Abstract
Twenty-five dicentric small supernumerary marker chromosomes (sSMC) derived from #13/21, #14, #15, #18, and #22 were studied by immunohistochemistry for their centromeric activity. Centromere protein (CENP)-B was applied as marker for all centromeres and CENP-C to label the active ones. Three different ‘predominant’ activation patterns could be observed, i.e., centric fusion or either only one or all two centromeres were active. In one inherited case, the same activation pattern was found in mother and son. In acrocentric-derived sSMC, all three activation patterns could be present. In contrary, in chromosome 18-derived sSMC, only the fusion type was observed. In concordance with previous studies a certain centromeric plasticity was observed in up to 13% of the cells of an individual case. Surprisingly, the obtained data suggests a possible influence of the sSMC carrier’s gender on the implementation of the predominant activation pattern; especially, only one active centromere was found more frequently in female than in male carriers. Also, it might be suggested that dicentric sSMC with one active centromere could be less stable than such with two active ones—centromeric plasticity might have an influence here, as well. Also, centromere activity in acrocentric-derived dicentrics could be influenced by heteromorphisms of the corresponding short arms. Finally, evidence is provided that the closer the centromeres of a dicentric are and if they are not fused, the more likely it was that both of them became active. In concordance and refinement with previous studies, a distance of 1.4 Mb up to about 13 Mb the two active centromere state was favored, while centromeric distance of over ∼15 Mb lead to inactivation of one centromere. Overall, here, the first and largest ever undertaken study in dicentric sSMC is presented, providing evidence that the centromeric activation pattern is, and parental origin may be of interest for their biology. Influence of mechanisms similar or identical to meiotic imprinting in the centromeric regions of human chromosomes might be present. Furthermore, centromeric activation pattern could be at least in parts meaningful for the clinical outcome of dicentric sSMC, as sSMC stability and mosaicism can make the difference between clinically normal and abnormal phenotypes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For polar movements of chromosomes during mitosis interaction of microtubules with a special centromere structure, the kinetochore, are necessary. A normal human chromosome harbors only one active centromere and derivative chromosomes with two or more centromeres (dicentrics or multicentrics) are generally thought to have only one active centromere during cell division. It is assumed that several active centromeres on one piece of DNA should lead to faulty alignments; two centromeres on one chromatid could orient to opposite poles, which would result in anaphase bridges and tearing of the chromosome. However, when the two centromeres are close together, there is little room for torsion between them, and stable dicentrics can be formed (Niebuhr 1972; Daniel and Lam-Po-Tang 1976; Dewald et al. 1979; Ing and Smith 1983; Rivera et al. 1989). Also, it was already shown that the presence of two functional kinetochores on a single chromosome does not invariably lead to chromosome instability and loss (Sullivan and Willard 1998). During human meiosis, rarely but regularly, a U-type exchange between different (Liehr et al. 2004) or within the same chromosome (Murmann et al. 2009; Sheth et al. 2009 take place. This leads to stable dicentric, mostly acrocentric-derived derivative chromosomes, so called dicentric small supernumerary marker chromosome (sSMC). At present, about 2.7 million carriers of such sSMC are alive, about two-thirds of which harbor dicentric, often mentioned as inverted duplicated (inv dup) sSMC (Liehr and Weise 2007).
Anticentromeric antibodies were identified in the sera of patients with the calcinosis, Raynaud’s syndrome, esophageal dysmotility, sclerodactyly, and telangiectasia (CREST) variety of scleroderma (Moroi et al. 1980). These sera recognize both centromeres in normal and dicentric chromosomes except for the Y chromosome (Merry et al. 1985; Earnshaw and Migeon 1985; Peretti et al. 1986; Rivera et al. 1989; Wandall 1989; Haaf and Schmid 1990). Different proteins were recognized by the CREST sera and their location in the centromere determined: centromere protein (CENP)-A is a centromere-specific histone similar to H3 (Palmer et al. 1991), CENP-B is distributed in the centromere region beneath the kinetochores (Cooke et al. 1990) where it binds to a recognition sequence in human alpha-satellite DNA (Masumoto et al. 1989), CENP-C is a component of the inner kinetochore plate (Saitoh et al. 1992), and CENP-D is similar to the RCC1 protein, a negative regulator of mitosis (Bischoff et al. 1990). However, only CENP-C differentiates between active and inactive centromeres (Earnshaw et al. 1989).
Here, we studied for the centromere activity in 25 dicentric sSMC.
Material and methods
Studied cases
Carnoy-fixed cell suspensions derived from peripheral blood of 25 carriers with a dicentric sSMC were included in the present study. As summarized in Fig. 1 and Table 1, three sSMC each were derived from chromosome 13 or 21 and chromosome 22, four each from chromosome 14 and 18, and the remainder from chromosome 15. The female-to-male ratio was 13 to 12. In 11 cases, the sSMC were de novo, in five maternally derived, in one case familial (maternal and paternally transmitted), and in the rest of the cases the origin was not known. An sSMC was present in 20-100% of the studied peripheral blood cells. The clinical data of the studied cases is available at (Liehr 2010).
Molecular cytogenetics
The origin and genetic content of each sSMC was determined by fluorescence in situ hybridization (FISH) as reported previously, using different multicolor-FISH approaches. Centromere-specific FISH in its different variants was applied (cenM-FISH: Liehr et al. 2006; acrocenM-FISH: Trifonov et al. 2003; subcenM-FISH: Liehr et al. 2006). Additionally, in inv dup (15) cases, the commercially available probe LSI UBE3A (Abbott) located in 15q13 was used according to manufacturer’s instructions.
Immunohistochemistry
The immunoistochemical tests were done on Carnoy-fixed cell suspension as previously published (Earnshaw et al. 1989). A rabbit polyclonal to CENP-B (Abcam, Cambridge, UK) was used to stain all centromeres (dilution 1:50). The specific staining of the active centromeres was performed with the anti-CENP-C antibody guinea pig serum (1:100; Ando et al. 2002). FITC-labeled goat anti-rabbit IgG and CyTM3-conjugated AffiniPure Goat Anti-Guinea Pig IgG (Dianova, Hamburg, Germany) were applied as secondary antibodies.
Chromosome banding was achieved by DAPI counterstaining (4′6-diaminidino-2-phenylindoleb, Sigma). Per case between 50 and 200 metaphases with sSMC were evaluated. An Axioplan 2 fluorescence microscope (Zeiss, Jena, Germany), a standard CCD camera (IMAC) and the software ISIS (Metasystems, Altlussheim, Germany) were used for the analysis.
Statistics
Statistical analysis was performed using Student’s t test and one-way analysis of variance (ANOVA) method. Statistical significance was defined as p < 0.05.
Results
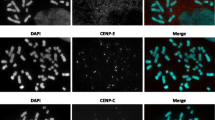
As summarized in Table 1 and Fig. 2 in the 25 studied cases, three different activation pattern of the two centromeres of each sSMC were found: in six cases, two centromeres were active (2× active); in nine cases, only one of the two centromeres was active (1× active); and in ten cases, the centromeres were fused to one active unit (fusion); for examples of the three patterns, see Fig. 3.
Examples for the three different activation pattern: both centromeres active (2× active) were found in case 15-O-q11.1/1-22–CENP-B (green labeling all centromeres) and CENP-C (red labeling only active centromeres) label simultaneously the centromeres on normal chromosomes 15 (#15) and both centromeres of the dicentric sSMC. In case 15-O-q11.1/1-63 and 22-O-q11.1/1-5, only the sSMC are shown; the first shows only one active centromere (1× active), the latter a typical fusion of green and red signal
Results of correlations of the three detected activation pattern fusion, 1× active and 2× active with different parameters are shown in Fig. 4. No statistically significant differences could be found when comparing de novo and parentally inherited sSMC (Fig. 4a); after Student’s t test all P values were between 0.101 and 0.599 (detailed results not shown).
a-c Comparison of the three observed three predominant immunohistochemical pattern fusion, 1× active and 2× active with inheritance (inherited or de novo, a), gender (male or female, b) and sSMC stability (mosaic or non-mosaic, c). d Alignment of chromosome size (expressed in chromosomal band) and only one active centromere
However, gender-dependent activation patterns were found: the states fusion and 2× active are found more frequently in male, while 1× active is present in seven female compared to only two male (Fig. 4a). This is a statistically significant difference with a P value of 0.012 according to Student’s t test. Fusion and 2× active P values were 0.372 and 0.247.
Statistically significant differences were also found for sSMC mosaicism and centromere activity (Fig. 4c). A fusion or two active centromeres (2× active) was present mostly in non-mosaic: Student’s t test P values were 0.003 (fusion), 0.011 (2× active), and <0.001 (1× active). This means sSMC with 2× active were less stable, while in almost all of the cases with only one active centromere (1× active) the sSMC was stable in >90% of the peripheral blood cells. This was supported by ANOVA test showing a statistically significant difference for mosaic and non-mosaic cases: F = 10.744, P = <0.001.
Finally, there was a possible correlation of the distance of both centromeres and their activity. The closer the centromeres were located, the more likely either they were fused or two of them were active (see Fig. 4d).
Discussion
The present study is the first systematic study performed in not less than 25 dicentric sSMC. Only one comparable larger study was done in 15 cases with Robertsonian translocations (Page and Shaffer 1998) using also CENP-B as all-centromere marker and CENP-C for active centromeres (Earnshaw et al. 1989). CENP-C is either necessary for anaphase chromosome movement or for mediating a signal which triggers centromere function during anaphase (Fukagawa and Brown 1997), however, it is necessary but not sufficient for the formation of a functional centromere (Fukagawa et al. 1999).
In the studied 25 dicentric sSMC, three different activation patterns could be present (fusion, 1× active, 2× active). In concordance with previous studies (Wandall 1994; Page and Shaffer 1998; Higgins et al. 2005), a certain centromeric plasticity could be observed; i.e., in between 0% and 13% of the cells of an individual case the sSMC showed another centromere-activity pattern than the majority, which is called in the following ‘predominant pattern’ (see Table 1, second to last column). Notable, centromeric plasticity was not observed if a fusion was present. Case 15-O-q11.1/1-16 and son of case 15-O-q11.1/1-16 provide first evidence that in familial cases an identical activation pattern could be retained. This is especially remarkable as in the mother (case 15-O-q11.1/1-16) the cells with an sSMC were by far less frequent in the peripheral blood than in the son. However, it is known that sSMC can be lost in peripheral blood throughout live time (Liehr et al. 2004). As visible in Table 1, all four inv dup(18)(q11.1) cases showed functional centromeric fusion, while in the acrocentric-derived sSMC in principle all three activation patterns could be present.
Here, for the first time, evidence was provided that the predominant centromere activation pattern is dependant from the gender of the sSMC carrier: fusion and 2× active are found more frequently in male, while 1× active is more frequent in female. This still could be due to an ascertainment bias and low the number of examined cases; further studies are necessary to verify that suggestion, even though the difference was statistically significant. If true, this observation might be helpful to explain the fact that familial sSMC are transmitted predominantly via the maternal line (Liehr 2006). It is considered that a dicentric chromosome with only one active centromere is more stable than one with two active centromeres (Therman et al. 1986). If the latter would be applicable also to dicentric sSMC during meiosis, the fact that dicentric sSMC with one active centromere are more frequently present in female could be of interest. Controversy, the present data show an adverse influence of 1× active at least on the mitotic sSMC stability (Fig. 4c). Additionally, four of the six sSMC with two active centromeres were mitotically stable (no mosaic formation) in the present study which might also be due in parts to the here and previously observed centromeric plasticity (Wandall 1994; Page and Shaffer 1998; Higgins et al. 2005).
A study in larger dicentric X chromosomes showed that the centromere activity is correlated to the centromere distance, i.e., the closer the centromeres, the more likely it was that both of them became active (between 4 and 12 Mb), while in two distant centromeres, only one was active (34 MB; Sullivan and Willard 1998). Here, in principle, this trend was confirmed (see Table 1 and Fig. 4d), even though reliable data on the centromere distance was available only for five of the cases. If centromeric distance was over 15.4 Mb, only one centromere was active (cases 15-CWw-148, 15-W-q13/2-4 and 15-W-q14/1-6). In those cases where the centromeric distance was between 1.4 and ∼4.5 Mb (22-Wces-5-95 and 15-O-q11.2∼12/1-2), two active centromeres could be observed. In the remainder 20 cases, the sSMC consisted according to molecular cytogenetics in their q-arms only of heterochromatic material and were broken in band q10 to q11.1. Both cases with cytogenetic breakpoint in q10 showed fusion pattern of centromere activity; besides, also eight cases with breaks in q11.1 had fusion pattern. The remainder ten q11.1 cases showed two clearly separated centromeric signals using the CENP-B antibody; four of those showing predominantly the 2× active pattern applying CENP-C antibody. Here, centromere plasticity may be not enough to explain this variance. However, it is a well-known fact that the acrocentric short arms are highly heteromorphic. Rearrangements in the short arms like reported previously (Lau et al. 1979; Schmid et al. 1994; Friedrich et al. 1996; Verma et al. 1996; Reddy and Sulcova 1998) may be causative for the observed variance. Thus, this might explain also, that cases with the same karyotype, like 47,XN,+inv dup(15)(q11.1) could have any of the three pattern fusion (case 15-O-q11.1/1-45), 1× active (case 15-O-q11.1/1-16) and 2× active (case 15-O-q11.1/1-11).
Conclusion
By this first and largest ever undertaken study in dicentric sSMC, evidence is provided that the centromeric activation pattern is of interest for the biology of sSMC maintenance and stability in individuals. Influence of mechanisms similar or identical to meiotic imprinting could act upon also the centromeric regions of human chromosomes, as possibly visible in the male-to-female ration in dicentric sSMC cases with one active centromere only. Furthermore, interesting clinical impact of mosaicism in sSMC carriers was reported: sSMC with known adverse prognosis if present in 100% of the cells of a carrier were observed to be harmless if present in small mosaic state only (Bonati et al. 2005; Loitzsch and Bartsch 2006; Guichet et al. 2009). Thus, understanding of centromeric activity and possible influence on sSMC stability have to be studied further also for the establishment of proper genotype-phenotype correlations in sSMC.
Abbreviations
- ANOVA:
-
Analysis of variance
- CENP:
-
Centromere protein
- MB:
-
Megabasepair
- sSMC:
-
Small supernumerary marker chromosome
References
Ando S, Yang H, Nozaki N, Okazaki T, Yoda K (2002) CENP-A, -B, and -C chromatin complex that contains the I-type alpha-satellite array constitutes the prekinetochore in HeLa cells. Mol Cell Biol 22:2229–2241
Bischoff FR, Maier G, Tilz G, Ponstingl H (1990) A 47-kDa human nuclear protein recognized by antikinetochore autoimmune sera is homologous with the protein encoded by RCC1, a gene implicated in onset of chromosome condensation. Proc Natl Acad Sci U S A 87:8617–8621
Bonati MT, Finelli P, Giardino D, Gottardi G, Roberts W, Larizza L (2005) Trisomy 15q25.2-qter in an autistic child: genotype-phenotype correlations. Am J Med Genet A 133:184–188
Cooke CA, Bernat RL, Earnshaw WC (1990) CENP-B: a major human centromere protein located beneath the kinetochore. J Cell Biol 110:1475–1488
Daniel A, Lam-Po-Tang PR (1976) Structure and inheritance of some heterozygous Robertsonian translocation in man. J Med Genet 13:381–388
Dewald GW, Boros SJ, Conroy MM, Dahl RJ, Spurbeck JL, Vitek HA (1979) A tdic(5;15)(p31;p11) chromosome showing variation for constriction in the centromeric regions in a patient with the cri du chat syndrome. Cytogenet Cell Genet 24:15–26
Earnshaw WC, Migeon BR (1985) Three related centromere proteins are absent from the inactive centromere of a stable isodicentric chromosome. Chromosoma 92:290–296
Earnshaw WC, 3rd Ratrie Ratrie, Stetten G (1989) Visualization of centromere proteins CENP-B and CENP-C on a stable dicentric chromosome in cytological spreads. Chromosoma 98:1–12
Friedrich U, Caprani M, Niebuhr E, Therkelsen AJ, Jørgensen AL (1996) Extreme variant of the short arm of chromosome 15. Hum Genet 97:710–713
Fukagawa T, Brown WR (1997) Efficient conditional mutation of the vertebrate CENP-C gene. Hum Mol Genet 6:2301–2308
Fukagawa T, Pendon C, Morris J, Brown W (1999) CENP-C is necessary but not sufficient to induce formation of a functional centromere. EMBO J 18:4196–4209
Guichet A, Boisseau P, Ingster O, Guardiola P, Couteleau A, Bonneau D (2009) Large inv dup(15) characterized by FISH and aCGH in a child with no abnormal phenotype at 2 years old. Chromosome Res 17(1):178, 11.4
Haaf T, Schmid M (1990) Y isochromosome associated with a mosaic karyotype and inactivation of the centromere. Hum Genet 85:486–490
Higgins AW, Gustashaw KM, Willard HF (2005) Engineered human dicentric chromosomes show centromere plasticity. Chromosome Res 13:745–762
Ing PS, Smith SD (1983) Cytogenetic studies of a patient with mosaicism of isochromosome 13q and a dicentric (Y;13) translocation showing differential centromeric activity. Clin Genet 24:194–199
Lau YF, Wertelecki W, Pfeiffer RA, Arrighi FE (1979) Cytological analyses of 14p+ variant by means of N-banding and combinations of silver staining and chromosome bandings. Hum Genet 46:75–82
Liehr T (2006) Familial small supernumerary marker chromosomes are predominantly inherited via the maternal line. Genet Med 8:459–462
Liehr T (2010) Small supernumerary marker chromosome homepage. Retrieved from http://www.med.uni-jena.de/fish/sSMC/00START.htm Accessed 9 April 2010
Liehr T, Weise A (2007) Frequency of small supernumerary marker chromosomes in prenatal, newborn, developmentally retarded and infertility diagnostics. Int J Mol Med 19:719–731
Liehr T, Claussen U, Starke H (2004) Small supernumerary marker chromosomes (sSMC) in humans. Cytogenet Genome Res 107:55–67
Liehr T, Mrasek K, Weise A, Dufke A, Rodríguez L, Martínez Guardia N, Sanchís A, Vermeesch JR, Ramel C, Polityko A, Haas OA, Anderson J, Claussen U, von Eggeling F, Starke H (2006) Small supernumerary marker chromosomes—progress towards a genotype-phenotype correlation. Cytogenet Genome Res 112:23–34
Loitzsch A, Bartsch O (2006) Healthy 12-year-old boy with mosaic inv dup(15)(q13). Am J Med Genet A 140:640–643
Masumoto H, Masukata H, Muro Y, Nozaki N, Okazaki T (1989) A human centromere antigen (CENP-B) interacts with a short specific sequence in alphoid DNA, a human centromeric satellite. J Cell Biol 109:1963–1973
Merry DE, Pathak S, Hsu TC, Brinkley BR (1985) Anti-kinetochore antibodies: use as probes for inactive centromeres. Am J Hum Genet 37:425–430
Moroi Y, Peebles C, Fritzler MJ, Steigerwald J, Tan EM (1980) Autoantibody to centromere (kinetochore) in scleroderma sera. Proc Natl Acad Sci U S A 77:1627–1631
Murmann AE, Conrad DF, Mashek H, Curtis CA, Nicolae RI, Ober C, Schwartz S (2009) Inverted duplications on acentric markers: mechanism of formation. Hum Mol Genet 18:2241–2256
Niebuhr E (1972) Dicentric and monocentric Robertsonian translocations in man. Humangenetik 16:217–226
Page SL, Shaffer LG (1998) Chromosome stability is maintained by short intercentromeric distance in functionally dicentric human Robertsonian translocations. Chromosome Res 6:115–122
Palmer DK, O'Day K, Trong HL, Charbonneau H, Margolis RL (1991) Purification of the centromere-specific protein CENP-A and demonstration that it is a distinctive histone. Proc Natl Acad Sci U S A 88:3734–3738
Peretti D, Maraschio P, Lambiase S, Lo Curto F, Zuffardi O (1986) Indirect immunofluorescence of inactive centromeres as indicator of centromeric function. Hum Genet 73:12–16
Reddy KS, Sulcova V (1998) The mobile nature of acrocentric elements illustrated by three unusual chromosome variants. Hum Genet 102:653–662
Rivera H, Zuffardi O, Maraschio P, Caiulo A, Anichini C, Scarinci R, Vivarelli R (1989) Alternate centromere inactivation in a pseudodicentric (15;20)(pter;pter) associated with a progressive neurological disorder. J Med Genet 26:626–630
Saitoh H, Tomkiel J, Cooke CA, 3rd Ratrie H, Maurer M, Rothfield NF, Earnshaw WC (1992) CENP-C an autoantigen in scleroderma, is a component of the human inner kinetochore plate. Cell 115:115–125
Schmid M, Nanda I, Steinlein C, Epplen JT (1994) Amplification of (GACA)n simple repeats in an exceptional 14p+ marker chromosome. Hum Genet 93:375–382
Sheth F, Ewers E, Kosyakova N, Weise A, Sheth J, Patil S, Ziegler M, Liehr T (2009) A neocentric isochromosome Yp present as additional small supernumerary marker chromosome–evidence against U-type exchange mechanism? Cytogenet Genome Res 125:115–116
Sullivan BA, Willard HF (1998) Stable dicentric X chromosomes with two functional centromeres. Nat Genet 20:227–228
Therman E, Trunca C, Kuhn EM, Sarto GE (1986) Dicentric chromosomes and the inactivation of the centromere. Hum Genet 72:191–195
Trifonov V, Seidel J, Starke H, Martina P, Beensen V, Ziegler M, Hartmann I, Heller A, Nietzel A, Claussen U, Liehr T (2003) Enlarged chromosome 13 p-arm hiding a cryptic partial trisomy 6p22.2-pter. Prenat Diagn 23:427–430
Verma RS, Kleyman SM, Conte RA (1996) Molecular characterization of an unusual variant of the short arm of chromosome 15 by FISH-technique. Jpn J Hum Genet 41:307–311
Wandall A (1989) Kinetochore development in two dicentric chromosomes in man. A light and electron microscopic study. Hum Genet 82:137–141
Wandall A (1994) A stable dicentric chromosome: both centromeres develop kinetochores and attach to the spindle in monocentric and dicentric configuration. Chromosoma 103:56–62
Acknowledgments
Supported in parts by the DAAD and the Prochance 2008, Jena.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Hans-Joachim Lipps.
Rights and permissions
About this article
Cite this article
Ewers, E., Yoda, K., Hamid, A.B. et al. Centromere activity in dicentric small supernumerary marker chromosomes. Chromosome Res 18, 555–562 (2010). https://doi.org/10.1007/s10577-010-9138-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10577-010-9138-7