Abstract
The centromere is a specialized chromosomal locus required for accurate chromosome segregation. A specific histone H3 variant, CENP-A, assembles at centromeres. CENP-A is required for kinetochore protein assembly and is an epigenetic marker for the maintenance of a functional centromere. Human CENP-A chromatin normally assembles on α-satellite DNA (alphoid DNA), a centromeric repetitive sequence. Using alphoid DNA arrays, human artificial chromosomes (HACs) have been constructed in human HT1080 cells and used to dissect the requirements for CENP-A assembly on DNA sequence. However, centromere formation is not a simple genetic event. In other commonly used human cell lines, such as HeLa and U2OS cells, no functional de novo centromere formation occurs efficiently with the same centromeric alphoid DNA sequences. Recent studies using protein tethering combined with the HAC system and/or genetic manipulation have revealed that epigenetic chromatin regulation mechanisms are also involved in the CENP-A chromatin assembly pathway and subsequent centromere/kinetochore formation. We summarize the DNA sequence requirements for CENP-A assembly and discuss the epigenetic regulation of human centromeres.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The centromere is a specialized chromosomal locus required for accurate chromosome segregation. The kinetochore structure is composed of more than 100 protein components that assemble on the centromere locus during the mitotic phase. The kinetochore interacts with microtubules and regulates the chromosomal segregation process via interactions with the mitotic checkpoint proteins (Cleveland et al. 2003; Musacchio and Salmon 2007; Allshire and Karpen 2008).
Almost 34 years have passed since eukaryotic artificial chromosomes were first generated in budding yeast. The first artificial chromosomes were constructed by combining origins of DNA replication with the functional centromere DNA elements in circular plasmids. Currently, artificial chromosomes with functional centromeres can be generated in human cells. During this process, centromere-specific repetitive DNA sequences (α-satellite DNA) are required for de novo assembly. However, human artificial chromosome formation is also strongly correlated with epigenetic regulation (e.g., chromatin modifications), as it is at natural centromeres, where evidence for epigenetic regulation came from the discovery of centromere inactivation (Earnshaw and Migeon 1985) and from the formation of human neocentromeres on non-α-satellite DNA if the natural centromeres are lost or deleted (Fukagawa and Earnshaw 2014). In this review, we focus on current advancements in de novo formation and maintenance of functional human centromeres and discuss how epigenetic chromatin modifications regulate centromere assembly, activation, and inactivation, including neocentromere formation.
The first eukaryotic artificial chromosome with a point centromere in budding yeast
In budding yeast (Saccharomyces cerevisiae) cells, the first autonomous replicating sequence (ARS) had been identified in part of the TRP1 gene as an element that enhanced the transformation efficiency of bacterial plasmid DNA in yeast cells (Stinchcomb et al. 1979; Struhl et al. 1979). Plasmid DNA containing ARS element replicates efficiently, accumulating as multicopy plasmids without integrating into the host chromosomes. However, such ARS-containing plasmids, which cannot properly segregate at mitosis, accumulate in the mother cells and are rapidly lost from cell population during growth without selection.
Clarke and Carbon (1980) identified the first functional centromere DNA element (CEN) near the CDC10 locus of chromosome III, which had been mapped genetically. Introduction of the cloned CEN DNA into a circular plasmid bearing an ARS element caused that plasmid to undergo classical Mendelian segregation mechanism with equally distribution into daughter cells and maintenance of a copy number of one per cell. The centromere and replication origin were both required for stable maintenance of this circular minichromosome.
Normal eukaryotic chromosomes are linear in structure and have telomere repeats at both chromosomal ends. The first linear artificial chromosome was constructed by Murray and Szostak (1983) in budding yeast. A linear DNA fragment containing a centromere, ARS, and selectable marker was capped at both ends with Tetrahymena rDNA repeats containing telomere structures. After transformation into yeast cells, this synthetic DNA construct formed a minichromosome, that segregated and stably maintained as a linear yeast artificial chromosome (YAC) (Fig. 1a, left).
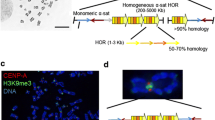
Centromere DNAs, protein components, and artificial chromosomes. a Structures of yeast centromeres and yeast artificial chromosomes (YACs). b Structures of human chromosome X and 21 alphoid DNA. The higher order repeats (HOR/type I) and monomeric (type II) alphoid DNA repeats. The HOR unit of chromosome 21 is composed of 11 alphoid monomer units containing five CENP-B boxes and one pseudo-CENP-B box. Red-shaded sequences are critical for CENP-B binding. Blue-shaded sequences are changed in the pseudo CENP-B box. No CENP-B binding is present on the pseudo-box. In the CENP-B box mutants of 11mer, wild-type CENP-B boxes (white ovals) are substituted with pseudo-box sequences (black ovals). c The assembly of human artificial chromosomes (HACs). d Properties of CENP-B protein. e A possible pathway for the CENP-A nucleosome in kinetochore protein assembly
The minimum length of centromere DNA of S. cerevisiae called as the conserved DNA element (CDE) is a quite short ∼120 bp (Fitzgerald-Hayes et al. 1982). The essential centromere-specific histone H3 variant Cse4 assembles as a single nucleosome on CDE II with other core histones, including H2A, H2B, and H4 (Furuyama and Biggins 2007; Camahort et al. 2009). This centromere-specific histone H3, CENP-A (also known in some organisms as CenH3), is highly conserved among eukaryotes, from yeast to humans and plants (Earnshaw and Rothfield 1985; Talbert et al. 2012; Earnshaw et al. 2013). In S. cerevisiae, the Cse4/CENP-A nucleosome cooperatively recruits kinetochore proteins for assembly with another CDE I binding protein (i.e., CBF1) and CDE III-binding protein complex CBF3 (Kitagawa and Hieter 2001). The defining factor of the yeast point centromere is that it is solely defined by the DNA sequence. Any DNA molecule carrying the 125-bp CDEI, CDEII, and CDEIII sequences capable to assemble a centromere, and a single DNA base mutation in CDEIII can inactivate the centromere (Hegemann et al. 1988).
A unique core centromere sequence surrounded by heterochromatin is required for YAC formation in fission yeast
In fission yeast (Schizosaccharomyces pombe), the centromere-competent DNA is considerably larger than in S. cerevisiae. S. pombe centromere DNA is 35–110 kb in length and consists of a unique central core sequence (cnt, 4∼7 kb) surrounded by repetitive sequences (imr, dg, and dh) (Nakaseko et al. 1986; Chikashige et al. 1989). For de novo formation of an artificial minichromosome from the input naked DNA, both the unique core and surrounding repetitive sequences are required (Hahnenberger et al. 1989) (Fig. 1a, right).
The S. pombe centromere has a specialized chromatin structure—when S. pombe DNA is cleaved with micrococcal nuclease, the central core DNA exhibits a smear pattern rather than a nucleosomal ladder (Takahashi et al. 1992), and this is required for functional kinetochore assembly (Takahashi et al. 2000). Multiple nucleosomes containing Cnp1, the S. pombe CENP-A, and other centromere-related proteins are assembled at cnt and imr DNA (Takahashi et al. 2000; Allshire and Karpen 2008; Ogiyama and Ishii 2012). It is not clear the extent to which these are interspersed with nucleosomes containing canonical histone H3. In contrast, the outer repeat region (dg and dh), consists of H3 nucleosomes where H3 is lysine 9 di-methylated (H3K9me2) and recruits Swi6/HP1, Clr4/Suv39, and other heterochromatin factors (Grewal and Jia 2007). Although the central core including outer repeat sequences is required for de novo artificial chromosome formation and Cnp1/CENP-A assembly, the outer repeat sequences can be substituted with an artificially induced heterochromatin structure (Folco et al. 2008; Kagansky et al. 2009). Heterochromatin is therefore important for artificial chromosome formation in S. pombe. Heterochromatin may be required for the enrichment of cohesin between the sister chromatids through HP1 binding (Bernard et al. 2001; Nonaka et al. 2002) and/or the assembly or maintenance of CENP-A chromatin (Folco et al. 2008; Kagansky et al. 2009).
Human centromeric DNA and protein components
A characteristic feature of the centromere DNA of all normal human chromosomes is α-satellite DNA (alphoid DNA). Alphoid DNA is a highly repetitive sequence with a monomer unit length of approximately 171 bp (Willard and Waye 1987) and clustered in regular and irregular arrays extending to 0.5∼5 Mbp in all human centromeres.
In human cells, centromere proteins were identified before the demonstration of a functional human DNA sequence as a centromere. Discovery of the first centromere proteins was made possible when it was shown that autoantigens recognized by sera from patients with scleroderma-spectrum disease were located at centromeres (Moroi et al. 1980). These antigens were subsequently identified as CENP-A, CENP-B, and CENP-C (Earnshaw and Rothfield 1985). CENP-B, the first centromere protein cloned in any species (Earnshaw et al. 1987), which recognizes the 17-bp CENP-B box motif in alphoid DNA (Masumoto et al. 1989; Muro et al. 1992), has a unique N-terminal DNA-binding domain (Pluta et al. 1992; Yoda et al. 1992) and a self-dimerization domain at the carboxyl terminus (Kitagawa et al. 1995). CENP-A, the essential centromeric H3, is highly conserved among eukaryotes and forms a nucleosome structure (Palmer et al. 1987; Howman et al. 2000; Tachiwana et al. 2011—though this is controversial—see discussion in Fukagawa and Earnshaw 2014). CENP-A is required for kinetochore assembly as an epigenetic marker of centromere chromatin (Black and Cleveland 2011). CENP-C is also an essential protein that bridges the inner and outer plates of the kinetochore (Saitoh et al. 1992; Przewloka et al. 2011; Screpanti et al. 2011).
With the development of proteomics approaches, many other CENPs have been identified. CENP-A chromatin-associated proteins, which have variously been termed the Interphase Centromere Complex (ICEN), CENP-A nucleosome-associated complex (NAC)/CENP-A nucleosome distal (CAD), or constitutive centromere-associated network (CCAN) proteins, form the centromere-specific chromatin structure (Obuse et al. 2004; Izuta et al. 2006; Foltz et al. 2006; Okada et al. 2006). Numerous kinetochore proteins, including the KNL-1/Mis12 complex/Ndc80 (KMN) complex, and other checkpoint proteins assemble on this centromere chromatin during the mitotic phase (Cheeseman et al. 2006; Musacchio and Salmon 2007).
The CENP-B box is found both in the centromeric repetitive DNA of human alphoid DNA and mouse centromeric minor satellite DNA, which has an otherwise unrelated DNA sequence (Masumoto et al. 1989; Kipling et al. 1995). When mapped in detail, the centromeric alphoid DNA of chromosome 21 and chromosome X was found to consist of two domains. The inner, centromere core, on which the kinetochore assembles, is composed of a homogeneous repeating array of higher-order repeat (HOR) units. This is flanked by highly divergent alphoid monomeric units (Fig. 1b). In the homogeneous HOR locus (also termed the type I alphoid locus), chromosome-specific multimers of the 171-bp unit constitute the HOR unit, and the HOR units extend over mega-base pairs of a homogeneous locus (Choo et al. 1991; Ikeno and Masumoto 1994; Schueler et al. 2001; Aldrup-Macdonald and Sullivan 2014; Miga et al. 2014). In the monomeric repeating locus (also termed the type II alphoid locus), 171-bp alphoid monomers exhibit a divergence of ∼85 % and have no regular repeat structure. All human chromosomes have slightly different alphoid HOR units and monomeric loci around the centromeres. CENP-B boxes appear in all type I/HOR loci except the Y chromosome alphoid HOR locus (Masumoto et al. 1989; Earnshaw et al. 1991). Interestingly, the absence of CENP-B box motifs in Y chromosome centromeres is common among humans, chimpanzees, and mice (Pertile et al. 2009).
Human artificial chromosome formation with centromeric repetitive DNA
It has been technically difficult to investigate whether the entire alphoid repeat loci are required for centromere function or if only a part of the alphoid repeat is sufficient for de novo centromere formation. However, top-down approaches for determining the functional centromeric DNA sequence have been carried out. The human X and Y chromosomes were minimized using the targeted telomere sequence-mediated chromosome fragmentation method until centromere function was lost. This top–down approach indicated that centromere function is associated with the alphoid repeats because the minimum stable X and Y chromosomes contained alphoid DNA (Brown et al. 1994; Farr et al. 1995). The smallest stable derivative of the X chromosome that was obtained by this approach had 1.8 Mb of alphoid DNA (Mills et al. 1999).
In a bottom-up approach, Willard et al. cloned chromosome 17 alphoid DNA HOR unit in a BAC vector and extended the repeat structure in tandem using an in vitro ligation method up to several hundred kilobases in length. Extended alphoid DNAs with a selection marker DNA fragment were co-transfected with total human genomic DNA and telomere sequences as a mixture of DNAs into human fibrosarcoma HT1080 cells. A mega-base-sized minichromosome formed as a consequence of heterogeneous multimerization of input DNAs from this mixture. This minichromosome was stably maintained without selection as the first human artificial chromosome (HAC) (Harrington et al. 1997) (Fig. 1c, top).
In another approach, 80–100 kb of type I/HOR or type II/monomeric alphoid DNA fragments from chromosome 21 were cloned into a YAC vector. These alphoid YAC DNAs were retrofitted with human telomere sequences at both ends, purified from yeast cells and transfected into human HT1080 cells. HAC formation occurred efficiently with the type I/HOR alphoid YAC DNA as mega-base-sized multimers of the input DNA lacking detectable host chromosomal DNA. In contrast, no HAC formation occurred with the type II/monomeric alphoid YAC. CENP-A, CENP-B, CENP-C, and CENP-E (a kinetochore motor protein) assembled on the HACs, which segregated equally into daughter cells at mitosis (Ikeno et al. 1998; Masumoto et al. 1998; Tsuduki et al. 2006) (Fig. 1c bottom).
In all cases, the introduced alphoid HOR DNA multimerized and formed stable mega-base-sized HACs (Ikeno et al. 1998; Mejia et al. 2001; Grimes et al. 2002). Telomere sequences were not required for HAC formation when the input DNA was circular (Ebersole et al. 2000). In that case, the circular input DNA formed circular HACs multimerizing the input DNAs. Although HAC DNA exhibits normally controlled replication coordinated with the cell cycle, no requirement for specific origin of replication sequences has been identified in these experiments.
Thus, although YAC and HAC formation with de novo functional centromere assembly from introduced naked centromeric DNAs has occurred both in yeast and human cells, crucial differences exist. HAC formation is always accompanied by multimerization of input DNA, and the HAC acquires regulated replication without defined origin sequences. HAC formation is not the only fate of the input alphoid HOR DNA. The other fate is integration into the host chromosomes as arrays that lack centromere activity. One explanation is that a larger chromosome size is required for stable mitotic maintenance as a HAC. Such a size effect is also observed for YAC stability (Murray and Szostak 1983; Hahnenberger et al. 1989) and for truncated human X chromosomes in DT40 cells (Mills et al. 1999). A second possible explanation is that multiple internal initiations of replication may be necessary. Recent analysis has shown that alphoid DNAs have the potential to initiate replication. Endogenous alphoid HOR DNA is a mega-base homogeneous repeat locus, and this size is much larger than the conceivable distance for the progression of a single replication fork. In addition, the selectable marker gene (Bsr) on the HAC vector arm works efficiently as a replication origin sequence on the HAC (Erliandri et al. 2014). But why does the same input alphoid HOR DNA undergo different fates in human cells? This interesting point for the epigenetic regulation of repetitive DNA with chromatin modification is discussed later in this review.
Alphoid HOR DNA with CENP-B boxes is required for de novo centromere formation
To investigate the importance of alphoid HOR DNA and CENP-B boxes in HAC formation and de novo centromere assembly, point mutations, which lose their binding capacity to CENP-B, were introduced to all CENP-B boxes in a chromosome 21 alphoid HOR DNA array, which was then introduced into human cells. No HAC formation occurred when all CENP-B boxes were mutated, and CENP-A assembly was diminished on the input mutant alphoid HOR DNA (Ohzeki et al. 2002; Basu et al. 2005). Thus, the CENP-B box is required for de novo CENP-A assembly and HAC formation. However, neither HAC formation nor CENP-A assembly was observed using a synthetic repeat DNA composed of a CENP-B box and a pBR-based non-alphoid GC-rich DNA fragment (Ohzeki et al. 2002). Thus, some aspect of alphoid HOR DNA, in addition to the CENP-B box, is required for CENP-A assembly and HAC formation. The requirement for CENP-B boxes appears at first paradoxical, as the CENP-B gene is not essential for life in the mouse, possibly because de novo centromere formation does not normally occur outside laboratory conditions.
The CENP-B box in the chromosome 21 HOR unit appears once per alphoid dimer sequence (340 bp). When the density of the CENP-B box was reduced to once per 11mer from the HOR unit by nucleotide substitution, CENP-A assembly decreased and the HAC formation activity was lost (Okamoto et al. 2007). In contrast, artificially increasing the CENP-B box density in the chromosome 17 HOR (to once per alphoid monomer) increased the efficiency of HAC formation (Basu et al. 2005). These results indicate that the CENP-B binding somehow attracts CENP-A assembly in a quantitative manner. The CENP-A density may be a threshold for further centromere/kinetochore assembly.
HACs are multimers of input alphoid DNA, but a minimum length of the input alphoid DNA is also important for HAC centromere formation. When a series of YAC and BAC vectors containing several different lengths of the alphoid HOR DNA were created, efficient HAC formation was observed only when the alphoid HOR DNA was more than 30 kb in length. Shorter arrays of 10 kb did not form HACs with stable, functional centromeres, even though the 10-kb array had CENP-A assembly activity (Okamoto et al. 2007). On the other hand, extension of the alphoid HOR DNA array to 240 kb did not significantly improve the frequency of HAC formation. Continuous regional occupancy of CENP-A chromatin of at least 30 kb in length without interruption by vector DNA may also be a requirement for functional centromere assembly.
Involvement of CENP-B in de novo CENP-A and heterochromatin assembly
CENP-B protein is a key factor in de novo HAC formation. CENP-B has homology to transposases and is conserved among at least S. pombe and mammals. CENP-B function is divergent among species. Three CENP-B homologs exist in S. pombe, and these mutants are related to heterochromatin formation at the centromere repeat and transposon (Nakagawa et al. 2002; Cam et al. 2008).
Striking results were reported from three laboratories, CENP-B gene knockout mice are viable, centromere/kinetochore function is maintained, and chromosomes are segregated without CENP-B (Hudson et al. 1998; Perez-Castro et al. 1998; Kapoor et al. 1998). Thus, either CENP-B is not required for maintenance of an established centromere or its function is redundant. To further test the requirement for CENP-B in de novo centromere formation, human alphoid HOR DNA was transfected into mouse embryonic fibroblasts (MEFs) and de novo CENP-A assembly and artificial chromosome formation occurred (Okada et al. 2007). This de novo CENP-A assembly activity was lost when the CENP-B gene was knocked out. Significantly, CENP-A assembly on the introduced alphoid HOR DNA recovered when the CENP-B amino terminal domain was expressed in the knockout MEFs in an add-back experiment (Okada et al. 2007). Thus, CENP-B protein contributes to de novo assembly of CENP-A on the input alphoid DNA. Surprisingly, add-back of full-length CENP-B induced strong heterochromatic modification (H3K9me3) at sites of ectopic integration of the input alphoid HOR DNA and CENP-A assembly was suppressed (Okada et al. 2007). These observations suggest that CENP-B exerts a dual antagonistic role on centromeric satellite DNA, balancing de novo centromere assembly and heterochromatin-induced inactivation depending on the overall chromatin context of the surrounding environment (Fig. 1d).
Interactions between CENP-A, CENP-B, and CENP-C may explain why CENP-B is required for de novo centromere assembly but not for maintenance of established centromeres. All three proteins co-immunoprecipitate with alphoid DNA (Ando et al. 2002; Suzuki et al. 2004) and they interact with one another. For example, CENP-B interacts with CENP-C in yeast two-hybrid analysis (Suzuki et al. 2004). The situation with CENP-A is more complex. The highly conserved H3-like histone-fold domain of CENP-A is flanked by unique N-terminal and C-terminal residues (Black and Cleveland 2011). CENP-C binds the C-terminal residues of nucleosomal CENP-A (Carroll et al. 2010). Recently, a functional interaction between CENP-B and the CENP-A N-terminal tail domain was reported (Fachinetti et al. 2013).
Knockout of CENP-A is lethal (Oegema et al. 2001; Régnier et al. 2005). CENP-A is a relatively stable protein, and after its expression is shut off, pre-existing CENP-A persists at the centromeres, only gradually disappears, possibly by replicative dilution. These cells maintain kinetochore function for a few cell cycles, but then rapidly lose all kinetochore protein assembly when the CENP-A level reaches a lower threshold (Fachinetti et al. 2013). Expression of exogenous CENP-A can rescue this kinetochore loss; either the N-terminal tail or the C-terminal residues are required for suppression of this kinetochore loss. CENP-A lacking the N-terminal tail rescues kinetochore assembly, probably through CENP-C interactions, but in these cells CENP-B levels at centromeres fall by about a half. Consistently, CENP-A lacking the C-terminal residues rescues kinetochore assembly, but when CENP-B is depleted by RNAi, chromosome segregation errors increase dramatically (Fachinetti et al. 2013).
These results suggest two possible kinetochore recruiting pathways: one leading from the CENP-A C-terminal tail to CENP-C, and the other from the CENP-A N-terminal tail to CENP-B. It is thought that CENP-B recruits CENP-C or other centromere proteins (Fig. 1e). These two possible kinetochore recruiting pathways provide an answer for why a protein required for de novo centromere and HAC formation on alphoid HOR DNA is not required for the function and the maintenance of the established centromere itself. Once assembled, the centromere maintains its function as the CENP-A C-terminal tail interacts with CENP-C without CENP-B. However, during de novo kinetochore formation, kinetochore, and centromere function require a strong link between alphoid HOR DNA (through the CENP-B box), which binds CENP-B and subsequent CENP-B-CENP-A N-terminal tail interactions (Masumoto et al. 2004; Okada et al. 2007; Fachinetti et al. 2013).
The evolutionarily conserved absence of the CENP-B box from Y chromosome centromeres may reveal as-yet undiscovered aspects of the regulation of this sex-determining chromosome. In addition, CENP-B does not always interact with CENP-A and CENP-C, especially in heterochromatic regions of the inner centromere. How such interactions are controlled is an intriguing question for future study.
Epigenetic regulation at the centromere: neocentromere and dicentric inactivation
Although de novo centromere assembly and HAC formation under experimental conditions is highly dependent on the DNA sequence, in patients, rare examples can be found where the natural centromere is damaged or lost, and a new centromere (“neocentromere”) has formed on a chromosomal arm (du Sart et al. 1997; Fukagawa and Earnshaw 2014). Neocentromeres form on a wide range of DNA sequences and lack alphoid DNA or CENP-B. Nonetheless, they assemble CENP-A chromatin and functional kinetochores assemble and are maintained epigenetically at neocentromeres (Alonso et al. 2007). This is a strong evidence that centromeres are determined epigenetically and not simply by the existence of the underlying DNA sequence. Further evidence supporting this was the finding that DNA cloned from a human neocentromere was not able to seed de novo HAC formation in HT1080 cells (Saffery et al. 2001).
Experimental formation of neocentromeres has also been demonstrated following deleting of the endogenous centromeres in S. pombe, Neurospora, Candida, and chicken DT40 cells. Deletion of the centromere using Cre-loxP recombination in S. pombe and DT40 cells yielded neocentromeres with low efficiency (frequency of approximately 10−4 and 10−6, respectively; Ishii et al. 2008; Shang et al. 2013).
Another epigenetic centromere event is dicentric inactivation. Typically, dicentric chromosomes resulted from breakage or other mechanism are highly unstable. This is because the two centromeres of the resulting chromosome can attach to opposite spindle poles, resulting in the chromosome being stretched and breaking at anaphase (McClintock 1941; Stimpson et al. 2012). The broken chromosome end accelerates the chromosome fusion event for the repair reaction, which generates a new dicentric chromosome for the vicious cycle. Rarely, the dicentric chromosome is stabilized by inactivating one of the two centromeres (Distèche et al. 1972). Therefore, centromere inactivation is important for maintaining dicentric chromosome integrity after rearrangement. The known dicentric inactivation mechanisms are DNA deletion and epigenetic silencing of the centromere (Earnshaw and Migeon 1985; Merry et al. 1985; Earnshaw et al. 1989; Stimpson et al. 2012).
It is not yet known how CENP-A assembly is nucleated at the neocentromere, acquires and maintains a functional size, or how the dicentric centromere is inactivated epigenetically. Understanding such epigenetic centromere-regulating mechanisms may help to elucidate why and how species have different and divergent centromere DNA sequences.
Heterochromatin negatively regulates CENP-A and kinetochore assembly
Integration of input alphoid HOR DNA into ectopic chromosomal sites is another common fate of the transfected DNA during HAC formation assays. CENP-A and kinetochore assembly is generally suppressed at the ectopic sites, even though the same input alphoid HOR DNA has the capacity to undergo de novo centromere assembly and HAC formation in other cells in the same experiment. These ectopic integration sites may be a good model to understand epigenetic inactivation of centromere DNA.
A common chromatin modification observed at ectopic alphoid HOR integration sites is the constitutive heterochromatic modification H3K9me3 (Nakano et al. 2003, 2008; Nakashima et al. 2005; Okamoto et al. 2007; Okada et al. 2007). Interestingly, in normal human embryonic fibroblasts, centromeric H3K9me3 modification and CENP-B levels increase during the cellular senescence process, while at the same time, levels of CENP-A decrease at the centromeres (Maehara et al. 2010). This could suggest that increasing levels of H3K9me3 might inactivate centromere function and inhibit CENP-A assembly.
This question could be answered using the alphoidtetO-HAC with a conditional centromere, which was developed by a collaboration of three laboratories based on synthetic alphoid dimer HOR repeats in which one monomer contains a CENP-B box while the adjacent monomer contains a tetracycline operator (tetO) at the corresponding position in the alphoid DNA sequence (Fig. 2). Any desired tet repressor (tetR) fusion protein can then be tethered at this tetO site. For example, the tet transcriptional silencer (tTS) is a tetR fusion protein containing the KRAB-AB silencing domain of the Kid1 protein (SDKid1). Tethering of the tTS to the tetO sites of the alphoidtetO-HAC recruits a repressive complex including H3K9-metylatransferase SETDB1 through protein–protein interactions. Thus, tethering the tTS to the alphoidtetO-HAC causes an increase in the heterochromatic H3K9me3 modification at centromeres, accompanied by a decrease in CENP-A assembly, and eventual missegregation of the HAC (Nakano et al. 2008). A subsequent study revealed that tethering the full KAP1 protein reduces other centromere chromatin protein assembly in the order, CENP-H → CENP-C → CENP-A (Cardinale et al. 2009). Thus, heterochromatin-associated modifications are involved in hierarchal negative regulation of centromere assembly (Bergmann et al. 2012b).
Models of de novo centromere formation and epigenetic maintenance. Top, schematic diagram of de novo centromere formation and chromatin assembly balance. CENP-B nucleates de novo CENP-A assembly in a quantitative manner on input alphoid DNA. CENP-B or CENP-A also interacts with CENP-C, which may enhance centromere protein assembly. H3K9-trimetylase not only inhibits new CENP-A assembly but also disrupts CENP-C assembly. Middle, once established, centromere chromatin is maintained with epigenetic mechanisms. CCAN protein assembly including CENP-TWSX and CENP-C is a platform for further mitotic kinetochore protein assembly during metaphase. CENP-A chromatin is replenished in the G1 phase (Jansen et al. 2007). CENP-C interacts with M18BP1 protein, a subunit of the human Mis18 protein complex (Moree et al. 2011; Dambacher et al. 2012). The Mis18 complex may recruit the CENP-A deposition factor HJURP. CENP-A replenishment requires its conserved domain, the CENP-A targeting domain (CATD). During S-phase, the CENP-A density would be diluted by DNA replication. Bottom, artificial tethering of HAT and/or Suv39 as tetR fusion proteins opens the way to manipulate the assembly balance of the centromere chromatin on the alphoidtetO DNA
In mouse pericentromeric constitutive heterochromatin, the H3K9me3 modification is introduced by Suv39h1 and Suv39h2 (Peters et al. 2001). In MEFs with Suv39h1/2 knocked out, de novo CENP-A assembly on the transfected alphoid DNA is enhanced (Okada et al. 2007). Similarly, Suv39h1 depletion by siRNA in HeLa cells results in a low, but significant, level of CENP-A assembly at ectopically integrated alphoid HOR DNA (Ohzeki et al. 2012). Thus, Suv39h1 H3K9-trimethyase and heterochromatin appear to be involved in an intrinsic centromere inactivation mechanism.
Acetylation positively regulates CENP-A assembly
Depletion of Suv39h1 by siRNA in HeLa cells was not sufficient to induce functional kinetochore assembly at the ectopic alphoid DNA integration sites. Thus, depletion of one of the factors repressing CENP-A assembly was apparently not enough to induce de novo functional centromere formation. It appeared that some positive regulation would be necessary.
In HT1080 cells, de novo centromere assembly and HAC formation is relatively efficient. However, neither de novo HAC formation nor stable CENP-A assembly on exogenous alphoid DNA occurs in many other commonly used human cell lines, including TIG7, U2OS, and HeLa cells. In exploring this phenomenon, we noticed that in HT1080 cells, the level of heterochromatic H3K9me3 at endogenous alphoid DNA loci is relatively low and histone H3K9 acetylation (H3K9ac) is increased. In contrast, in TIG7, U2OS, and HeLa cells, higher levels of H3K9me3 modification are observed at endogenous centromeric alphoid DNA loci, and H3K9ac is low (Ohzeki et al. 2012).
The importance of acetylation for de novo centromere assembly was subsequently demonstrated in HeLa cells. Tethering the histone acetyl transferase (HAT) domain of p300 or PCAF to an ectopic integrated alphoidtetO DNA array containing high levels of H3K9me3 induced de novo hyper-assembly of CENP-A (Ohzeki et al. 2012). This HAT-assisted CENP-A hyper-assembly occurred across the entire ∼5 Mb region of the ectopic integrated alphoidtetO array within 2 h during G1 phase. Later, during mitosis, de novo kinetochore proteins were observed to assemble on the array and interact with bundles of spindle microtubules.
De novo HAC formation has never been observed in HeLa or U2OS cells following simple transfection of input alphoidtetO DNA. Strikingly, transient tethering of the histone acetyl transferase (HAT) domain of p300 or PCAF to the input alphoidtetO DNA following transfection did permit de novo formation of HACs with functional centromeres. It therefore appears that even transient HAT tethering can break an initial barrier (presumably due to hyper H3K9me3 levels at centromeres) for de novo centromere establishment on input alphoid DNA in HeLa cells. Once formed, these HACs were maintained stably without further assistance from the tethered HAT, even under strong centromeric heterochromatin pressure in HeLa cells (Ohzeki et al. 2012) (Fig. 2). Thus, the centromere itself can acquire a system to overcome such strong heterochromatin pressure.
Involvement of HAT activity in the pathway depositing newly synthesized CENP-A at centromeres has been suggested in several reports. hMis18 alpha is a subunit of the human Mis18 complex (Fujita et al. 2007), which assembles at centromeres only in telophase to G1. This complex functions upstream of the CENP-A/Histone H4 chaperone, HJURP, in CENP-A targeting (Dunleavy et al. 2009; Foltz et al. 2009). New CENP-A deposition is lost following hMis18α depletion, but hyperacetylation induced by histone deacetylase inhibitor TSA treatment can suppress this hMis18α depletion phenotype (Fujita et al. 2007). Indeed, during mitotic exit, a brief pulse of histone acetylation can be detected on centromeric alphoid DNA (Ohzeki et al. 2012). PCAF, p300 HATs (Craig et al. 2003), and MYST-HAT family complexes (Ohta et al. 2010) associate with endogenous centromeres. Intrinsic HAT activity may therefore cooperate with other factors during centromere assembly or functional maintenance.
Tethering of the CENP-A deposition factors from the Mis18 complex or HJURP can also induce CENP-A targeting and subsequent kinetochore assembly on the alphoidtetO DNA (Ohzeki et al. 2012). Indeed, tethering of HJURP can deposit CENP-A and induce subsequent kinetochore assembly, even on Lac operator (LacO) repeats in the absence of alphoid DNA sequence (Barnhart et al. 2011). CENP-A assembly on such LacO repeats can maintain centromere function without the tethering of additional initial factors (Hori et al. 2013). Established CENP-A chromatin is both a platform for kinetochore assembly and an epigenetic landmark for centromere maintenance.
Consistent with this view, the assembly of CENP-A chromatin can be bypassed if two proteins that link the inner and outer (microtubule-binding) elements of the kinetochore (CENP-T, and part of CENP-C) are tethered to ectopic arrays in vertebrate cells (Gascoigne et al. 2011; Hori et al. 2013). These synthetic kinetochores appear to be fully functional without the assembly of CENP-A or other inner kinetochore (CCAN) components.
Other epigenetic modifications and transcription for centromere/kinetochore assembly
Transcription-related chromatin modifications are also involved in centromere/kinetochore assembly. A euchromatic modification, H3K4me2, is associated with transcriptionally competent chromatin. H3K4me2 is also observed between blocks of CENP-A chromatin at endogenous centromeres (Sullivan and Karpen 2004). Tethering of LSD1, a H3K4me2 demethylase, on the tetO-HAC reduces assembly of the HJURP and consequent loss of new CENP-A assembly (Bergmann et al. 2011).
Monomethylation on H4K20 (H4K20me1) is also associated with both transcriptional activity and repression. H4K20me1 is specifically associated with CENP-A nucleosomes, both in endogenous centromeres in HeLa and DT40 cells and at neocentromeres in the latter (Hori et al. 2014). This modification appears to somehow license CENP-A chromatin for subsequent kinetochore assembly, and the assembly of centromere proteins CENP-H and CENP-T is diminished when PHF8, a demethylase for H4K20me1, is tethered to endogenous centromeres by fusion to CENP-U in human cells (Hori et al. 2014).
Surprisingly, elongating RNA polymerase II has been detected at centromeres in metaphase, where its transcriptional activity may be important for CENP-C assembly (Chan and Wong 2012). The level of this transcriptional activity must be finely balanced, however. On the alphoidtetO-HAC centromere, induction of strong transcriptional activity by the tethering of VP16, a strong transcriptional activator, not only blocks new CENP-A assembly but also strips the centromere of pre-existing CENP-A. In this experiment, VP16 raised centromeric transcript levels by >100-fold, to the level of a housekeeping gene. In this context, the HAC was rapidly lost due to failure of mitotic segregation. In contrast, weak (∼10×) transcriptional activation of alphoid arrays caused by tethering the activation domain of NF-KappaB p65 is compatible with CENP-A assembly (Bergmann et al. 2012a). H3K9me3 and acetylation are also involved in transcriptional regulation. It is not clear if the weak transcription is required for CENP-A assembly. However, differential histone H3.3 or H3.1 deposition is indeed coupled with transcription or replication machinery respectively (Tagami et al. 2004).
Other transcription-related factors, such as FACT or RSF1, also co-purify with CENP-A chromatin and involved in centromere regulation. RSF1 appears to be required for stable incorporation of CENP-A into chromatin (Perpelescu et al. 2009). Tethering of RSF also recruits CENP-S and CENP-X (Helfricht et al. 2013). FACT in yeast cells prevents ectopic CENP-A assembly through a ubiquitin-related degradation pathway (Choi et al. 2012; Dayter and Biggins 2014).
Balance between centromere nucleation and suppression
The size of the CENP-A-bound region of alphoid DNA has been suggested to be 30∼100 kb (Okamoto et al. 2007; Alonso et al. 2007; Shang et al. 2013), which is much smaller than the type I alphoid HOR. Thus, endogenous centromeres and HAC centromeres derived from a simple alphoid HOR DNA have a large number of non-CENP-A chromatin domains (Grimes et al. 2004; Okamoto et al. 2007), most of which are heterochromatin.
What is the role of heterochromatin in HAC formation? Heterochromatin assembly may act to enhance cohesion or suppress the CENP-A chromatin assembly from extending into the whole area of a large repetitive DNA. Disruption of heterochromatin during HAC formation by embedding an additional promoter in the vector arm results in intact de novo centromere assembly, but HAC formation is lost, presumably due to a loss of cohesin enrichment through HP1 binding (Nakashima et al. 2005). Another role of heterochromatin may be to control kinetochore size. Forced introduction of H3K9me3 via the tethering of Suv39h1 on the alphoidtetO-HAC centromere disrupts or cancels centromere protein assembly. In contrast, forced introduction of acetylation by tethering HATs results in extensive CENP-A chromatin and kinetochore assembly across an entire ∼5 Mb alphoidtetO DNA integration site (Ohzeki et al. 2012) (Fig. 2). Such large kinetochore cannot satisfy the mitotic checkpoint, probably due to spindle abnormalities or formation of functional dicentric chromosomes. A balance between centromere nucleation and suppression by heterochromatin therefore appears be important to control either kinetochore size or assembly.
S. pombe has a tRNA genes, which act as barrier elements, between heterochromatin and centromeres at the DNA level. Deletion of these genes induces heterochromatin invasion into the centromere domain, and this results in meiotic chromosomal instability (Scott et al. 2006). However, in humans and many other species, centromere assembly occurs on homogeneous, simple, repetitive DNAs. Maintenance of a mechanism balancing centromere nucleation and suppression by the heterochromatin is important. Centromere/kinetochore size can be adjusted by artificially embedding a boundary element between two different synthetic alphoid HOR DNAs. Boundary elements that function in this way include human HS4, human gamma satellite DNA, and tRNA genes, all of which prevent heterochromatin from spreading to adjacent marker genes (Kim et al. 2009; Ebersole et al. 2011). Development of conditional protein tethering to pericentromeric heterochromatin using combined tetO and/or LacO systems may increase our understanding of the interplay between centromeric chromatin and pericentromeric heterochromatin.
Concluding remarks
HAC formation is a powerful tool for understanding how chromosomes form and are organized. However, there is not a simple 1:1 correlation between input DNA and the generated HAC. More technical advancements and understanding of principal events that occur during dynamic HAC formation in vivo are necessary.
With the HAC formation assay, knowledge about centromere chromatin and heterochromatin has been accumulating. HACs can also be used for studies of replication, telomeres, and other chromosomal functions (Weuts et al. 2012; Wakai et al. 2014). The meiotic behavior of artificial mammalian chromosomes is also of interest.
HACs are also potentially useful as gene delivery vectors for transgenic animals and variety of cells (Ikeno et al. 2009; Hasegawa et al. 2014). Genome editing technologies are rapidly advancing, and any genomic locus could be loaded into a HAC via direct cloning through homologous recombination. In particular, the conditional alphoidtetO-HAC with a controllable kinetochore (Nakano et al. 2008; Kim et al. 2011; Kononenko et al. 2014) is useful for a hit-and-run gene expression, such as is useful during induction of pluripotency or direct reprogramming of cells (Hiratsuka et al. 2011). Advancement of chromosome transfer technology will accelerate such HAC applications.
Abbreviations
- Alphoid DNA:
-
Alpha satellite DNA
- ARS:
-
Autonomous replicating sequence
- CAD:
-
CENP-A distal
- CCAN:
-
Constitutive centromere-associated network
- CDE:
-
Conserved DNA element
- CENP:
-
Centromere protein
- HAC:
-
Human artificial chromosome
- HOR:
-
Higher order repeat
- ICEN:
-
Interphase Centromere Complex
- KMN:
-
KNL-1/Mis12 complex/Ndc80
- NAC:
-
CENP-A nucleosome-associated complex
- YAC:
-
Yeast artificial chromosome
References
Aldrup-Macdonald ME, Sullivan BA (2014) The past, present, and future of human centromere genomics. Genes 5(1):33–50
Allshire RC, Karpen GH (2008) Epigenetic regulation of centromeric chromatin: old dogs, new tricks? Nat Rev Genet 9(12):923–937
Alonso A, Fritz B, Hasson D, Abrusan G, Cheung F, Yoda K, Radlwimmer B, Ladurner AG, Warburton PE (2007) Co-localization of CENP-C and CENP-H to discontinuous domains of CENP-A chromatin at human neocentromeres. Genome Biol 8(7):R148
Ando S, Yang H, Nozaki N, Okazaki T, Yoda K (2002) CENP-A, -B, and -C chromatincomplex that contains the I-type alpha-satellite array constitutes the prekinetochore in HeLa cells. Mol Cell Biol 22(7):2229–2241
Barnhart MC, Kuich PH, Stellfox ME, Ward JA, Bassett EA, Black BE, Foltz DR (2011) HJURP is a CENP-A chromatin assembly factor sufficient to form a functional de novo kinetochore. J Cell Biol 194(2):229–243
Basu J, Stromberg G, Compitello G, Willard HF, Van Bokkelen G (2005) Rapid creation of BAC-based human artificial chromosome vectors by transposition with synthetic alpha-satellite arrays. Nucleic Acids Res 33(2):587–596
Bergmann JH, Rodríguez MG, Martins NM, Kimura H, Kelly DA, Masumoto H, Larionov V, Jansen LE, Earnshaw WC (2011) Epigenetic engineering shows H3K4me2 is required for HJURP targeting and CENP-A assembly on a synthetic human kinetochore. EMBO J 30(2):328–340
Bergmann JH, Jakubsche JN, Martins NM, Kagansky A, Nakano M, Kimura H, Kelly DA, Turner BM, Masumoto H, Larionov V, Earnshaw WC (2012a) Epigenetic engineering: histone H3K9 acetylation is compatible with kinetochore structure and function. J Cell Sci 125(Pt 2):411–421
Bergmann JH, Martins NM, Larionov V, Masumoto H, Earnshaw WC (2012b) HACking the centromere chromatin code: insights from human artificial chromosomes. Chromosome Res 20(5):505–519
Bernard P, Maure JF, Partridge JF, Genier S, Javerzat JP, Allshire (2001) Requirement of heterochromatin for cohesion at centromeres. Science 294(5551):2539–2542
Black BE, Cleveland DW (2011) Epigenetic centromere propagation and the nature of CENP-a nucleosomes. Cell 144(4):471–479
Brown KE, Barnett MA, Burgtorf C, Shaw P, Buckle VJ, Brown WR (1994) Dissecting the centromere of the human Y chromosome with cloned telomeric DNA. Hum Mol Genet 3(8):1227–1237
Cam HP, Noma K, Ebina H, Levin HL, Grewal SI (2008) Host genome surveillance for retrotransposons by transposon-derived proteins. Nature 451(7177):431–436
Camahort R, Shivaraju M, Mattingly M, Li B, Nakanishi S, Zhu D, Shilatifard A, Workman JL, Gerton JL (2009) Cse4 is part of an octameric nucleosome in budding yeast. Mol Cell 35(6):794–805
Cardinale S, Bergmann JH, Kelly D, Nakano M, Valdivia MM, Kimura H, Masumoto H, Larionov V, Earnshaw WC (2009) Hierarchical inactivation of a synthetic human kinetochore by a chromatin modifier. Mol Biol Cell 20(19):4194–4204
Carroll CW, Milks KJ, Straight AF (2010) Dual recognition of CENP-A nucleosomes is required for centromere assembly. J Cell Biol 189(7):1143–1155
Chan FL, Wong LH (2012) Transcription in the maintenance of centromere chromatin identity. Nucleic Acids Res 40(22):11178–11188
Cheeseman IM, Chappie JS, Wilson-Kubalek EM, Desai A (2006) The conserved KMN network constitutes the core microtubule-binding site of the kinetochore. Cell 127:983–997
Chikashige Y, Kinoshita N, Nakaseko Y, Matsumoto T, Murakami S, Niwa O, Yanagida M (1989) Composite motifs and repeat symmetry in S. pombe centromeres: direct analysis by integration of NotI restriction sites. Cell 57(5):739–751
Choi ES, Strålfors A, Catania S, Castillo AG, Svensson JP, Pidoux AL, Ekwall K, Allshire RC (2012) Factors that promote H3 chromatin integrity during transcription prevent promiscuous deposition of CENP-A (Cnp1) in fission yeast. PLoS Genet 8(9):e1002985
Choo KH, Vissel B, Nagy A, Earle E, Kalitsis P (1991) A survey of the genomic distribution of alpha satellite DNA on all the human chromosomes, and derivation of a new consensus sequence. Nucleic Acids Res 19(6):1179–1182
Clarke L, Carbon J (1980) Isolation of a yeast centromere and construction of functional small circular chromosomes. Nature 287:504–509
Cleveland DW, Mao Y, Sullivan KF (2003) Centromeres and kinetochores: from epigenetics to mitotic checkpoint signaling. Cell 112(4):407–421
Craig JM, Earle E, Canham P, Wong LH, Anderson M, Choo KH (2003) Analysis of mammalian proteins involved in chromatin modification reveals new metaphase centromeric proteins and distinct chromosomal distribution patterns. Hum Mol Genet 12(23):3109–3121
Dambacher S, Deng W, Hahn M, Sadic D, Fröhlich J, Nuber A, Hoischen C, Diekmann S, Leonhardt H, Schotta G (2012) CENP-C facilitates the recruitment of M18BP1 to centromeric chromatin. Nucleus 3(1):101–110
Deyter GM, Biggins S (2014) The FACT complex interacts with the E3 ubiquitin ligase Psh1 to prevent ectopic localization of CENP-A. Genes Dev 28(16):1815–1826
Distèche C, Hagemeijer A, Frederic J, Progneaux D (1972) An abnormal large human chromosome identified as an end-to-end fusion of two X’s by combined results of the new banding techniques and microdensitometry. Clin Genet 3(5):388–395
du Sart D, Cancilla MR, Earle E, Mao JI, Saffery R, Tainton KM, Kalitsis P, Martyn J, Barry AE, Choo KH (1997) A functional neo-centromere formed through activation of a latent human centromere and consisting of non-alpha-satellite DNA. Nat Genet 16(2):144–153
Dunleavy EM, Roche D, Tagami H, Lacoste N, Ray-Gallet D, Nakamura Y, Daigo Y, Nakatani Y, Almouzni-Pettinotti G (2009) HJURP is a cell-cycle-dependent maintenance and deposition factor of CENP-A at centromeres. Cell 137(3):485–497
Earnshaw WC, Migeon BR (1985) Three related centromere proteins are absent from the inactive centromere of a stable isodicentric chromosome. Chromosoma 92(4):290–296
Earnshaw WC, Rothfield N (1985) Identification of a family of human centromere proteins using autoimmune sera from patients with scleroderma. Chromosoma 91(3–4):313–321
Earnshaw WC, Sullivan KF, Machlin PS, Cooke CA, Kaiser DA, Pollard TD, Rothfield NF, Cleveland DW (1987) Molecular cloning of cDNA for CENP-B, the major human centromere autoantigen. J Cell Biol 104(4):817–829
Earnshaw WC, Ratrie H 3rd, Stetten G (1989) Visualization of centromere proteins CENP-B and CENP-C on a stable dicentric chromosome in cytological spreads. Chromosoma 98(1):1–12
Earnshaw WC, Bernat RL, Cooke CA, Rothfield NF (1991) Role of the centromere/kinetochore in cell cycle control. Cold Spring Harb Symp Quant Biol 56:675–685
Earnshaw WC, Allshire RC, Black BE, Bloom K, Brinkley BR, Brown W, Cheeseman IM, Choo KH, Copenhaver GP, Deluca JG, Desai A, Diekmann S, Erhardt S, Fitzgerald-Hayes M, Foltz D, Fukagawa T, Gassmann R, Gerlich DW, Glover DM, Gorbsky GJ, Harrison SC, Heun P, Hirota T, Jansen LE, Karpen G, Kops GJ, Lampson MA, Lens SM, Losada A, Luger K, Maiato H, Maddox PS, Margolis RL, Masumoto H, McAinsh AD, Mellone BG, Meraldi P, Musacchio A, Oegema K, O’Neill RJ, Salmon ED, Scott KC, Straight AF, Stukenberg PT, Sullivan BA, Sullivan KF, Sunkel CE, Swedlow JR, Walczak CE, Warburton PE, Westermann S, Willard HF, Wordeman L, Yanagida M, Yen TJ, Yoda K, Cleveland DW (2013) Esperanto for histones: CENP-A, not CenH3, is the centromeric histone H3 variant. Chromosome Res 21(2):101–106
Ebersole TA, Ross A, Clark E, McGill N, Schindelhauer D, Cooke H, Grimes B (2000) Mammalian artificial chromosome formation from circular alphoid input DNA does not require telomere repeats. Hum Mol Genet 9(11):1623–1631
Ebersole T, Kim JH, Samoshkin A, Kouprina N, Pavlicek A, White RJ, Larionov V (2011) tRNA genes protect a reporter gene from epigenetic silencing in mouse cells. Cell Cycle 10(16):2779–2791
Erliandri I, Fu H, Nakano M, Kim JH, Miga KH, Liskovykh M, Earnshaw WC, Masumoto H, Kouprina N, Aladjem MI, Larionov V (2014) Replication of alpha-satellite DNA arrays in endogenous human centromeric regions and in human artificial chromosome. Nucleic Acids Res
Fachinetti D, Folco HD, Nechemia-Arbely Y, Valente LP, Nguyen K, Wong AJ, Zhu Q, Holland AJ, Desai A, Jansen LE, Cleveland DW (2013) A two-step mechanism for epigenetic specification of centromere identity and function. Nat Cell Biol 15(9):1056–1066
Farr CJ, Bayne RA, Kipling D, Mills W, Critcher R, Cooke HJ (1995) Generation of a human X-derived minichromosome using telomere-associated chromosome fragmentation. EMBO J 14(21):5444–5454
Fitzgerald-Hayes M, Clarke L, Carbon J (1982) Nucleotide sequence comparisons and functional analysis of yeast centromere DNAs. Cell 29(1):235–244
Folco HD, Pidoux AL, Urano T, Allshire RC (2008) Heterochromatin and RNAi are required to establish CENP-A chromatin at centromeres. Science 319(5859):94–97
Foltz DR, Jansen LE, Black BE, Bailey AO, Yates JR 3rd, Cleveland DW (2006) The human CENP-A centromeric nucleosome-associated complex. Nat Cell Biol 8(5):458–469
Foltz DR, Jansen LE, Bailey AO, Yates JR 3rd, Bassett EA, Wood S, Black BE, Cleveland DW (2009) Centromere-specific assembly of CENP-a nucleosomes is mediated by HJURP. Cell 137(3):472–484
Fujita Y, Hayashi T, Kiyomitsu T, Toyoda Y, Kokubu A, Obuse C, Yanagida M (2007) Priming of centromere for CENP-A recruitment by human hMis18alpha, hMis18beta, and M18BP1. Dev Cell 12(1):17–30
Fukagawa T, Earnshaw WC (2014) The centromere: chromatin foundation for the kinetochore machinery. Dev Cell 30(5):496–508
Furuyama S, Biggins S (2007) Centromere identity is specified by a single centromeric nucleosome in budding yeast. Proc Natl Acad Sci U S A 104(37):14706–14711
Gascoigne KE, Takeuchi K, Suzuki A, Hori T, Fukagawa T, Cheeseman IM (2011) Induced ectopic kinetochore assembly bypasses the requirement for CENP-A nucleosomes. Cell 145(3):410–422
Grewal SI, Jia S (2007) Heterochromatin revisited. Nat Rev Genet 8(1):35–46
Grimes BR, Rhoades AA, Willard HF (2002) Alpha-satellite DNA and vector composition influence rates of human artificial chromosome formation. Mol Ther 5(6):798–805
Grimes BR, Babcock J, Rudd MK, Chadwick B, Willard HF (2004) Assembly and characterization of heterochromatin and euchromatin on human artificial chromosomes. Genome Biol 5(11):R89
Hahnenberger KM, Baum MP, Polizzi CM, Carbon J, Clarke L (1989) Construction of functional artificial minichromosomes in the fission yeast Schizosaccharomyces pombe. Proc Natl Acad Sci U S A 86(2):577–581
Harrington JJ, Van Bokkelen G, Mays RW, Gustashaw K, Willard HF (1997) Formation of de novo centromeres and construction of first-generation human artificial microchromosomes. Nat Genet 15(4):345–355
Hasegawa Y, Ishikura T, Hasegawa T, Watanabe T, Suzuki J, Nakayama M, Okamura Y, Okazaki T, Koseki H, Ohara O, Ikeno M, Masumoto H (2014) Generating a transgenic mouse line stably expressing human MHC surface antigen from a HAC carrying multiple genomic BACs. Chromosoma. doi:10.1007/s00412-014-0488-3
Hegemann JH, Shero JH, Cottarel G, Philippsen P, Hieter P (1988) Mutational analysis of centromere DNA from chromosome VI of Saccharomyces cerevisiae. Mol Cell Biol 8(6):2523–2535
Helfricht A, Wiegant WW, Thijssen PE, Vertegaal AC, Luijsterburg MS, van Attikum H (2013) Remodeling and spacing factor 1 (RSF1) deposits centromere proteins at DNA double-strand breaks to promote non-homologous end-joining. Cell Cycle 12(18):3070–3082
Hiratsuka M, Uno N, Ueda K, Kurosaki H, Imaoka N, Kazuki K, Ueno E, Akakura Y, Katoh M, Osaki M, Kazuki Y, Nakagawa M, Yamanaka S, Oshimura M (2011) Integration-free iPS cells engineered using human artificial chromosome vectors. PLoS One 6(10):e25961
Hori T, Shang WH, Takeuchi K, Fukagawa T (2013) The CCAN recruits CENP-A to the centromere and forms the structural core for kinetochore assembly. J Cell Biol 200(1):45–60
Hori T, Shang WH, Toyoda A, Misu S, Monma N, Ikeo K, Molina O, Vargiu G, Fujiyama A, Kimura H, Earnshaw WC, Fukagawa T (2014) Histone H4 Lys 20 monomethylation of the CENP-A nucleosome is essential for kinetochore assembly. Dev Cell 29(6):740–749
Howman EV, Fowler KJ, Newson AJ, Redward S, MacDonald AC, Kalitsis P, Choo KH (2000) Early disruption of centromeric chromatin organization in centromere protein A (Cenpa) null mice. Proc Natl Acad Sci U S A 97(3):1148–1153
Hudson DF, Fowler KJ, Earle E, Saffery R, Kalitsis P, Trowell H, Hill J, Wreford NG, de Kretser DM, Cancilla MR, Howman E, Hii L, Cutts SM, Irvine DV, Choo KH (1998) Centromere protein B null mice are mitotically and meiotically normal but have lower body and testis weights. J Cell Biol 141(2):309–319
Ikeno M, Masumoto H, Okazaki T (1994) Distribution of CENP-B boxes reflected in CREST centromere antigenic sites on long-range alpha-satellite DNA arrays of human chromosome 21. Hum Mol Genet 3(8):1245–1257
Ikeno M, Grimes B, Okazaki T, Nakano M, Saitoh K, Hoshino H, McGill NI, Cooke H, Masumoto H (1998) Construction of YAC-based mammalian artificial chromosomes. Nat Biotechnol 16(5):431–439
Ikeno M, Suzuki N, Hasegawa Y, Okazaki T (2009) Manipulating transgenes using a chromosome vector. Nucleic Acids Res 37:e44
Ishii K, Ogiyama Y, Chikashige Y, Soejima S, Masuda F, Kakuma T, Hiraoka Y, Takahashi K (2008) Heterochromatin integrity affects chromosome reorganization after centromere dysfunction. Science 321(5892):1088–1091
Izuta H, Ikeno M, Suzuki N, Tomonaga T, Nozaki N, Obuse C, Kisu Y, Goshima N, Nomura F, Nomura N, Yoda K (2006) Comprehensive analysis of the ICEN (Interphase Centromere Complex) components enriched in the CENP-A chromatin of human cells. Genes Cells 11(6):673–684
Jansen LE, Black BE, Foltz DR, Cleveland DW (2007) Propagation of centromeric chromatin requires exit from mitosis. J Cell Biol 176(6):795–805
Kagansky A, Folco HD, Almeida R, Pidoux AL, Boukaba A, Simmer F, Urano T, Hamilton GL, Allshire RC (2009) Synthetic heterochromatin bypasses RNAi and centromeric repeats to establish functional centromeres. Science 324(5935):1716–1719
Kapoor M, de Montes de Oca Luna R, Liu G, Lozano G, Cummings C, Mancini M, Ouspenski I, Brinkley BR, May GS (1998) The CENPB gene is not essential in mice. Chromosoma 107(8):570–576
Kim JH, Ebersole T, Kouprina N, Noskov VN, Ohzeki J, Masumoto H, Mravinac B, Sullivan BA, Pavlicek A, Dovat S, Pack SD, Kwon YW, Flanagan PT, Loukinov D, Lobanenkov V, Larionov V (2009) Human gamma-satellite DNA maintains open chromatin structure and protects a transgene from epigenetic silencing. Genome Res 19(4):533–544
Kim JH, Kononenko A, Erliandri I, Kim TA, Nakano M, Iida Y, Barrett JC, Oshimura M, Masumoto H, Earnshaw WC, Larionov V, Kouprina N (2011) Human artificial chromosome (HAC) vector with a conditional centromere for correction of genetic deficiencies in human cells. Proc Natl Acad Sci U S A 108(50):20048–20053
Kipling D, Mitchell AR, Masumoto H, Wilson HE, Nicol L, Cooke HJ (1995) CENP-B binds a novel centromeric sequence in the Asian mouse Mus caroli. Mol Cell Biol 15(8):4009–4020
Kitagawa K, Hieter P (2001) Evolutionary conservation between budding yeast and human kinetochores. Nat Rev Mol Cell Biol 2(9):678–687
Kitagawa K, Masumoto H, Ikeda M, Okazaki T (1995) Analysis of protein-DNA and protein-protein interactions of centromere protein B (CENP-B) and properties of the DNA-CENP-B complex in the cell cycle. Mol Cell Biol 15(3):1602–1612
Kononenko AV, Bansal R, Lee NC, Grimes BR, Masumoto H, Earnshaw WC, Larionov V, Kouprina N (2014) A portable BRCA1-HAC (human artificial chromosome) module for analysis of BRCA1 tumor suppressor function. Nucleic Acids Res
Maehara K, Takahashi K, Saitoh S (2010) CENP-A reduction induces a p53-dependent cellular senescence response to protect cells from executing defective mitoses. Mol Cell Biol 30(9):2090–2104
Masumoto H, Masukata H, Muro Y, Nozaki N, Okazaki T (1989) A human centromere antigen (CENP-B) interacts with a short specific sequence in alphoid DNA, a human centromeric satellite. J Cell Biol 109(5):1963–1973
Masumoto H, Ikeno M, Nakano M, Okazaki T, Grimes B, Cooke H, Suzuki N (1998) Assay of centromere function using a human artificial chromosome. Chromosoma 107(6–7):406–416
Masumoto H, Nakano M, Ohzeki J (2004) The role of CENP-B and alpha-satellite DNA: de novo assembly and epigenetic maintenance of human centromeres. Chromosome Res 12(6):543–556
McClintock B (1941) The stability of broken ends of chromosomes in Zea mays. Genetics 26(2):234–282
Mejía JE, Willmott A, Levy E, Earnshaw WC, Larin Z (2001) Functional complementation of a genetic deficiency with human artificial chromosomes. Am J Hum Genet 69(2):315–326
Merry DE, Pathak S, Hsu TC, Brinkley BR (1985) Anti-kinetochore antibodies: use as probes for inactive centromeres. Am J Hum Genet 37(2):425–430
Miga KH, Newton Y, Jain M, Altemose N, Willard HF, Kent WJ (2014) Centromere reference models for human chromosomes X and Y satellite arrays. Genome Res 24(4):697–707
Mills W, Critcher R, Lee C, Farr CJ (1999) Generation of an approximately 2.4 Mb human X centromere-based minichromosome by targeted telomere-associated chromosome fragmentation in DT40. Hum Mol Genet 8(5):751–761
Moree B, Meyer CB, Fuller CJ, Straight AF (2011) CENP-C recruits M18BP1 to centromeres to promote CENP-A chromatin assembly. J Cell Biol 194(6):855–871
Moroi Y, Peebles C, Fritzler MJ, Steigerwald J, Tan EM (1980) Autoantibody to centromere (kinetochore) in scleroderma sera. Proc Natl Acad Sci U S A 77(3):1627–1631
Muro Y, Masumoto H, Yoda K, Nozaki N, Ohashi M, Okazaki T (1992) Centromere protein B assembles human centromeric alpha-satellite DNA at the 17-bp sequence, CENP-B box. J Cell Biol 116(3):585–596
Murray AW, Szostak JW (1983) Construction of artificial chromosomes in yeast. Nature 305(5931):189–193
Musacchio A, Salmon ED (2007) The spindle-assembly checkpoint in space and time. Nat Rev Mol Cell Biol 8(5):379–393
Nakagawa H, Lee JK, Hurwitz J, Allshire RC, Nakayama J, Grewal SI, Tanaka K, Murakami Y (2002) Fission yeast CENP-B homologs nucleate centromeric heterochromatin by promoting heterochromatin-specific histone tail modifications. Genes Dev 16(14):1766–1778
Nakano M, Okamoto Y, Ohzeki J, Masumoto H (2003) Epigenetic assembly of centromeric chromatin at ectopic alpha-satellite sites on human chromosomes. J Cell Sci 116:4021–4034
Nakano M, Cardinale S, Noskov VN, Gassmann R, Vagnarelli P, Kandels-Lewis S, Larionov V, Earnshaw WC, Masumoto H (2008) Inactivation of a human kinetochore by specific targeting of chromatin modifiers. Dev Cell 14(4):507–522
Nakaseko Y, Adachi Y, Funahashi S, Niwa O, Yanagida M (1986) Chromosome walking shows a highly homologous repetitive sequence present in all the centromere regions of fission yeast. EMBO J 5(5):1011–1021
Nakashima H, Nakano M, Ohnishi R, Hiraoka Y, Kaneda Y, Sugino A, Masumoto H (2005) Assembly of additional heterochromatin distinct from centromere-kinetochore chromatin is required for de novo formation of human artificial chromosome. J Cell Sci 118(Pt 24):5885–5898
Nonaka N, Kitajima T, Yokobayashi S, Xiao G, Yamamoto M, Grewal SI, Watanabe Y (2002) Recruitment of cohesin to heterochromatic regions by Swi6/HP1 in fission yeast. Nat Cell Biol 4(1):89–93
Obuse C, Yang H, Nozaki N, Goto S, Okazaki T, Yoda K (2004) Proteomics analysis of the centromere complex from HeLa interphase cells: UV-damaged DNA binding protein 1 (DDB-1) is a component of the CEN-complex, while BMI-1 is transiently co-localized with the centromeric region in interphase. Genes Cells 9(2):105–120
Oegema K, Desai A, Rybina S, Kirkham M, Hyman AA (2001) Functional analysis of kinetochore assembly in Caenorhabditis elegans. J Cell Biol 153(6):1209–1226
Ogiyama Y, Ishii K (2012) The smooth and stable operation of centromeres. Genes Genet Syst 87(2):63–73
Ohta S, Bukowski-Wills JC, Sanchez-Pulido L, Alves Fde L, Wood L, Chen ZA, Platani M, Fischer L, Hudson DF, Ponting CP, Fukagawa T, Earnshaw WC, Rappsilber J (2010) The protein composition of mitotic chromosomes determined using multiclassifier combinatorial proteomics. Cell 142(5):810–821
Ohzeki J, Nakano M, Okada T, Masumoto H (2002) CENP-B box is required for de novo centromere chromatin assembly on human alphoid DNA. J Cell Biol 159(5):765–775
Ohzeki J, Bergmann JH, Kouprina N, Noskov VN, Nakano M, Kimura H, Earnshaw WC, Larionov V, Masumoto H (2012) Breaking the HAC Barrier: histone H3K9 acetyl/methyl balance regulates CENP-A assembly. EMBO J 31(10):2391–2402
Okada M, Cheeseman IM, Hori T, Okawa K, McLeod IX, Yates JR 3rd, Desai A, Fukagawa T (2006) The CENP-H-I complex is required for the efficient incorporation of newly synthesized CENP-A into centromeres. Nat Cell Biol 8(5):446–457
Okada T, Ohzeki J, Nakano M, Yoda K, Brinkley WR, Larionov V, Masumoto H (2007) CENP-B controls centromere formation depending on the chromatin context. Cell 131(7):1287–1300
Okamoto Y, Nakano M, Ohzeki J, Larionov V, Masumoto H (2007) A minimal CENP-A core is required for nucleation and maintenance of a functional human centromere. EMBO J 26(5):1279–1291
Palmer DK, O’Day K, Wener MH, Andrews BS, Margolis RL (1987) A 17-kD centromere protein (CENP-A) copurifies with nucleosome core particles and with histones. J Cell Biol 104(4):805–815
Perez-Castro AV, Shamanski FL, Meneses JJ, Lovato TL, Vogel KG, Moyzis RK, Pedersen R (1998) Centromeric protein B null mice are viable with no apparent abnormalities. Dev Biol 201(2):135–143
Perpelescu M, Nozaki N, Obuse C, Yang H, Yoda K (2009) Active establishment of centromeric CENP-A chromatin by RSF complex. J Cell Biol 185(3):397–407
Pertile MD, Graham AN, Choo KH, Kalitsis P (2009) Rapid evolution of mouse Y centromere repeat DNA belies recent sequence stability. Genome Res 19(12):2202–2213
Peters AH, O’Carroll D, Scherthan H, Mechtler K, Sauer S, Schöfer C, Weipoltshammer K, Pagani M, Lachner M, Kohlmaier A, Opravil S, Doyle M, Sibilia M, Jenuwein T (2001) Loss of the Suv39h histone methyltransferases impairs mammalian heterochromatin and genome stability. Cell 107(3):323–337
Pluta AF, Saitoh N, Goldberg I, Earnshaw WC (1992) Identification of a subdomain of CENP-B that is necessary and sufficient for localization to the human centromere. J Cell Biol 116(5):1081–1093
Przewloka MR, Venkei Z, Bolanos-Garcia VM, Debski J, Dadlez M, Glover DM (2011) CENP-C is a structural platform for kinetochore assembly. Curr Biol 21(5):399–405
Régnier V, Vagnarelli P, Fukagawa T, Zerjal T, Burns E, Trouche D, Earnshaw W, Brown W (2005) CENP-A is required for accurate chromosome segregation and sustained kinetochore association of BubR1. Mol Cell Biol 25(10):3967–3981
Saffery R, Wong LH, Irvine DV, Bateman MA, Griffiths B, Cutts SM, Cancilla MR, Cendron AC, Stafford AJ, Choo KH (2001) Construction of neocentromere-based human minichromosomes by telomere-associated chromosomal truncation. Proc Natl Acad Sci U S A 98(10):5705–5710
Saitoh H, Tomkiel J, Cooke CA, Ratrie H 3rd, Maurer M, Rothfield NF, Earnshaw WC (1992) CENP-C, an autoantigen in scleroderma, is a component of the human inner kinetochore plate. Cell 70(1):115–125
Schueler MG, Higgins AW, Rudd MK, Gustashaw K, Willard HF (2001) Genomic and genetic definition of a functional human centromere. Science 294(5540):109–115
Scott KC, Merrett SL, Willard HF (2006) A heterochromatin barrier partitions the fission yeast centromere into discrete chromatin domains. Curr Biol 16(2):119–129
Screpanti E, De Antoni A, Alushin GM, Petrovic A, Melis T, Nogales E, Musacchio A (2011) Direct binding of Cenp-C to the Mis12 complex joins the inner and outer kinetochore. Curr Biol 21(5):391–398
Shang WH, Hori T, Martins NM, Toyoda A, Misu S, Monma N, Hiratani I, Maeshima K, Ikeo K, Fujiyama A, Kimura H, Earnshaw WC, Fukagawa T (2013) Chromosome engineering allows the efficient isolation of vertebrate neocentromeres. Dev Cell 24(6):635–648
Stimpson KM, Matheny JE, Sullivan BA (2012) Dicentric chromosomes: unique models to study centromere function and inactivation. Chromosome Res 20(5):595–605
Stinchcomb DT, Struhl K, Davis RW (1979) Isolation and characterisation of a yeast chromosomal replicator. Nature 282(5734):39–43
Struhl K, Stinchcomb DT, Scherer S, Davis RW (1979) High-frequency transformation of yeast: autonomous replication of hybrid DNA molecules. Proc Natl Acad Sci U S A 76(3):1035–1039
Sullivan BA, Karpen GH (2004) Centromeric chromatin exhibits a histone modification pattern that is distinct from both euchromatin and heterochromatin. Nat Struct Mol Biol 11(11):1076–1083
Suzuki N, Nakano M, Nozaki N, Egashira S, Okazaki T, Masumoto H (2004) CENP-B interacts with CENP-C domains containing Mif2 regions responsible for centromere localization. J Biol Chem 279(7):5934–5946
Tachiwana H, Kagawa W, Shiga T, Osakabe A, Miya Y, Saito K, Hayashi-Takanaka Y, Oda T, Sato M, Park SY, Kimura H, Kurumizaka H (2011) Crystal structure of the human centromeric nucleosome containing CENP-A. Nature 476(7359):232–235
Tagami H, Ray-Gallet D, Almouzni G, Nakatani Y (2004) Histone H3.1 and H3.3 complexes mediate nucleosome assembly pathways dependent or independent of DNA synthesis. Cell 116(1):51–61
Takahashi K, Murakami S, Chikashige Y, Funabiki H, Niwa O, Yanagida M (1992) A low copy number central sequence with strict symmetry and unusual chromatin structure in fission yeast centromere. Mol Biol Cell 3(7):819–835
Takahashi K, Chen ES, Yanagida M (2000) Requirement of Mis6 centromere connector for localizing a CENP-A-like protein in fission yeast. Science 288(5474):2215–2219
Talbert PB, Ahmad K, Almouzni G, Ausió J, Berger F, Bhalla PL, Bonner WM, Cande WZ, Chadwick BP, Chan SW, Cross GA, Cui L, Dimitrov SI, Doenecke D, Eirin-López JM, Gorovsky MA, Hake SB, Hamkalo BA, Holec S, Jacobsen SE, Kamieniarz K, Khochbin S, Ladurner AG, Landsman D, Latham JA, Loppin B, Malik HS, Marzluff WF, Pehrson JR, Postberg J, Schneider R, Singh MB, Smith MM, Thompson E, Torres-Padilla ME, Tremethick DJ, Turner BM, Waterborg JH, Wollmann H, Yelagandula R, Zhu B, Henikoff S (2012) A unified phylogeny-based nomenclature for histone variants. Epigenetics Chromatin 5:7
Tsuduki T, Nakano M, Yasuoka N, Yamazaki S, Okada T, Okamoto Y, Masumoto H (2006) An artificially constructed de novo human chromosome behaves almost identically to its natural counterpart during metaphase and anaphase in living cells. Mol Cell Biol 26(20):7682–7695
Wakai M, Abe S, Kazuki Y, Oshimura M, Ishikawa F (2014) A human artificial chromosome recapitulates the metabolism of native telomeres in mammalian cells. PLoS One 9(2):e88530
Weuts A, Voet T, Verbeeck J, Lambrechts N, Wirix E, Schoonjans L, Danloy S, Marynen P, Froyen G (2012) Telomere length homeostasis and telomere position effect on a linear human artificial chromosome are dictated by the genetic background. Nucleic Acids Res 40(22):11477–11489
Willard HF, Waye JS (1987) Hierarchical order in chromosome-specific human alpha satellite DNA. Trends Genet 3:192–198
Yoda K, Kitagawa K, Masumoto H, Muro Y, Okazaki T (1992) A human centromere protein, CENP-B, has a DNA binding domain containing four potential alpha helices at the NH2 terminus, which is separable from dimerizing activity. J Cell Biol 119(6):1413–1427
Acknowledgments
We would like to express our gratitude to Drs. Beth Sullivan and Natalay Kouprina for giving us the opportunity to write this review article. Due to space constraints, it was not possible to cite all of the excellent work in this area and we regret omitting mention of many valuable studies in the field of human artificial chromosome technology/biology. This work was supported in part by MEXT KAKENHI Japan (grant numbers 23247030 and 23114008 to H.M.), the Kazusa DNA Research Institute Foundation (H.M.) and Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research, USA (V.L.). W.C.E. is a Principal Research Fellow of The Wellcome Trust [grant number 073915].
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editors: Natalay Kouprina and Vladimir Larionov.
Rights and permissions
About this article
Cite this article
Ohzeki, Ji., Larionov, V., Earnshaw, W.C. et al. Genetic and epigenetic regulation of centromeres: a look at HAC formation. Chromosome Res 23, 87–103 (2015). https://doi.org/10.1007/s10577-015-9470-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10577-015-9470-z