Abstract
AMPA receptors are tetrameric ionic glutamate receptors, which mediate 90% fast excitatory synaptic transmission induced by excitatory glutamate in the mammalian central nervous system through the activation or inactivation of ion channels. The alternation of synaptic AMPA receptor number and subtype is thought to be one of the primary mechanisms that involve in synaptic plasticity regulation and affect the functions in learning, memory, and cognition. The increasing of surface AMPARs enhances synaptic strength during long-term potentiation, whereas the decreasing of AMPARs weakens synaptic strength during the long-term depression. It is closely related to the AMPA receptor as well as its subunits assembly, trafficking, and degradation. The dysfunction of any step in these precise regulatory processes is likely to induce the disorder of synaptic transmission and loss of neurons, or even cause neuropsychiatric diseases ultimately. Therefore, it is useful to understand how AMPARs regulate synaptic plasticity and its role in related neuropsychiatric diseases via comprehending architecture and trafficking of the receptors. Here, we reviewed the progress in structure, expression, trafficking, and relationship with synaptic plasticity of AMPA receptor, especially in anxiety, depression, neurodegenerative disorders, and cerebral ischemia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inotropic glutamate receptors (iGluRs) including three major types as AMPA receptors, NMDA receptors, kainate receptors and other pathologic states types like delta. Most of them share a similar architecture, activated by binding to L-glutamates which are released from presynaptic membrane thereby triggering a series of complex cascades including ion channels opening and rapid depolarization. It contributes to rapidly excitatory synaptic transmission as well as synaptic plasticity in the mammalian brain and mediates learning and memory function (Greger and Mayer 2019). The AMPA receptor, named after its agonist α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic, is formed via the assembly of four homologous or heterogeneous subunits together with its auxiliary subunits proteins (Mihály 2019). In mammalian brain, the architecture and composition of the AMPA receptor are dynamic, manifested as the spatial and temporal specificity of subunits distribution. Besides, along with development stage changes, the subunits constitute AMPA receptors are in dynamic alternation such as the typical difference of the receptor composition between mature and immature hippocampus. By utilizing the High-throughput Proteomics analysis, GluA2 subunits were observed dominantly in cortex, hippocampus, striatum, and thalamus, same as the GluA1 and GluA3 subunits. However, GluA4 constitute a great proportion of AMPA receptors in the cerebellum (Schwenk et al. 2014).
Glutamate is a major excitatory neurotransmitter in the brain and spinal cord, which binds to iGluRs then excites neurons. Nevertheless, different receptors have various ionic permeability. NMDA receptors channel is permeable to Na+, K+ and Ca2+ but less responsive to glutamate. Low concentration of Glu cannot induce NMDA-gated ion channel opened, which requires the participation of glycine (Gly). On the other side, Mg2+ is another important site regulating NMDAR, and inhibition of Mg2+ on the receptor performs voltage dependent (Yu et al. 2019). In contrast, the non-NMDA receptors including KA and AMPAR are more sensitive to Glu but mainly permeable to Na+ and K+ (Mihály 2019). Importantly, due to the specific RNA editing site occurred in GluA2, AMPARs exhibit different biophysical properties of the receptors with or without GluA2. The AMPARs containing GluA2 are Ca2+ impermeable (CI) which contributes to maintain low calcium concentration in the postsynaptic cytoplasm, whereas GluA2 lacking means Ca2+ permeable (CP). Thus, the levels of GluA2 transcription, translation, and/or expression on cell membranes play a considerable role in neurotransmission and synaptic plasticity under physiological and pathological conditions like calcium overload. Both of them involve GluA1 phosphorylation and cytokines regulation such as TNF-α increasing the expression of CP-AMPARs on the retinal ganglion cell (Summers et al. 2019; Cueva Vargas et al. 2015). How to understand the dynamic regulation of excitatory synaptic AMPA receptors is the core of the synaptic plasticity mechanism. Dysfunction of assembly, transport, and anchoring at the postsynaptic membrane of AMPA receptors may cause synaptic transmission disorders, leading to some neuropsychiatric diseases. In this current review, we provide an overview of the progress in composition and trafficking of AMPARs and discuss the role of AMPARs in synaptic plasticity and related diseases.
Advances in the AMPAR Receptor Architecture and Constitution
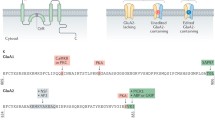
α-Amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptors (AMPARs) are ligand-gated ion channels composed of core subunits GluA1-4 and co-assembled with auxiliary subunits (Greger and Mayer 2019; Mihály 2019). The AMPA receptors are mainly distributed in cortex (52%), hippocampus (23%) as well as the cerebellum (17%), and expressed in both neurons and colloidal cells (Schwenk et al. 2014). As one significant postsynaptic ionotropic glutamate receptor subtypes in central nervous system (CNS), AMPARs characteristic domain architecture is layered and flexible. The AMPAR subunits composition is essential for the functions, properties, and trafficking mechanisms of receptors, impacts synaptic activity and plasticity, neural network formation and processing (Henley and Wilkinson 2016). In adult hippocampal neurons, GluA1/GluA2 and GluA2/GluA3 heteromer predominate, whereas GluA2/GluA4 heteromer dominate in immature hippocampus (Yang et al. 2008). Also, there is a small proportion of GluA1 homomer in brain. By specifically inhibiting GluA1 homologous AMPA receptors, researchers found that GluA1 homomer mediated quite a bit of proportion in synaptic responses in mouse hippocampal primary neurons (Takemoto et al. 2017). Each subunit consists of three extracellular and transmembrane structural domains with high homology: the amino-terminal domain (ATD)/N-terminal domain (NTD), the ligand-binding domain (LBD), transmembrane domain (TMD); and distinct intracellular structural domains, carboxyl-terminal domain (CTD) (Fig. 1a).
Schematics of the AMPAR subunit GluA2 and its auxiliary subunits. a Domain organization of the GluA2, embedded within the membrane. Four domains are bordered by dashed lines: ATD/NTD contains the N-terminal extending from the membrane; LBD consists of S1 and S2 segments with the flip/flop (the purple segment) and R/G editing site (labeled in red) on it; TMD composites of four hydrophobic helices (M1–M4), among them M2 is tilting to the cytoplasm with Q/R editing site (labeled in red) on it; CTD contains the C-tail facing to the cytoplasm. b Schematic of the TARPs, containing transmembrane domains composed of four α-helices (TM1–TM4), extracellular domain (ECD), N-terminal, and C-tail facing the cytoplasm. ECD contains an ECH (labeled in red), five β-sheets (β1–5), and four loops (β1–β2, β3–β4, β4-TM2, TM3-β5). c Schematic of the CNIH2/3, containing four transmembrane helices (TM1–TM4) with a CNIH2/3 specific segment (labeled in red) and two loops (loop1, loop2), together with both N terminus and C terminus extending from the membrane
ATD/NTD and LBD
The architecture of NTD exhibits as a dimer composed of two non-equivalence pairs of subunits in a diagonally opposite position respectively, named as AC and BD (in GluA2-containing AMPARs) (Greger and Mayer 2019). This domain has a signal sequence, which initiates the assembly of AMPAR tetramer after the formation of dimer. There is a balance between AMPARs homomerization and heteromerization since the limited capacity to drive AMPARS heteromerization of NTD. Besides, due to the different affinities between distinct subunit interfaces, the assembly modes are alternative. However, the heteromerization is usually prior to homomerization, and non-GluA2 is usually prior to heteromerization with GluA2 (Rossmann et al. 2011). The LBD, a dimer consisting of two segments (S1 and S2) with an alternatively spliced flip/flop exon, binds to glutamate and induces a rapid conformation change (Sakakura et al. 2019). The conformation of LBD is presumed to be associated with the regulation of AMPARs desensitization (Amin et al. 2017). The two segments (S1, S2) of LBD are split by ion channel, leading to the intertwined between the folding of LBD and ion channel domain (Greger and Esteban 2007). Accordingly, by impacting the GluA1/GluA2 splice variant assembly, splicing of the alternative flip/flop exons in LBD has an additional regulatory role in excitatory signaling (Penn et al. 2012). The dimeric NTD with bipartite densities asymmetrically stacks on top of LBD and elongates from the membrane, manifesting like the top of the capital letter Y (Nakagawa et al. 2005). It is the most common but not the only configuration in the resting state. The R/G editing (the arginine is replaced by Glycine) site sits at the twofold symmetric subunit interface, required during the formation of functional AMPAR tetramers and related with the desensitization of receptors. These two domains constitute each subunit extracellular domain (ECD), accounting for 70–80% of the molecular mass of the tetramer, and essential in the assembly of AMPAR dimerization and tetramerization (Gan et al. 2016).
TMD and CTD
The TMD belongs to the pore loop channel superfamily. It contains three hydrophobic membrane-spanning segments (M1, M3, and M4), a non-membrane spanning re-entrant loop (M2), and a Q/R editing site which refers to the Glutamine (Q, CAG) replaced by arginine (R, CGG). These four segments are symmetrically distributed in the transmembrane region around a fourfold rotational axis. (Henley and Wilkinson 2013). The M1 polymerizes with S1 following the LBD binding to glutamates. The formed complex called pre-M1 locates outside of the cytomembrane and initiates conformation change. The M3 belongs to the inner transmembrane helix, longer than the outer (M1). The M3 helix intertwines with M1 to form a pathway for ion current that faces outside of the cell close by membrane-aqueous solution boundary (Sobolevsky et al. 2009). The M4 is also an outer transmembrane segment surrounding the M3 segment and interacting with M3 in the closed state mostly. Additionally, it also interacts with the M1 segments of individual adjacent subunits and the pre-M1 of the same subunit. All of the interaction has a crucial impact on AMPARs assembly (Amin et al. 2017). Unlike M1, M3, and M4 segments which all belong to transmembrane helices, the M2 is a pore-loop helix, tilting to the inside of the cell membrane with a certain angle. At resting state, there are loop regions between M1 and M2 and also M2 and M3. While in active state, the loop region between M1 and M2 (M1/M2 loop) moved (Zachariassen et al. 2016). The Q/R site located at the M2 loop region regulates many specific processes and change properties of the subunits. For example, when the Q is replaced, the edited (R) subunit with positive charge prevents the passage of Ca2+ and reduce the signal-channel conductance (Huettner 2015). The CTD is like a tail of AMPA receptor in the intracellular side and extends into cytoplasm. This domain is the sites of post-translational modification of AMPA receptor, including phosphorylation, ubiquitination, and palmitoylation (Fig. 2). The C-tail binds to scaffold proteins or cytoskeleton proteins and has a crucial role in the regulation of receptor functions including signaling, anchoring, receptor trafficking, and channel opening (Greger and Esteban 2007; Shepherd and Huganir 2007).
Auxiliary Subunits
In addition to pore-forming subunits (Glu1-4), the auxiliary subunits which are structurally irrelevant membrane proteins also involve in the composition of the AMPA receptor complexes. The type of auxiliary subunit proteins includes transmembrane AMPA receptor regulatory proteins (TARPs), cornichon homologs (CNIHs), cysteine-knot AMPA receptor germ cell-specific gene 1-like protein (GSG1L), modulating protein family (CKAMP) (Kamalova and Nakagawa 2020), Synapse Differentiation Induced Gene 1 (SynDIG1) (Kalashnikova et al. 2010), and Synapse Differentiation Induced Gene 4 (SynDIG4)/proline-rich transmembrane protein 1 (Prrt1) (Matt et al. 2018). Distinct auxiliary subunits have different regulating functions for AMPA receptors. Two major classes auxiliary subunit of AMPA receptor are TARPs and CNIHs, specifically TARPs. They are widely distributed in hippocampus, cortex, and cerebellum. The canonical TARP homologs are classified γ-2, γ-3, γ-4, and γ-8 as Type I, γ-5 and γ-7 as type II in accordance with functional distinctions, belonging to the claudin homolog family (Chen et al. 2017). Accordingly, the overall structure of TARPs is close to claudins. They are composed of four transmembrane helices (TM1-4) with both N-terminal and a long C-terminal tail in the intracellular domain. The extracellular domain (ECD) is composed of an extracellular helix (ECH), five β-sheets (β1–5), and four loops (β1–β2, β3–β4, β4-TM2, and TM3-β5) (Fig. 1b) (Nakamura et al. 2019). The structural biology studies show usually every four, two, or one TARPs binds to AMPA receptor to form the complexes. For instance, the typical AMPA receptor auxiliary protein γ-2 type TARP (also known as stargazin) has fourfold symmetric ensemble encircles TMD of AMPA receptor (AMPAR: stargazin = 4:4 stoichiometry) (Chen et al. 2017). Stargazin is expressed in hippocampus, cortex, cerebellum, and spinal cord, especially in the cerebellum. It regulates properties of AMPA receptors as well as trafficking and gating. For example, stargazin is associated with functional AMPA receptors in lamina II neurons since it plays a part in hyperalgesia and is involved in synaptic transmission in an input-specific way at the same time (Sullivan et al. 2017). The prototypical cornichon homologs of AMPA receptor auxiliary proteins belong to CNIH2 and CNIH3 (Schwenk et al. 2009). Unlike the TARPs, the overall protein structure is composed of four hydrophobic transmembrane helices (TM1-4) but both C-terminus and N-terminus are in the extracellular domain. Interestingly, CNIHs seem to have only three transmembrane helices for a long time because of a specific segment connecting TM2 and TM3, making them a single transmembrane helix (Fig. 1c) (Kamalova and Nakagawa 2020; Nakagawa 2019; Wudick et al. 2018). There is a difference between HEK (human embryonic kidney) cells and neurons in the expression of CNIHs, as well as the regulating function and properties. In HEK cells, CNIH2 has been detected on cell surface, which was believed to raise AMPAR surface expression, moreover, slow the deactivation and desensitization of AMPARs compared to HEK cell TARPs. However, the expression of CNIHs was barely detected in brain. Accordingly, it was speculated that CNIHs may not as essential as TARPs in AMPA receptors regulation in brain region (Shi et al. 2010). GSG1L is also a member of claudin families, thus it shares a similar structure with TARPs. However, there are differences in architectures between them, making AMPARs functional diversity (Kamalova and Nakagawa 2020). The two auxiliary subunits may compete when binding to AMPA receptors (Schwenk et al. 2012). Both CKAMP and SynDIG1/4 have only one transmembrane helix. The CKAMP44 N-terminal containing a cysteine-knot is in extracellular domain and the C-terminal is in intracellular, while SynDIG1/4 is precisely the opposite (Kamalova and Nakagawa 2020). SynDIG1 affects the number and strength of the excitatory synapse by binding to AMPA receptors through C-terminal where contains a membrane-associated domain (Kalashnikova et al. 2010), while SynDIG4 is thought to be the basis of regulation higher-order cognitive plasticity (Matt et al. 2018).
Synthesis and Trafficking of the AMPA Receptors
AMPA receptors are synthesized in the endoplasmic reticulum (ER) just as most membrane proteins. Moreover, there is also an endoplasmic reticulum synthesizing AMPA receptors pathway in dendrites, which could shorten the distance of AMPA receptor transport to dendritic spine and enable synapse respond rapidly to signal transmission. After AMPA receptor oligopeptide chains synthesized, they cannot just enter Golgi apparatus directly. Primarily, these oligopeptide chains are required to become tetramers through post-translation processing like folding and polymerization in the ER. The RNA level editing (i.e. R/G editing or Q/R editing) is involved in the tetramer formation. Evidences suggested that edited GluA2 impeded the folding and assembly of subunit. However partly edited GluA2 was still able to assemble as homotetramer and exit the ER. While the fully edited GluA2 required co-assembly with other subunits like GluA1 to exit the ER. Particularly, the desensitization of AMPAR are more likely depending on the glutamate binding during formation. The tetramer desensitization competence is sensed by ER quality-control machinery to decide its dwell time (Greger and Esteban 2007). Subsequently, the mature tetramers release from Golgi apparatus through microtubule transport pathway and are discharged and transported to dendrites and dendritic spines (Fig. 3) (Hanley 2014b). Besides RNA editing, there are also other factors affect the formation of AMPARs and release from the ER including the intracellular Ca2+ releasing, CaMKII (calmodulin-dependent protein kinase II) active strengthening, and the mediation of AMPARS auxiliary subunits like TARPs and CNIHs (Greger and Esteban 2007).
Schematic of the recycling and degradation pathway of AMPARs. Excitatory neurotransmitter glutamates released from the active zone/AZ (orange shaded area) of the presynaptic membrane act on AMPARS and NMDARs in the postsynaptic membrane. NMDARs-dependent synaptic plasticity triggered by Ca2+ influx and promotes the synthesis and assembly of AMPARs in ER and GC. Intracellular AMPARs are transported to the postsynaptic membrane, subsequently undergoing exocytosis and laterally moving the AMPAR assemblies are ultimately fixed on the PSD that opposite to AZ (grey shaded area) with the cooperation of anchoring proteins like PSD-95. After detaching from PSD, AMPARs laterally move to the endocytic zone/EZ (purple shaded area) and be encapsulated to form endocytic vesicles that are trafficked to EEs/sorting endosomes subsequently. The AMPARs are sorted into three pathways in EEs: ① returning to the plasma membrane via recycling endosomes fissured from the tubular structure of EEs; ② degraded in lysosomes by the trafficking of late endosomes formed from the maturation of the cisternal parts of EEs,③ transported to the GC retrogradely. Abbreviations: Pre presynaptic membrane, Post postsynaptic membrane, ER endoplasmic reticulum, GC Golgi complex, EE (s) early endosome (s), PSD postsynaptic density
AMPA receptors are dynamically transported proteins which can be altered by long-term or short-term neuronal activity changes thereby inserting into or removing from the postsynaptic membrane rapidly (Kessels and Malinow 2009). The trafficking of synaptic AMPA receptor includes the following steps: transportation and exocytosis of intracellular AMPARs; lateral diffusion on the extra-synaptic cell surface and insertion into the synaptic sites; fixation on the postsynaptic membrane; recycling or degradation (Fig. 3) (Opazo and Choquet 2011).
AMPARs Intracellular Transportation and Insertion
AMPA receptors released from Golgi apparatus are transported to the dendritic trunk, the base of dendritic spine, and the plasma membrane of dendritic spine of extra-synapse by an actin proteins-depending microtubule transport pathway. Considering distribution difference between distinct subunits, there is a discussion about whether the transportation of AMPA receptors belongs to the subunit-depending mechanism. For example, AMPA receptors are mainly in the form of GluA1/GluA2 or GluA2/GluA3 heterotetramers in cortical and hippocampal neurons. Whereas GluA4 is less expressed in adult brain tissues but mainly expressed in inhibitory interneurons (Kessels and Malinow 2009; Pelkey et al. 2015). The AMPA receptors are cargo to the plasma membrane by exocytosis, in the process which is highly dynamic and driven by actin proteins. The actin proteins could bind to actin cytoskeleton thereby drives AMPARs-containing vesicles to the destination, termed as an actin-dependent mechanism (Hanley 2014a). Such proteins as MyoV and MyoVI are the two most representative myosin motor proteins. They are important in the process of vesicles to membrane surface by transporting the cargo to the cell periphery directly or promoting intracellular vesicles away from the plasma membrane (Hartman et al. 2011). Plus, the function of MyoVI is required to be combined with GluA1 subunit (Wu et al. 2002). Besides, the diverse C-tail length of distinct AMPA receptor subunits also affects receptor transportation. Generally, the AMPAR subunits with long C-terminal tail like GluA1 and GluA4 can be transported and inserted into the postsynaptic membrane rapidly, which is crucial in activity-dependent synaptic enhancement such as LTP. The AMPAR subunits with short C-terminal tail like GluA2 and GluA3is inserted and removed from the synapse cyclically mainly in an activity-independent manner, to maintain the number of AMPA receptors in postsynaptic membrane (Kessels and Malinow 2009). In that case, the AMPA receptor containing the GluA1 could be transported rapidly from ER to cell membrane surface, while containing GluA2 and GluA3 subunits is transported more slowly. Therefore, under the activity-dependent condition, the GluA1-containing AMPA receptors are transferred firstly to the synapse rapidly and then replaced by GluA2/3-containing receptors, leading to an increasing number of synaptic AMPA receptors during LTP (Keifer and Zheng 2010). Additional pathways are also involved in the secretion of synaptic AMPARs, such as the overexpression of the TARPs or the activation of protein kinase A (PKA), showing a strong function in the transportation of intracellular AMPA receptor and insertion into the postsynaptic membrane (Man et al. 2007; Schnell et al. 2002).
AMPARs Fixation in Postsynaptic Membrane
The spatial position of the AMPA receptor is unstable after insertion into the postsynaptic membrane, that is, it will move rapidly in the plasma membrane plane or be delivered from the plasma membrane. Therefore, the local cytoskeleton proteins or cytoskeletons are needed to bind to AMPA receptor to anchor it at the postsynaptic density (PSD), and consequently stabilize the number of the receptors in the postsynaptic membrane (Kessels and Malinow 2009). PSD is a specialized fiber region of the cytoskeleton in the excitatory postsynaptic terminal, opposite the active zone (AZ) in presynaptic membrane terminal. This electrodense structure is rich in the postsynaptic density protein of 95 kDa (PSD-95), which is a scaffolding protein that belongs to the Membrane-associated guanylate kinases (MAGUKs) family and could influence the trafficking of AMPA receptors directly or indirectly (Jeanneret et al. 2018). Same as the other PSD-95-like subfamily members PSD-93, SAP-97, and SAP-102, the PSD-95 protein contains PDZ regions in N-terminal, which are known as the Gly-Leu-Gly-Phe repeat sequences (GLGF repeat sequences). As a protein–protein mutual recognition modulator, the PDZ region can recognize specific C-terminal sequences of receptors and play a profound role in synaptic signal transduction and neuromuscular junctions (Kim and Sheng 2004; Elias and Nicoll 2007). PSD-95 reduces the activity of the AMPA receptor and anchors it at the postsynaptic membrane through binding to TARPs directly. In another word, the level of PSD-95 proteins would directly affect the number of the AMPA receptor in the postsynaptic membrane (Xu 2011). Given the previous experiment that PSD-95 KO mice exhibited the enhancement of LTP and the weakening of LTD, it is postulated that the PSD-95 regulation may be one of molecular mechanisms in excitatory synaptic plasticity (Yao et al. 2004). In contrast to this detection result in PSD-95 KO mice, the PSD-93 KO mice exhibited LTP disruption but without obvious change in LTD (Carlisle et al. 2008), which indicated that PSD-95 and PSD-93 share a similar function in the AMPA receptor anchoring but play opposite roles in the regulation of LTP. A study showed the importance that TARPs bind directly to both AMPARs and PSD-MAGUKs to the anchoring of AMPARs at the synapse (Elias and Nicoll 2007). Lately, Zeng et al. found that PSD-95 has a higher affinity with the C-terminal tail of the TARPs (TARPs-CT), rather than PDZ binding motif (PBM, usually the last 4–6 residues of TARPs) binding to PSD-95 mostly. The entire C-terminal tail is involved in the interaction of TARPs and PSD-MAGUKs, especially PSD-95 (Zeng et al. 2019). Such high affinity of TARPs-CT/PSD-95 complex makes TARPs remaining binding to PSD-95 even this interaction being interrupted and AMPARs being free from the postsynaptic membrane (Bats et al. 2007). Moreover, among the process of TARPs-CT/PSD-95 interaction, a series of protein kinases are involved. For instance, through regulating serine residue phosphorylation in stargazin C-terminal, the calmodulin-dependent protein kinase II (CaMKII) could enhance affinity between TARPs-CT and PSD-95, thereby strengthen AMPA receptors stability on the postsynaptic membrane (Sumioka et al. 2010). The phosphorylation sites induced by CaMKII are mainly located in the GluA1 C-tail. In addition, the other auxiliary subunit proteins of AMPARs including CNIH2/3, CKAMP44, SynDIG1, GSG1L, and Sol-2 also engage in the anchoring of AMPA receptors at the postsynaptic membrane, ensuring the stability of AMPA receptors number in the postsynaptic membrane.
Endocytosis, Recycling, and Degradation of AMPARs
According to the distinct mediating mechanisms, endocytosis of AMPARs can be classified into clathrin-dependent endocytosis and dynamin-dependent endocytosis. Both of them play a role in LTD by decreasing the expression of surface AMPARs. Through the complex endosomal sorting, the AMPA receptors can be either reversely transported to plasma membrane and re-inserting postsynaptic membrane, or degraded by lysosomes or proteasomes, which is known as clathrin-mediated endocytosis (CME) (van der Sluijs and Hoogenraad 2011; Parkinson and Hanley 2018). Primarily, AMPA receptors need to be released from PSD. The AMPARs internalization is regulated by the phosphatases such as protein phosphate-1 (PP1) which is targeted to the receptors through the PP1-anchoring protein (Gao et al. 2018), as well as the stimulation of NMDAR which contributes to the association between TARP and phospholipids thereby disrupting TARP/PSD-95 interaction (Sumioka et al. 2010). Subsequently, AMPA receptors are encapsulated by endocytic vesicles and transported to an endocytic zone adjacent to PSD (known as the EZ region), in a process called AMPARs internalization. After that, the receptors are trafficked into early endosomes and assigned to different trafficking pathways. That's why early endosomes are also known as sorting endosomes. Through recycling endosomes, part of the receptors return to plasma membrane and re-activated by postsynaptic membrane insertion by exocytosis and anchoring. While the receptors marked for degradation are carried by late endosomes and degraded by fusion with lysosomes. Beyond the two pathways, the receptor can also be retrogradely transported to trans-Golgi network (TGN). The whole process is directed by a complex and interconnected network, called the endosomal–lysosomal system. According to respective functions, the system is divided into distinct compartments: early endosomes /sorting endosomes, recycling endosomes, late endosomes, and lysosomes (Parkinson and Hanley 2018; Vagnozzi and Praticò 2019). Early endosomes (EEs) are short-lived with endomembrane and heterogeneous structures, which are expanded and form a network of tubular, cisternal, and tubulovesicular subdomains by continuously fusing with cargo endocytic vesicles. Recycling endosomes are fissured from the tubular structure of the early endosomes, while the cisternal parts of EEs mature into the late endosomes (LEs), which finally mature into the lysosomes (Kaur and Lakkaraju 2018). In recent years, the retromer complex, which exists in all eukaryotic cells, has become a novel target in the study of neurodegenerative filed. The dysfunction of the retromer complex lead to trafficking, recycling, and degradation functional disturbance of the endosomal–lysosomal system, which may be related to the pathogenesis of neurodegenerative diseases, characteristically by the two typical disorders Alzheimer disease (AD) and Parkinson disease (PD) (Vagnozzi and Praticò 2019).
In the meanwhile, the AMPA receptor endocytosis is regulated by a variety of interacting proteins and signaling molecules. Endocytic adaptor protein complex AP2 recruits and concentrates GluA2-containing AMPA receptors that are shed from the membrane via binding to GluA2 subunits or TARPs in specific plasma membrane regions. Through binding to the appendage domain of the AP2 subunit (α-adaptin) directly, the protein interacting with C-Kinase 1 (PICK1) promoted the endocytosis of GluA2 subunits and reduced the levels of GluA2-containing AMPA receptors in the postsynaptic membrane thereby enhanced the transformation of synaptic AMPA receptors to CP-AMPARs. This process required the activity-regulated cytoskeleton-associated protein (Arc/Arg3.1) as immobilized bait (Goo et al. 2018). The glutamate receptor-interacting protein (GRIP) family is an important synaptic stabilizing protein in the PSD. It plays the same role as the PSD-95 and competes the same sites on the GluA2 with PICK1, disturbing the interaction of GluA2/PICK1. Additionally, the N-ethylmaleimide sensitive fusion protein (NSF) also binds to GluA2, preventing internalization of the AMPA receptors by disturbing the binding of GluA2 and AP2 (Hanley 2018). Furthermore, in vitro experiments showed that AMPAR endocytosis can also be triggered by glutamate receptor agonists, such as group I metabotropic glutamate receptors (mGluRs). They are member of metabotropic glutamate receptors and involved in mGluR-mediated AMPAR endocytosis. When studying the functions of ubiquitination in mGluR1-mediated endocytosis, it was observed that enhanced endocytosis of the mGluR-mediated AMPAR through specifically inhibiting ubiquitination by acute knockdown of the ubiquitin ligase siAH-1A. But the research also suggested that there were ubiquitin-independent mGluR-mediated internalization pathway (Gulia et al. 2017). This indicates that ubiquitination plays a crucial role not only in protein degradation but also in ionotropic and metabotropic glutamate receptor endocytosis.
In general, various signaling molecules and interacting proteins work together on AMPA receptor to determine its endocytosis/exocytosis ratio, maintain the number of synaptic AMPA receptors and the synaptic transmission efficiency thereby making the receptor adapt to various changes in synaptic plasticity.
Synaptic Plasticity and AMPARs
Synaptic Plasticity is a Prototypical Property in the Mammalian Brain
Neurons connect through synapses, which can be strengthened or weakened in response to the enhancement or weakening of their activities, manifesting as adaptive behavior. Due to the adjustable nature of the connection strength between neurons, the morphology and function of synapses can undergo transient or lasting changes namely synaptic plasticity, which is a prototypical property of mammalian brains. Synaptic plasticity refers specifically to an experience-dependent modification of the strength or potency of synaptic transmission at existing synapses. In other words, synaptic plasticity is a typical manifestation of neural network re-modification and processing (Citri and Malenka 2008). According to the span temporal domains of these changes, the synaptic plasticity can be categorized as short-term synaptic plasticity (STP) and long-term synaptic plasticity. The STP has two functionally opposite forms, short-term facilitation (STF) and short-term depression (STD), which lasts on tens-to-hundreds milliseconds to several minutes and results a temporal modification of synaptic efficiency. The STP functions as a "memory buffer" reflecting the history of presynaptic activity and impacting the information processing of temporally-selective neurons (Wu et al. 2013; Motanis et al. 2018). Compared to STP, the long-term synaptic plasticity is range from minutes to hours, days, or even months. Long-term potentiation (LTP) and long-term depression (LTD) are two forms of long-term synaptic plasticity, which are currently recognized as the biological basis of learning and memory at the cellular level (Sumi and Harada 2020). The LTP, first discovered in the rabbit hippocampus by Terje Lømo in 1966, is a phenomenon that persistent enhancement in the transmission of signals between two neurons by simultaneously stimulating both LTP is specifically divided into two different phases, the early phase LTP (EP-LTP) and the late phase LTP (LP-LTP). The former is thought to be initiated by the increasing cytoplasmic calcium levels and relevant to the short-term memory. While the latter is proposed to be relevant to long term memory and is a structure changed process involving synapse enlargement itself in which required the protein synthesis and brain-derived neurotrophic factor (BDNF) (Lisman 2017). Whereas LTD is an activity-dependent reduction of neuronal synapses lasting several hours or even longer following intense synaptic stimulation (cerebellar Purkinje cells) or continues weak synaptic stimulation (hippocampus), which was first observed by Ito M et al. in Japan when stimulated parallel fibers (PF) and climbing fibers (CF) simultaneously in the cerebellum. LTD in cerebellum is believed to be significant for motor learning (Yamaguchi et al. 2016). Interestingly, concerning whether LTD can reverse the preexisting LTP, it's noticeable that this perhaps only applies to NMDAR-dependent LTD, while metabotropic glutamate receptor-dependent LTD appears to be a more complex process that not simply involves a reversal of LTP (Bosch et al. 2014; Grasselli and Hansel 2014). Except for LTP and LTD, there are other forms of synaptic plasticity in the response to information. For instance, the homeostatic plasticity could maintain the excitatory/inhibitory (E/I) balance through regulating the neuronal excitability toward a setting level (Monday et al. 2018). Evidences suggested that dysregulation of synaptic plasticity is associated with neurodegenerative disorders (NDDs) like AD, PD, or Huntington's disease (HD) and neuropsychiatric disorders like Schizophrenia, Autism, or Intellectual disability also drug addiction (Zhang et al. 2020; Zaman et al. 2017; Zhu et al. 2017).
The Role of AMPARs in the Synaptic Plasticity
AMPA receptors mediate the rapid transmission of excitatory neurotransmitter glutamate in mammalian CNS through the activation and inactivation of fast Na+ channels. AMPA receptors are modified by the changes in long-term or short-term neuronal activity and rapidly insert or disengage from the postsynaptic membrane. These dynamic trafficking patterns make the receptors a significant role in various forms of synaptic plasticity by changing the number of synaptic AMPA receptors. The increase of synaptic AMPA receptors enhances synaptic strength during LTP, while the decrease of weakens synaptic strength during the LTD (Huganir and Nicoll 2013). What is more, the AMPA receptors composed of different subunits have distinct mechanisms of trafficking, thus, the composition of the receptors is also significant for synaptic plasticity. By replacing the C-terminal tail of GluA1 and GluA2 in embryonic stem cells through the homologous recombination strategy, Zhou and his colleagues bred three strains mutate mice: GluA1C2KI mice, in which the endogenous CTD of GluA1 was replaced with the CTD of GluA2; GluA2C1KI mice, in which did the opposite; the GluA1C2KI and GluA2C1KI double-mutant mice with swapping GluA1 C-tail and GluA2 C-tail (Fig. 4) (Zhou et al. 2018). Compared to these mutant mice, the previous GluA mouse models or GluA1/2 exogenously expressing in vitro experiments would make the surface AMPA receptors reduced thereby making the mechanism of the impaired LTP confused (Granger et al. 2013). In contrast, they had no significant changes in the basal parameters of the AMPARs expression and trafficking except the target parameter. The LTP was absent in GluA1C2KI mice, while enhanced in GluA2C1KI mice, demonstrating that the CTD of GluA1 is indispensable in LTP. Besides, the NMDAR-dependent LTD was completely impaired in the GluA2C1KI mice but not glutamate receptor-dependent LTD, certifying the importance of the GluA2 C-tail in the NMDAR-dependent LTD (Zhou et al. 2018). The glutamate receptor-dependent LTD remained intact in both GluA1C2KI and GluA2C1KI mice. However, the mGluR-LTD also can be triggered following AMPARs endocytosis similarly to NMDAR-dependent LTD (Collingridge et al. 2010). Presumably there may be other domains of AMPA receptors involved in the mGluR-LTD. Besides, as mentioned above, the GluA2/3-containing AMPA receptors with short-tail replace the long-tailed GluA1-containing receptors after the latter inserting into the postsynaptic membrane, leading to an increase in the number of synaptic AMPA receptors during LTP (Keifer and Zheng 2010), suggesting that GluA2/3 also involves in LTP. By elevated glycine to stimulate the rat hippocampal neurons thereby to induce LTP, Nadia J and colleagues observed that the number of GluA1-containing AMPARs in the postsynaptic membrane increased rapidly. Meanwhile GluA2 interacted with PICK1 in the endosomal compartments. The interaction between them was not disrupted until a while after a stimulus (about 5 to 20 min), subsequently GluA2 was trafficked to the postsynaptic membrane. These results may be related to transient alterations mechanism of the synaptic AMPARs calcium permeability during the early phase of LTP (Jaafari et al. 2012). Lately, Nicolas C et al. found the GluA3-containing AMPARs rather than GluA1-containing AMPARs are involved in LTP at cerebellar Purkinje cells which are related to motor learning. Compared to classic LTP relates to the declarative memory forming occurring at the hippocampal neurons, GluA3-containing AMPARs-depending cerebellar LTP is induced by single-channel opening of GluA3-containing AMPARs through cAMP-dependent pathway (Gutierrez-Castellanos et al. 2017). However, whether this mechanism applies to the LTP at the hippocampal neurons is still unclear. Although the prevailing theory is that GluA2 is dominant in the LTD, studies favored that the C-tail phosphorylation site of GluA3 such as S885 is capable to interact with PICK1 to promote the internalization of the subunit, and is conserved with GluA2 S880 residue (Fig. 2) (Xia et al. 1999; Diering and Huganir 2018). As another long-tailed AMPA receptor, GluA4-containing receptors play a similar role in LTP as GluA1-containing receptors except that GluA4-containing receptor is required for LTP in the immature hippocampus through binding to PKA alone (Yasuda et al. 2003).
AMPA receptors act as substrates for a series of protein and kinases. These proteins and kinases bind to the corresponding sites on the C-terminal of the receptor distinct subunits respectively, which is important in LTP and LTD as well as other forms of synaptic plasticity. Some of them have been extensively studied like 4.1 N (one neuronal form of the erythrocyte membrane cytoskeleton protein), protein kinase C (PKC), protein kinase A (PKA) and CaMKII which all could bind to the C-terminal of GluA1, meanwhile PICK1, NSF, GRIP and PKC all could bind to the C-terminal of GluA2 (Fig. 2). They could regulate the trafficking and properties of AMPARs through distinct modifications especially phosphorylation and dephosphorylation, affecting synaptic plasticity. CaMKII, called the "molecule of memory", is indispensable to the production of LTP. Ca2+ acts firstly on CaMKII after enters the postsynaptic cytoplasm via the NMDA receptor thereby initiates LTP following the complex cascade reactions. When Ca2+ bound to the CaM binding domain of CaMKII, auto-phosphorylation occurred at the T286 in the inhibition domain in turn activating CaMKII. Auto-phosphorylated CaMKII remains part activity after Ca2+ detached and is able to phosphorylate adjacent CaMKII. Activated CaMKII phosphorylates GluA1 residues S831, which not only promotes the insertion of AMPA receptors into the postsynaptic membrane but also enhances its channel conductivity, ultimately facilitates LTP (Appleby et al. 2011; Baltaci et al. 2019). PKC-mediated phosphorylation of GluA1 S816 and S818 enhance the interaction between GluA1 and 4.1 N. This effect promotes GluA1 inserting into the postsynaptic membrane consequently strengthens LTP. But when GluA1 residues C811 being palmitoylation, the interaction between GluA1 and 4.1 N would be disrupted and finally impair the LTP (Lin et al. 2009). While PKC-mediated phosphorylation of GluA2 S880 interferes the interactions between GluA2 and GRIP without affecting GluA2 binding to PICK1, which is the mechanism of the LTD-induced GluA2 endocytosis (Bassani et al. 2013). Furthermore, the proteins or molecules involved in the AMPARs endocytosis mentioned above is useful in LTP/LTD more or less via CTD modification of distinct AMPAR subunits.
AMPARs in Anxiety, Depression, Neurodegenerative Disorders, and Cerebral Ischemia/Reperfusion Injury
AMPARs Play an Opposite Role in Anxiety and Depression
Both depression and anxiety are considered as stress-related disorders. Depression is associated with multiple factors, such as biochemical (e.g. reduction of the central monoamine transmitter 5-hydroxytryptamine (5-HT)), neuroendocrine (e.g. dysfunction of hypothalamus–pituitary–adrenal (HPA)), immunological (e.g. serum concentrations of inflammatory cytokines IL-6 and TNF-α), anatomical (e.g. atrophy of the hippocampus in patients with treatment-resistant depression (TRD)), genetic, and social-environmental factors (Ménard et al. 2016). Mark and colleagues proved that lab-induced stress could mimic the acute or chronic stress events in life and change the body's endocrine system function, which provided a theoretical foundation for preclinical experiment (Larson et al. 2001).Florian F et al. reviewed preclinical and clinical evidence of AMPA receptors in depression. Although the expression levels of receptors mRNA subunit and the subunit itself are alternative in brain regions and under different stress patterns (e.g. acute or chronic, short-term or long-term), signal transduction and AMPARs expression were changed in animal model or patient with depression. Also, the stress exposure length changes the expression level of GluA1 and GRIA1 (GluA1 genes) in hippocampus (Freudenberg et al. 2015). Accordingly there may be a strong association between AMPARs-mediated synaptic plasticity and depression. Dorian et al. found that acute stress exposure effected subunits phosphorylation, especially GluA1 and GluA2 related residues (e.g. GluA1 residues S831 and S845, GluA2 residues S880), which had a significant impact on synaptic plasticity (Caudal et al. 2010). Additionally, AMPARs are involved in structural changes of dendritic spines. Previous research suggested that blocking AMPARs could decrease the thorns density of CA3 thorny excrescences (a postsynaptic dendritic spine-like structure corresponding to the mossy fiber terminal of the presynaptic membrane) which is induced by corticosterone and increasing rapidly under the acute stress (Yoshiya et al. 2013). Based on these mechanisms, many classic and novel drug therapeutic targets, directly or indirectly involving AMPARs, have been prevailingly used in the treatment of antidepressants. Ketamine, a kind of NMDAR antagonists, indirectly increases extracellular glutamate by inhibition of NMDA receptors located on interneurons (mostly Gabaergic interneurons), which enhances the activation of AMPA receptors and AMPA-mediated synaptic strength. And Ampakines, acting as AMPAR agonist that has the effect of improving depression-like behavior of chronic mild stress (CMS) mice, is regarded as a potential antidepressant (Freudenberg et al. 2015). Considering the comorbidity and overlapping symptoms of anxiety and depression, some classic antidepressants such as monoamine-based antidepressants are commonly used to treat both disorders. However, there are some evidences showing the opposing role of AMPARs in the therapeutic effects of antidepressants. The AMPARs positive-allosteric modulators (PAMs) LY451646 showed an anti-depressive effect alone in CMS mice in both forced swim tests (FST) and tail suspension tests (TST). When used in combination with citalopram, it enhanced the antidepressant effect of citalopram, whereas the AMPARs non-competitive antagonist GYKI-53655 showed a depressogenic-like effect in TST. On the other hand, LY451646 reduced the anti-anxiety effect of citalopram in elevated zero mazes (EZM) and marble-burying test (MBT), whereas GYKI-53655 showed a remarkable anti-anxiety effect in EZM, MBT, and novelty-induced hypophagia (NIH) tests (Andreasen et al. 2015; Fitzpatrick et al. 2016). Besides, Jesper T et al. also demonstrated the anti-depressive effect of citalopram in FST and the anxiolytic-like effect in EZM and MAT. But they found the anxiogenic-like profile of citalopram in NIH tests (in a high-level dose, > 30 mg/kg) (Andreasen et al. 2015). These studies provide a new idea for the development of novel drugs targeting AMPARs for the ramified treatment of depression and anxiety.
AMPARs in Neurodegenerative Disorders
NDDs are described as the progressive loss of structure and function of synapse or neurons as well as the death of neurons, including AD, PD, and HD triggered by the toxic protein accumulations in the brain. AD is prevalent neurodegeneration that is characterized in pathology by the formation of β-amyloid (Aβ, an insoluble substance produced by the lysis of amyloid precursor protein (APP)) plaques and neurofibrillary tangles of aggregated hyper-phosphorylated tau proteins (a filamentous microtubule-associated protein) in brain parenchyma (Monday et al. 2018; Lashley et al. 2018). Previous findings suggested that Aβ down-regulated synaptic transmission by blocking the phosphorylation of GluA1 residues S845 and expression of AMPARs in some case (Monfort and Felipo 2010). However, recent studies suggested that Aβ applied intracellularly enhanced the excitatory postsynaptic currents (EPSCs) probably by facilitating the expression of Ca2+-permeable AMPARs (CP-AMPARs) and the PKA-mediated phosphorylation of GluA1 residues S845 (Whitcomb et al. 2015). In another study, the abnormal expression of CP-AMPARs and improving GluA1 S845 and GluA1 S831 phosphorylation levels were observed in young (1 month) APP/PS1 mice (double transgenic mice exhibiting a prominent elevation of Aβ production), whereas adult AD model mice (6 months) showed no apparent change in these parameters (Megill et al. 2015). Taken together, AMPARs are likely to play a significant role in the early stage of AD. And the mechanism may depend on the expression of CP-AMPARs and phosphorylation of GluA1 residues. Lately, Alejandro M et al. observed that synaptic AMPARs were reduced in both dendritic spines of hippocampus CA1 pyramidal cells and dendritic shafts of CA1 interneurons in 12 months APP/PS1 mice through the electron microscopic level technique. Incidentally, the two observed synapses are vulnerable to NDDs (Martín-Belmonte et al. 2020). Compared to 1 month APP/PS1 mice with no overt neuropathology, the 12 months APP/PS1 mice exhibited significant synapses loss, Aβ plagues formation, and memory impairment, indicating that there likely to be a connection between the reduction of synaptic AMPARs and memory impairment in AD later stage. Furthermore, the ubiquitination of GluA1, which is related to the degradation of AMPARs, was involved in the soluble Aβ-induced loss of surface AMPARs. Compared to WT mice, ubiquitin-deficient mutant mouse neurons exhibited that the effect of Aβ-induced reduction of synaptic AMPARs was blocked, while the phosphorylation of GluA1 was increased. Consistently, the Aβ adverse effect on synaptic AMPARs was inhibited in S845-deficient phosphomimetic mutant mice, while the ubiquitination of GluA1 was enhanced conversely (Guntupalli et al. 2017). This suggests that the soluble Aβ-induced cross-regulation between phosphorylation and GluA1 ubiquitination may be involved in AD pathogenesis. PD is characterized by the impairment of dopaminergic neurons in midbrain substantia nigra, with characteristic motor symptoms and non-motor symptoms like depression and cognitive impairment. Recently, the role of Parkin (a PD-related E3 ubiquitin ligase) in synaptic AMPARs was described to bind to scaffold protein Homer1 in PSD thereby to regulate the receptor endocytosis. The Parkin-deficient hippocampal neurons blocked the AMPARs endocytosis, and inhibited AMPAR-mediated currents as well as the LTD, which was likely to result from the incapacity of the described effect of Parkin-deficient neurons (Cortese et al. 2016; Zhu et al. 2018).
AMPARs in Ischemic Stroke
Cerebral ischemia is a catastrophic condition resulted from the rapid decline of blood flow to the brain thus causes oxygen and glucose deprivation (OGD) in the brain, which leads to injury or death of neurons, brain tissues, and cerebral infarction. More severely, there will be secondary ischemic cerebrovascular diseases such as ischemic stroke which is characterized by high morbidity, high disability, and high mortality rates (Collaborators 2019). After cerebral ischemia–reperfusion, immune cells such as neutrophils infiltrate the brain regarded as the hallmark of neuroinflammatory responses. It has been observed that the cerebral ischemia–reperfusion injury (CIRI) initiated excessive Ca2+ influx, the pattern called Ca2+ transients described as excitotoxicity which resulted in synapses dysfunction and neuronal death (Arundine and Tymianski 2004). The toxic Ca2+ influx mainly goes through the NMDAR-dependent pathway, which initiated downstream responses including the loss of AMPAR subunits GluA2 on the cell surface and synapse transformation AMPARs to CP-AMPARs. The transcription of GluA2 mRNA and the expression of surface GluA2 was both downregulated after OGD (following the GluA2 endocytosis). It was speculated that OGD induced interaction of PICK1 and AP consequently promoted GluA2 endocytosis and degradation. Notably, the GluA1-containing AMPARs were not affected by OGD-induced effect which indicated that there was a protective mechanism for surface GluA1 in OGD-induced endocytosis (Koszegi et al. 2017). Consistently, by BCAL (ligating the bilateral common carotid arteries) to induce transient ischemia in mice, Li et al. detected increasing of AMPARs in cortical spine (like CP-AMPARs) and the presence of Ca2+ transients in the cortex namely cortical hyperactivity after short term ischemia–reperfusion in vivo. Unsurprisingly, NMDARs antagonist applying could resist the impaired structures and synapses dysfunction (Li et al. 2020). Taken together, it is crucial in response to ischemia/reperfusion whether the GluA2 is released from the postsynaptic membrane and targeted to the lysosome. Thus, the mechanisms underlying the processes make a point in the improvement of CIRI. Lately, it was reported that NADPH oxidase played a role in the OGD-induced AMPAR subunits endocytosis and degradation. P38 MAPK (P38 Mitogen-activated Protein Kinases) was activated by oxidative stress after ischemia, which exhibited the capacity of facilitating the GluA2-containing AMPARs endocytosis induced by the receptor agonists. By inhibiting NADPH oxidase, the endocytosis of GluA1 and GluA2 induced by OGD/R was attenuated, as well as GluA2 degradation (Beske et al. 2015). Furthermore, the relationship between the trafficking and phosphorylation of GluA2 is also in response to the post-ischemia/reperfusion. Compared to the MCAO-induced ischemia–reperfusion model in rats, applying propofol showed neuroprotective effect after ischemia–reperfusion. For example, the expression of GluA2 on neurons surface was increased, along with phosphorylation down-regulation, which involved the Phosphoinositide-3-kinase (PI3K)/AKT pathway (a significant pathway in the regulating cell cycle) (Wang et al. 2019).
This accumulated evidence indicates us to consider the blocking AMPARs, specifically blocking CP-AMPARs as a potential therapeutic target of ischemia stroke. Recently, a noncompetitive AMPAR antagonist perampanel got increasing attention in the ischemia stroke therapy. Since its high specificity (inhibiting AMPARs without interrupting the function of the other two iGluRs in its IC50) and highly bioavailability (gastrointestinal tract absorption can reach 100%) was reported (Patsalos 2015). By oral administration of perampanel no matter pre- or post-administration of MCAO operation, the post-ischemia injury like neuroinflammation, cerebral infarction, and hydrocephalus was improved. The underlying mechanism was postulated to down-regulate the expression of inflammatory cytokine including IL-1β, TNF-α, and NO, and to promote the releasing of anti-inflammatory cytokines including IL-10 and TGF-β (Niu et al. 2018). Consistently, by intraperitoneal administration of perampanel, the improvement of post-ischemia injuries in cerebra mentioned above was observed as well. The perampanel exerted its neuroprotection by activating the PI3K/Akt pathway to protect brain injury against inflammation, oxidative stress, and apoptotic. Interestingly, the amelioration of perampanel was not only shown in MCAO-induced acute cerebral ischemic model (7 days following MCAO) but also cognitive impairments induced by chronic cerebral ischemic injury (30 days following MCAO) (Nakajima et al. 2018). Importantly, perampanel showed a neuroprotective effect in striatum and hippocampus slices of cerebral ischemia model rats at low dose without impairing fundamental synaptic structure and function (Mazzocchetti et al. 2020). In summary, the AMPAR antagonists such as perampanel are potentially effective medicines for ischemia stroke treatment. However, the precise mechanisms of the neuroprotective effect are supposed to figure out and more clinical evidences are required.
Conclusion
Herein, we reviewed the domain architecture of AMPA receptor together with the mechanism of its circuits in postsynaptic neurons briefly and highlighted the importance of the receptor in synaptic plasticity as well as the role in anxiety, depression, NDDs, and cerebral ischemia/reperfusion injury. The expression of surface AMPA receptors follows a precise cascade regulatory process, starting with the synthesis of the tetramers. Then the tetramers are transported and anchored to the postsynaptic membrane through exocytosis and interaction with scaffolding proteins, eventually degraded or entering the next cycle. Through regulation and modifications by distinct auxiliary subunits together with various protein and kinases, the expression and composition of the receptors are altered, which plays a considerable role in synapses transmission under physiological and pathological conditions.
Accumulative researches on anxiety, depression, and neurodegenerative disease have shown that there is a strong connection between these disorders and abnormalities during the AMPA receptor transportation. It indicates that the regulation of the transportation of AMPA receptor, as well as its subunits, is critical for neuroplasticity and cognitive function. Lately, given the significant alternation of AMPARs following the cerebral ischemia–reperfusion revealed, much more attention has been paid to AMPAR- targeted therapy for ischemic stroke. Accordingly, further study of transport, composition, and underlying molecular mechanism of AMPA receptor are required to provide a safe and effective therapeutic target for these nervous and mental diseases.
References
Amin JB, Salussolia CL, Chan K et al (2017) Divergent roles of a peripheral transmembrane segment in AMPA and NMDA receptors. J Gen Physiol 149:661–680. https://doi.org/10.1085/jgp.201711762
Andreasen JT, Fitzpatrick CM, Larsen M et al (2015) Differential role of AMPA receptors in mouse tests of antidepressant and anxiolytic action. Brain Res 1601:117–126. https://doi.org/10.1016/j.brainres.2015.01.001
Appleby VJ, Corrêa SAL, Duckworth JK et al (2011) LTP in hippocampal neurons is associated with a CaMKII-mediated increase in GluA1 surface expression. J Neurochem 116:530–543. https://doi.org/10.1111/j.1471-4159.2010.07133.x
Arundine M, Tymianski M (2004) Molecular mechanisms of glutamate-dependent neurodegeneration in ischemia and traumatic brain injury. Cell Mol Life Sci C 61:657–668. https://doi.org/10.1007/s00018-003-3319-x
Baltaci SB, Mogulkoc R, Baltaci AK (2019) Molecular mechanisms of early and late LTP. Neurochem Res 44:281–296. https://doi.org/10.1007/s11064-018-2695-4
Bassani S, Folci A, Zapata J, Passafaro M (2013) AMPAR trafficking in synapse maturation and plasticity. Cell Mol Life Sci 70:4411–4430. https://doi.org/10.1007/s00018-013-1309-1
Bats C, Groc L, Choquet D (2007) The interaction between stargazin and PSD-95 regulates AMPA receptor surface trafficking. Neuron 53:719–734. https://doi.org/10.1016/j.neuron.2007.01.030
Beske PH, Byrnes NM, Astruc-Diaz F, Jackson DA (2015) Identification of NADPH oxidase as a key mediator in the post-ischemia-induced sequestration and degradation of the GluA2 AMPA receptor subunit. J Neurochem 132:504–519. https://doi.org/10.1111/jnc.13005
Bosch M, Castro J, Saneyoshi T et al (2014) Structural and molecular remodeling of dendritic spine substructures during long-term potentiation. Neuron 82:444–459. https://doi.org/10.1016/j.neuron.2014.03.021
Carlisle HJ, Fink AE, Grant SGN, O’Dell TJ (2008) Opposing effects of PSD-93 and PSD-95 on long-term potentiation and spike timing-dependent plasticity. J Physiol 586:5885–5900. https://doi.org/10.1113/jphysiol.2008.163469
Caudal D, Godsil BP, Mailliet F et al (2010) Acute stress induces contrasting changes in AMPA receptor subunit phosphorylation within the prefrontal cortex, amygdala and hippocampus. PLoS ONE 5:e15282–e15282. https://doi.org/10.1371/journal.pone.0015282
Chen S, Zhao Y, Wang Y et al (2017) Activation and desensitization mechanism of AMPA receptor-TARP complex by Cryo-EM. Cell 170:1234-1246.e14. https://doi.org/10.1016/j.cell.2017.07.045
Citri A, Malenka RC (2008) Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology 33:18–41. https://doi.org/10.1038/sj.npp.1301559
Collingridge GL, Peineau S, Howland JG, Wang YT (2010) Long-term depression in the CNS. Nat Rev Neurosci 11:459–473. https://doi.org/10.1038/nrn2867
Cortese GP, Zhu M, Williams D et al (2016) Parkin deficiency reduces hippocampal glutamatergic neurotransmission by impairing AMPA receptor endocytosis. J Neurosci 36:12243–12258. https://doi.org/10.1523/JNEUROSCI.1473-16.2016
Cueva Vargas JL, Osswald IK, Unsain N et al (2015) Soluble tumor necrosis factor alpha promotes retinal ganglion cell death in glaucoma via calcium-permeable AMPA receptor activation. J Neurosci 35:12088–12102. https://doi.org/10.1523/JNEUROSCI.1273-15.2015
Diering GH, Huganir RL (2018) The AMPA receptor code of synaptic plasticity. Neuron 100:314–329. https://doi.org/10.1016/j.neuron.2018.10.018
Elias GM, Nicoll RA (2007) Synaptic trafficking of glutamate receptors by MAGUK scaffolding proteins. Trends Cell Biol 17:343–352. https://doi.org/10.1016/j.tcb.2007.07.005
Fitzpatrick CM, Larsen M, Madsen LH et al (2016) Positive allosteric modulation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid glutamate receptors differentially modulates the behavioural effects of citalopram in mouse models of antidepressant and anxiolytic action. Behav Pharmacol 27:549–555. https://doi.org/10.1097/FBP.0000000000000243
Freudenberg F, Celikel T, Reif A (2015) The role of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors in depression: Central mediators of pathophysiology and antidepressant activity? Neurosci Biobehav Rev 52:193–206. https://doi.org/10.1016/j.neubiorev.2015.03.005
Gan Q, Dai J, Zhou HX, Wollmuth LP (2016) The transmembrane domain mediates tetramerization of α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid (AMPA) Receptors. J Biol Chem 291:6595–6606. https://doi.org/10.1074/jbc.M115.686246
Gao J, Hu X-D, Yang H, Xia H (2018) Distinct roles of protein phosphatase 1 bound on neurabin and spinophilin and its regulation in AMPA receptor trafficking and LTD induction. Mol Neurobiol 55:7179–7186. https://doi.org/10.1007/s12035-018-0886-2
GBDS Collaborators 2016 (2019) Global, regional, and national burden of stroke, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 18:439–458. https://doi.org/10.1016/S1474-4422(19)30034-1
Goo BMSS, Sanstrum BJ, Holden DZY et al (2018) Arc/Arg3.1 has an activity-regulated interaction with PICK1 that results in altered spatial dynamics. Sci Rep 8:14675. https://doi.org/10.1038/s41598-018-32821-4
Granger AJ, Shi Y, Lu W et al (2013) LTP requires a reserve pool of glutamate receptors independent of subunit type. Nature 493:495–500. https://doi.org/10.1038/nature11775
Grasselli G, Hansel C (2014) Cerebellar long-term potentiation: Cellular mechanisms and role in learning. Int Rev Neurobiol 117:39–51. https://doi.org/10.1016/B978-0-12-420247-4.00003-8
Greger IH, Esteban JA (2007) AMPA receptor biogenesis and trafficking. Curr Opin Neurobiol 17:289–297. https://doi.org/10.1016/j.conb.2007.04.007
Greger IH, Mayer ML (2019) Structural biology of glutamate receptor ion channels: towards an understanding of mechanism. Curr Opin Struct Biol 57:185–195. https://doi.org/10.1016/j.sbi.2019.05.004
Gulia R, Sharma R, Bhattacharyya S (2017) A critical role for ubiquitination in the endocytosis of glutamate receptors. J Biol Chem 292:1426–1437. https://doi.org/10.1074/jbc.M116.752105
Guntupalli S, Jang SE, Zhu T et al (2017) GluA1 subunit ubiquitination mediates amyloid-β-induced loss of surface α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors. J Biol Chem 292:8186–8194. https://doi.org/10.1074/jbc.M116.774554
Gutierrez-Castellanos N, Da Silva-Matos CM, Zhou K et al (2017) Motor learning requires purkinje cell synaptic potentiation through activation of AMPA-receptor subunit GluA3. Neuron 93:409–424. https://doi.org/10.1016/j.neuron.2016.11.046
Hanley JG (2014a) Actin-dependent mechanisms in AMPA receptor trafficking. Front Cell Neurosci 8:1–8. https://doi.org/10.3389/fncel.2014.00381
Hanley JG (2014) Subunit-specific trafficking mechanisms regulating the synaptic expression of Ca2+-permeable AMPA receptors. Semin Cell Dev Biol 27:14–22. https://doi.org/10.1016/j.semcdb.2013.12.002
Hanley JG (2018) The regulation of AMPA receptor endocytosis by dynamic protein-protein interactions. Front Cell Neurosci 12:1–10. https://doi.org/10.3389/fncel.2018.00362
Hartman MA, Finan D, Sivaramakrishnan S, Spudich JA (2011) Principles of unconventional myosin function and targeting. Annu Rev Cell Dev Biol 27:133–155. https://doi.org/10.1146/annurev-cellbio-100809-151502
Henley JM, Wilkinson KA (2013) AMPA receptor trafficking and the mechanisms underlying synaptic plasticity and cognitive aging. Dialogues Clin Neurosci 15:11–27. https://doi.org/10.31887/DCNS.2013.15.1/jhenley
Henley JM, Wilkinson KA (2016) Synaptic AMPA receptor composition in development, plasticity and disease. Nat Rev Neurosci 17:337–350. https://doi.org/10.1038/nrn.2016.37
Huettner JE (2015) Glutamate receptor pores. J Physiol 593:49–59. https://doi.org/10.1113/jphysiol.2014.272724
Huganir RL, Nicoll RA (2013) AMPARs and synaptic plasticity: the last 25 years. Neuron 80:704–717. https://doi.org/10.1016/j.neuron.2013.10.025
Jaafari N, Henley JM, Hanley JG (2012) PICK1 mediates transient synaptic expression of GluA2-lacking AMPA receptors during glycine-induced AMPA receptor trafficking. J Neurosci 32:11618–11630. https://doi.org/10.1523/JNEUROSCI.5068-11.2012
Jeanneret V, Ospina JP, Diaz A et al (2018) Tissue-type plasminogen activator protects the postsynaptic density in the ischemic brain. J Cereb Blood Flow Metab 38:1896–1910. https://doi.org/10.1177/0271678X18764495
Kalashnikova E, Lorca RA, Kaur I et al (2010) SynDIG1: an activity-regulated, AMPA-receptor-interacting transmembrane protein that regulates excitatory synapse development. Neuron 65:80–93. https://doi.org/10.1016/j.neuron.2009.12.021
Kamalova A, Nakagawa T (2020) AMPA receptor structure and auxiliary subunits. J Physiol. https://doi.org/10.1113/JP278701
Kaur G, Lakkaraju A (2018) Early endosome morphology in health and disease. Adv Exp Med Biol 1074:335–343. https://doi.org/10.1007/978-3-319-75402-4_41
Keifer J, Zheng Z (2010) AMPA receptor trafficking and learning. Eur J Neurosci 32:269–277. https://doi.org/10.1111/j.1460-9568.2010.07339.x
Kessels HW, Malinow R (2009) Synaptic AMPA receptor plasticity and behavior. Neuron 61:340–350. https://doi.org/10.1016/j.neuron.2009.01.015
Kim E, Sheng M (2004) PDZ domain proteins of synapses. Nat Rev Neurosci 5:771–781. https://doi.org/10.1038/nrn1517
Koszegi Z, Fiuza M, Hanley JG (2017) Endocytosis and lysosomal degradation of GluA2/3 AMPARs in response to oxygen/glucose deprivation in hippocampal but not cortical neurons. Sci Rep 7:1–12. https://doi.org/10.1038/s41598-017-12534-w
Larson MR, Ader R, Moynihan JA (2001) Heart rate, neuroendocrine, and immunological reactivity in response to an acute laboratory stressor. Psychosom Med 63:493–501
Lashley T, Schott JM, Weston P et al (2018) Molecular biomarkers of Alzheimer’s disease: progress and prospects. Dis Model Mech. https://doi.org/10.1242/dmm.031781
Li Y, Ding R, Wang F et al (2020) Transient ischemia-reperfusion induces cortical hyperactivity and AMPAR trafficking in the somatosensory cortex. Aging (Albany, NY) 12:4299–4321. https://doi.org/10.18632/aging.102881
Lin D-T, Makino Y, Sharma K et al (2009) Regulation of AMPA receptor extrasynaptic insertion by 4.1N, phosphorylation and palmitoylation. Nat Neurosci 12:879–887. https://doi.org/10.1038/nn.2351
Lisman J (2017) Glutamatergic synapses are structurally and biochemically complex because of multiple plasticity processes: long-term potentiation, long-term depression, short-term potentiation and scaling. Philos Trans R Soc Lond B Biol Sci 372:20160260. https://doi.org/10.1098/rstb.2016.0260
Man H-Y, Sekine-Aizawa Y, Huganir RL (2007) Regulation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor trafficking through PKA phosphorylation of the Glu receptor 1 subunit. Proc Natl Acad Sci 104:3579–3584. https://doi.org/10.1073/pnas.0611698104
Martín-Belmonte A, Aguado C, Alfaro-Ruíz R et al (2020) Age-dependent shift of AMPA receptors from synapses to intracellular compartments in Alzheimer’s disease: immunocytochemical analysis of the CA1 hippocampal region in APP/PS1 transgenic mouse model. Front Aging Neurosci 12:1–17. https://doi.org/10.3389/fnagi.2020.577996
Matt L, Kirk LM, Chenaux G et al (2018) SynDIG4/Prrt1 is required for excitatory synapse development and plasticity underlying cognitive function. Cell Rep 22:2246–2253. https://doi.org/10.1016/j.celrep.2018.02.026
Mazzocchetti P, Mancini A, Sciaccaluga M et al (2020) Low doses of Perampanel protect striatal and hippocampal neurons against in vitro ischemia by reversing the ischemia-induced alteration of AMPA receptor subunit composition. Neurobiol Dis. https://doi.org/10.1016/j.nbd.2020.104848
Megill A, Tran T, Eldred K et al (2015) Defective age-dependent metaplasticity in a mouse model of Alzheimer’s disease. J Neurosci 35:11346–11357. https://doi.org/10.1523/JNEUROSCI.5289-14.2015
Ménard C, Hodes GE, Russo SJ (2016) Pathogenesis of depression: Insights from human and rodent studies. Neuroscience 321:138–162. https://doi.org/10.1016/j.neuroscience.2015.05.053
Mihály A (2019) The reactive plasticity of hippocampal ionotropic glutamate receptors in animal epilepsies. Int J Mol Sci. https://doi.org/10.3390/ijms20051030
Monday HR, Younts TJ, Castillo PE (2018) Long-term plasticity of neurotransmitter release: Emerging mechanisms and contributions to brain function and disease. Annu Rev Neurosci 41:299–322. https://doi.org/10.1146/annurev-neuro-080317-062155
Monfort P, Felipo V (2010) Amyloid-β impairs, and ibuprofen restores, the cGMP pathway, synaptic expression of AMPA receptors and long-term potentiation in the hippocampus. J Alzheimer’s Dis 22:795–809. https://doi.org/10.3233/JAD-2010-101092
Motanis H, Seay MJ, Buonomano DV (2018) Short-term synaptic plasticity as a mechanism for sensory timing. Trends Neurosci 41:701–711. https://doi.org/10.1016/j.tins.2018.08.001
Nakagawa T (2019) Structures of the AMPA receptor in complex with its auxiliary subunit cornichon. Science 366:1259–1263. https://doi.org/10.1126/science.aay2783
Nakagawa T, Cheng Y, Ramm E et al (2005) Structure and different conformational states of native AMPA receptor complexes. Nature 433:545–549. https://doi.org/10.1038/nature03328
Nakajima M, Suda S, Sowa K et al (2018) AMPA Receptor antagonist perampanel ameliorates post-stroke functional and cognitive impairments. Neuroscience 386:256–264. https://doi.org/10.1016/j.neuroscience.2018.06.043
Nakamura S, Irie K, Tanaka H et al (2019) Morphologic determinant of tight junctions revealed by claudin-3 structures. Nat Commun 10:1–10. https://doi.org/10.1038/s41467-019-08760-7
Niu HX, Wang JZ, Wang DL et al (2018) The orally active noncompetitive AMPAR antagonist perampanel attenuates focal cerebral ischemia injury in rats. Cell Mol Neurobiol 38:459–466. https://doi.org/10.1007/s10571-017-0489-x
Opazo P, Choquet D (2011) A three-step model for the synaptic recruitment of AMPA receptors. Mol Cell Neurosci 46:1–8. https://doi.org/10.1016/j.mcn.2010.08.014
Parkinson GT, Hanley JG (2018) Mechanisms of AMPA receptor endosomal sorting. Front Mol Neurosci 11:440. https://doi.org/10.3389/fnmol.2018.00440
Patsalos PN (2015) The clinical pharmacology profile of the new antiepileptic drug perampanel: a novel noncompetitive AMPA receptor antagonist. Epilepsia 56:12–27. https://doi.org/10.1111/epi.12865
Pelkey KA, Barksdale E, Craig MT et al (2015) Pentraxins coordinate excitatory synapse maturation and circuit integration of parvalbumin interneurons. Neuron 85:1257–1272. https://doi.org/10.1016/j.neuron.2015.02.020
Penn AC, Balik A, Wozny C et al (2012) Activity-Mediated AMPA receptor remodeling, driven by alternative splicing in the ligand-binding domain. Neuron 76:503–510. https://doi.org/10.1016/j.neuron.2012.08.010
Rossmann M, Sukumaran M, Penn AC et al (2011) Subunit-selective N-terminal domain associations organize the formation of AMPA receptor heteromers. EMBO J 30:959–971. https://doi.org/10.1038/emboj.2011.16
Sakakura M, Ohkubo Y, Oshima H et al (2019) Structural mechanisms underlying activity changes in an AMPA-type glutamate receptor induced by substitutions in its ligand-binding domain. Structure 27:1698-1709.e5. https://doi.org/10.1016/j.str.2019.09.004
Schnell E, Sizemore M, Karimzadegan S et al (2002) Direct interactions between PSD-95 and stargazin control synaptic AMPA receptor number. Proc Natl Acad Sci 99:13902–13907. https://doi.org/10.1073/pnas.172511199
Schwenk J, Harmel N, Zolles G et al (2009) Functional proteomics identify cornichon proteins as auxiliary subunits of AMPA receptors. Science 323:1313–1319. https://doi.org/10.1126/science.1167852
Schwenk J, Harmel N, Brechet A et al (2012) High-resolution proteomics unravel architecture and molecular diversity of native AMPA receptor complexes. Neuron 74:621–633. https://doi.org/10.1016/j.neuron.2012.03.034
Schwenk J, Baehrens D, Haupt A et al (2014) Regional diversity and developmental dynamics of the AMPA-receptor proteome in the mammalian brain. Neuron 84:41–54. https://doi.org/10.1016/j.neuron.2014.08.044
Shepherd JD, Huganir RL (2007) The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol 23:613–643. https://doi.org/10.1146/annurev.cellbio.23.090506.123516
Shi Y, Suh YH, Milstein AD et al (2010) Functional comparison of the effects of TARPs and cornichons on AMPA receptor trafficking and gating. Proc Natl Acad Sci 107:16315–16319. https://doi.org/10.1073/pnas.1011706107
Sobolevsky AI, Rosconi MP, Gouaux E (2009) X-ray structure, symmetry and mechanism of an AMPA-subtype glutamate receptor. Nature 462:745–756. https://doi.org/10.1038/nature08624
Sullivan SJ, Farrant M, Cull-Candy SG (2017) TARP γ-2 is required for inflammation-associated AMPA receptor plasticity within lamina II of the spinal cord dorsal horn. J Neurosci 37:6007–6020. https://doi.org/10.1523/JNEUROSCI.0772-16.2017
Sumi T, Harada K (2020) Mechanism underlying hippocampal long-term potentiation and depression based on competition between endocytosis and exocytosis of AMPA receptors. Sci Rep 10:14711. https://doi.org/10.1038/s41598-020-71528-3
Sumioka A, Yan D, Tomita S (2010) TARP phosphorylation regulates synaptic AMPA receptors through lipid bilayers. Neuron 66:755–767. https://doi.org/10.1016/j.neuron.2010.04.035
Summers KC, Bogard AS, Tavalin SJ (2019) Preferential generation of Ca (2+)-permeable AMPA receptors by AKAP79-anchored protein kinase C proceeds via GluA1 subunit phosphorylation at Ser-831. J Biol Chem 294:5521–5535. https://doi.org/10.1074/jbc.RA118.004340
Takemoto K, Iwanari H, Tada H et al (2017) Optical inactivation of synaptic AMPA receptors erases fear memory. Nat Biotechnol 35:38–47. https://doi.org/10.1038/nbt.3710
Vagnozzi AN, Praticò D (2019) Endosomal sorting and trafficking, the retromer complex and neurodegeneration. Mol Psychiatry 24:857–868. https://doi.org/10.1038/s41380-018-0221-3
van der Sluijs P, Hoogenraad CC (2011) New insights in endosomal dynamics and AMPA receptor trafficking. Semin Cell Dev Biol 22:499–505. https://doi.org/10.1016/j.semcdb.2011.06.008
Wang C, Wei Y, Yuan Y et al (2019) The role of PI3K-mediated AMPA receptor changes in post-conditioning of propofol in brain protection. BMC Neurosci 20:1–14. https://doi.org/10.1186/s12868-019-0532-6
Whitcomb DJ, Hogg EL, Regan P et al (2015) Intracellular oligomeric amyloid-beta rapidly regulates GluA1 subunit of AMPA receptor in the hippocampus. Sci Rep 5:1–12. https://doi.org/10.1038/srep10934
Wu H, Nash JE, Zamorano P, Garner CC (2002) Interaction of SAP97 with minus-end-directed actin motor myosin VI: Implications for AMPA receptor trafficking. J Biol Chem 277:30928–30934. https://doi.org/10.1074/jbc.M203735200
Wu S, Wong KYM, Tsodyks M (2013) Neural information processing with dynamical synapses. Front Comput Neurosci 7:188. https://doi.org/10.3389/fncom.2013.00188
Wudick MM, Portes MT, Michard E et al (2018) CORNICHON sorting and regulation of GLR channels underlie pollen tube Ca2+ homeostasis. Science 360:533–536. https://doi.org/10.1126/science.aar6464
Xia J, Zhang X, Staudinger J, Huganir RL (1999) Clustering of AMPA receptors by the synaptic PDZ domain containing protein PICK1. Neuron 22:179–187. https://doi.org/10.1016/S0896-6273(00)80689-3
Xu W (2011) PSD-95-like membrane associated guanylate kinases (PSD-MAGUKs) and synaptic plasticity. Curr Opin Neurobiol 21:306–312. https://doi.org/10.1016/j.conb.2011.03.001
Yamaguchi K, Itohara S, Ito M (2016) Reassessment of long-term depression in cerebellar Purkinje cells in mice carrying mutated GluA2 C terminus. Proc Natl Acad Sci U S A 113:10192–10197. https://doi.org/10.1073/pnas.1609957113
Yang Y, Bin WX, Frerking M, Zhou Q (2008) Delivery of AMPA receptors to perisynaptic sites precedes the full expression of long-term potentiation. Proc Natl Acad Sci U S A 105:11388–11393. https://doi.org/10.1073/pnas.0802978105
Yao W-D, Gainetdinov RR, Arbuckle MI et al (2004) Identification of PSD-95 as a regulator of dopamine-mediated synaptic and behavioral plasticity. Neuron 41:625–638. https://doi.org/10.1016/S0896-6273(04)00048-0
Yasuda H, Barth AL, Stellwagen D, Malenka RC (2003) A developmental switch in the signaling cascades for LTP induction. Nat Neurosci 6:15–16. https://doi.org/10.1038/nn985
Yoshiya M, Komatsuzaki Y, Hojo Y et al (2013) Corticosterone rapidly increases thorns of CA3 neurons via synaptic/extranuclear glucocorticoid receptor in rat hippocampus. Front Neural Circuits 7:191. https://doi.org/10.3389/fncir.2013.00191
Yu Y, Liu S, Wu X et al (2019) Mechanism of stiff substrates up-regulate cultured neuronal network activity. ACS Biomater Sci Eng 5:3475–3482. https://doi.org/10.1021/acsbiomaterials.9b00225
Zachariassen LG, Katchan L, Jensen AG et al (2016) Structural rearrangement of the intracellular domains during AMPA receptor activation. Proc Natl Acad Sci U S A 113:E3950–E3959. https://doi.org/10.1073/pnas.1601747113
Zaman T, De Oliveira C, Smoka M et al (2017) BK channels mediate synaptic plasticity underlying habituation in rats. J Neurosci 37:4540–4551. https://doi.org/10.1523/JNEUROSCI.3699-16.2017
Zeng M, Díaz-Alonso J, Ye F et al (2019) Phase separation-mediated TARP/MAGUK complex condensation and AMPA receptor synaptic transmission. Neuron 104:529-543.e6. https://doi.org/10.1016/j.neuron.2019.08.001
Zhang X, Zhao F, Wang C et al (2020) AVP (4–8) Improves cognitive behaviors and hippocampal synaptic plasticity in the APP/PS1 mouse model of Alzheimer’s disease. Neurosci Bull 36:254–262. https://doi.org/10.1007/s12264-019-00434-0
Zhou Z, Liu A, Xia S et al (2018) The C-terminal tails of endogenous GluA1 and GluA2 differentially contribute to hippocampal synaptic plasticity and learning. Nat Neurosci 21:50–65. https://doi.org/10.1038/s41593-017-0030-z
Zhu F, Wu Q, Li J et al (2017) A single dose of cocaine potentiates glutamatergic synaptic transmission onto locus coeruleus neurons. Cell Calcium 67:11–20. https://doi.org/10.1016/j.ceca.2017.07.007
Zhu M, Cortese GP, Waites CL (2018) Parkinson’s disease-linked Parkin mutations impair glutamatergic signaling in hippocampal neurons. BMC Biol 16:100. https://doi.org/10.1186/s12915-018-0567-7
Funding
This work was supported by the National Natural Science Foundation of China [Grant Numbers 81773924, 81730096]; the Drug Innovation Major Project [Grant Numbers 2018ZX09711001-002-007, 2018ZX09711001-003-005, 2018ZX09711001-009-013]; CAMS Innovation Fund for Medical Sciences (CIFMS) [Grant Number 2016-I2M-1-004].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wu, QL., Gao, Y., Li, JT. et al. The Role of AMPARs Composition and Trafficking in Synaptic Plasticity and Diseases. Cell Mol Neurobiol 42, 2489–2504 (2022). https://doi.org/10.1007/s10571-021-01141-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-021-01141-z