T, Wu DD, Fuchs J, Boudichevskaia A, Rubtsova M et al (2020) Supernumerary B chromosomes of Aegilops speltoides undergo precise elimination in roots early in embryo development. Nat Comm 11:2764. https://doi.org/10.1038/s41467-020-16594-x
Ruiz-Ruano FJ, Cabrero J, López-León MD, Sánchez A, Camacho JPM (2018) Quantitative sequence characterization for repetitive DNA content in the supernumerary chromosome of the migratory locust. Chromosoma 127:45–57. https://doi.org/10.1007/s00412-017-0644-7
Ruiz-Ruano FJ, Navarro-Domínguez B, López-León MD, Cabrero J, Camacho JPM (2019) Evolutionary success of a parasitic B chromosome rests on gene content. BioRxiv 1–25. https://doi.org/10.1101/683417
Rutishauser A, Röthlisberger E (1966) Boosting mechanism of B chromosomes in Crepis capillaris. Chromosomes Today 1:28–30
Sandler L, Novitski E (1957) Meiotic drive as an evolutionary force. Am Nat 91:105–110
Schrader F (1921) The chromosomes of Pseudococcus nipae. Biol Bull 40:259–270
Skinner SW (1983) Extrachromosomal sex ratio factors in the parasitoid wasp Nasonia (= Mormoniella) vitripennis. Ph.D. Thesis, University of Utah, Salt Lake City.
Svedberg J, Vogan AA, Rhoades NA, Sarmarajeewa D, Jacobson DJ, Lascoux M et al (2021) An introgressed gene causes meiotic drive in Neurospora sitophila. Proc Nat Acad Sci USA 118(17):e2026605118. https://doi.org/10.1073/pnas.2026605118
Tao Y, Araripe L, Kingan SB, Ke Y, Xiao H, Hartl DL (2007) A sex-ratio meiotic drive system in Drosophila simulans. II: An X-linked distorter. PLoS Biol 5(11):2576–2588. https://doi.org/10.1371/journal.pbio.0050293
Viseras E, Camacho J, Cano M, Santos J (1990) Relationship between mitotic instability and accumulation of B chromosomes in males and females of Locusta migratoria. Genome 33(1):23–29. https://doi.org/10.1139/g90-005
Wendel JF, Edwards MD, Stuber CW (1987) Evidence for multilocus genetic control of preferential fertilisation in maize. Heredity 58:297–301. https://doi.org/10.1038/hdy.1987.44
Werren JH (1991) The paternal-sex-ratio chromosome of Nasonia. Am Nat 137:392–402. https://doi.org/10.1086/285172
Werren JH, Skinner SW, Charnov EL (1981) Paternal inheritance of a daughterless sex ratio factor. Nature 293(5832):467–468. https://doi.org/10.1038/293467a0
Werren JH, Nur U, Eickbush D (1987) An extrachromosomal factor causing loss of paternal chromosomes. Nature 327(6117):75–76. https://doi.org/10.1038/327075a0
Wright S (1968) Evolution and the genetics of populations. Vol. 1: Genetic and biometric foundations. The University of Chicago Press, Chicago
Wu CI, Lyttle TW, Wu ML, Lin GF (1988) Association between a satellite DNA sequence and the Responder of Segregation Distorter in D. melanogaster. Cell 54:179–189. https://doi.org/10.1016/0092-8674(88)90550-8
Zimmering S, Sandler L, Nicoletti B (1970) Mechanisms of meiotic drive. Annu Rev Genet 4:409–436. https://doi.org/10.1146/annurev.ge.04.120170.002205
Zurita S, Cabrero J, López-León M, Camacho JPM (1998) Polymorphism regeneration for a neutralized selfish B chromosome. Evolution 52:274–277. https://doi.org/10.1111/j.1558-5646.1998.tb05163.x
Acknowledgements
I am highly grateful to all the researchers who have co-authored my publications on B chromosomes for more than 40 years, as well as the University of Granada, Junta de Andalucía and the Spanish Government for providing us the necessary funds. I am also grateful to Andreas Houben and an anonymous reviewer for useful comments and David Martinez for English corrections.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests.
Additional information
Responsible Editors: Stacey Hanlon and Amanda Larracuente
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Camacho, J.P.M. Non-Mendelian segregation and transmission drive of B chromosomes. Chromosome Res 30, 217–228 (2022). https://doi.org/10.1007/s10577-022-09692-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10577-022-09692-7