Abstract
The endoplasmic reticulum (ER) and mitochondria have both been shown to be critical in cellular homeostasis. The functions of the ER and mitochondria are independent but interrelated. These two organelles could form physical interactions, known as MAMs, to regulate physiological functions between ER and mitochondria to maintain Ca2+, lipid, and metabolite exchange. Several proteins are located in MAMs, including RNA-dependent protein kinase (PKR)-like ER kinase, inositol 1,4,5-trisphosphate receptors, phosphofurin acidic cluster sorting protein-2 and sigma-1 receptor to ensure regulation. Recent studies indicated that MAMs participate in inflammation and apoptosis in various conditions. All of these functions are crucial in determining cell fate following traumatic brain injury (TBI). We hypothesized that MAMs may associate with TBI and could contribute to mitochondrial dysfunction, ER stress, autophagy dysregulation, dysregulation of Ca2+ homeostasis, and oxidative stress. In this review, we summarize the latest understanding of MAM formation and their potential regulatory role in TBI pathophysiology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The interaction between different organelles is necessary for eukaryotic cells to maintain physiological functions. Therefore, the interaction between organelles has become the focus of current research. MAMs are structures that can regulate the physiological functions between ER and mitochondria. In the last century, scientists first saw the close contact between the ER and mitochondria in the electron microscope. However, they knew nothing about its function (Morre et al. 1971). In 1990, Vance et al. (Vance 1990) developed a protocol to isolate ER-like membranes that co-isolated with mitochondria from rat liver, which are now called mitochondria-associated membranes. The main function of MAMs is the regulation of Ca2+, lipid and metabolite exchange between ER and mitochondria (Marchi et al. 2014). Ca2+ homeostasis is essential for normal neuronal function and cell survival processes and the transfer of Ca2+ from the ER to mitochondria is mediated by IP3Rs, which are concentrated in the MAMs (Rizzuto et al. 1993). Alterations in MAMs will destroy intracellular Ca2+ homeostasis and ultimately induce apoptosis (Pizzo and Pozzan 2007). Furthermore, MAMs could also modulate cell function via the regulation of mitochondrial reactive oxygen species (ROS) production (Rodríguez-Arribas et al. 2016). Previous studies indicated that MAMs are associated with numerous pathophysiological conditions, including Alzheimer’s disease, Parkinson syndrome, and many other neurodegenerative disorders (Vance 2014). We propose that MAMs may play an important role in TBI pathophysiology. In this review, we will discuss current knowledge of MAMs, highlighting the roles of MAMs in the pathophysiology of TBI and discussing therapeutic opportunities for drug discovery.
Mitochondria-Associated ER Membranes
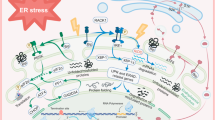
Organelles are wrapped by membrane to ensure their unique identities and specialized functions in eukaryotic cells. To implement a variety of physiological functions, these organelles need to communicate and cooperate with each other via ion, metabolite, and lipid exchange at their contact sites (Rodríguez-Arribas et al. 2016). MAMs, the best-characterized inter-organelle connections, were demonstrated to be a signaling hub in the regulation of cell processes, such as lipid exchange, Ca2+ transfer, mitochondrial morphology, autophagy, and apoptosis (Marchi et al. 2014; Rowland and Voeltz 2012) (Fig. 1).
Mitochondria-associated ER membranes. Several proteins reside in MAMs, including inositol 1,4,5-trisphosphate receptors (IP3Rs), the mitochondrial voltage-dependent anion channel (VDAC1), glucose-regulated protein 75 (Grp75), RNA-dependent protein kinase (PKR)-like ER kinase (PERK), Mitofusin-1/2, etc. In addition, several resident MAM proteins can regulate cell survival by governing apoptosis and inflammation. A tripartite complex that includes Grp75, the VDAC1, and IP3Rs, links the ER and the OMM and is the major Ca2+ transporter and channel between the ER and mitochondria. In addition, IP3Rs can cooperate with PML and Akt to regulate proapoptotic signals. B-cell receptor-associated protein of 31 kDa (BAP31) is an important regulator of ER–mitochondria crosstalk and interacts with the mitochondrial fission protein Fission 1 homolog (Fis1) and phosphofurin acidic cluster sorting protein-2 (PACS-2). Sarco/endoplasmic reticulum Ca2+ ATPase (SERCA), which is regulated by p53, can meditate Ca2+ release and reuptake at MAMs. Sigma-1 receptor (Sig1-R) can regulate inflammation by interacting with dehydroepiandrosterone (DHEA). In various conditions, the inflammasome could be meditated by ER stress, reactive oxygen species (ROS), VDAC, etc., which play an important role in the regulation of MAM-related inflammation
Structural Composition of the MAMs
The association between the ER and mitochondria could be observed by electron microscopy in animal cells and yeast. The distance between the ER and mitochondria was originally measured to be approximately 100 nm. With the development of high-speed digital imaging microscopy and electron tomography studies, scientists suggested that the contact sites were much smaller, approximately 10–30 nm wide (Csordas et al. 2006; Soltys and Gupta 1992). Several proteins have been found to be enriched at the MAMs. They not only participate directly in tethering, but are also involved in the processes regulated by MAMs (van Vliet et al. 2014). A tripartite complex, including glucose-regulated protein 75 (Grp75), the mitochondrial voltage-dependent anion channel (VDAC1), and IP3Rs, links the ER and the OMM and can regulate Ca2+ transfer from the ER to mitochondria (Szabadkai et al. 2006). The absence of IP3Rs does not affect the ER–mitochondrial linkage, while the absence of Grp75 in HeLa cells could destroy the physical tethering role of this Ca2+ channel (Csordas et al. 2006). In addition, Sig1-R, which regulates Ca2+ signaling and cell survival, is abundant in the MAMs (Hayashi and Su 2007). Another two proteins, PACS-2 and MFN2, are involved in the regulation of MAM formation and function. The mechanism of PACS-2 in the stabilization of the structural integrity of the ER–mitochondria membrane contact site (MCS) is not entirely clear. However, lacking PACS-2 causes B-cell receptor-associated protein of 31 kDa (BAP31)-dependent mitochondrial fragmentation and uncoupling from the ER (Simmen et al. 2005). BAP31 is not only the ER-resident protein that is involved in protein sorting, but is also an important regulator of ER–mitochondria crosstalk by interacting with the mitochondrial fission protein Fission 1 homolog (Fis1) (Grimm 2012). MFN2, together with mitofusin 1 (MFN1) and optic atrophy 1 (OPA1), could regulate mitochondrial fusion and Ca2+ signaling on the MAMs (Munoz et al. 2013). In addition, de Brito indicated that ER–mitochondria contact is reduced when MFN2 is deleted. However, this reduced contact can be rescued by upregulating the expression of MFN2, which demonstrates that MFN2 is essential for ER–mitochondria tethering (de Brito and Scorrano 2008). Another study that demonstrated that the absence of MFN2 can increase proximity between ER and mitochondria did not support de Brito’s finding (Filadi et al. 2015).
Mitochondria-Associated ER Membranes and Inflammation
In recent years, MAMs have been shown to be critical in inflammation. A link between inflammation and the ER–mitochondria interface was established for the first time in 2011. In this study, Zhou et al. demonstrated that ROS can promote NOD-like receptor family, pyrin domain-containing protein 3 (NLRP3) inflammasome activation, which explained the frequent association of MAMs with inflammatory diseases (Zhou et al. 2011).
NLRP3
The human NLR family is composed of 22 human genes (Schroder and Tschopp 2010). NLRP3 is a multiprotein complex of innate immune responses, and it is one of the most fully characterized and well-studied inflammasomes of NLRs which are composed of the NLRP3 protein, the adapter apoptosis-associated speck-like protein (ASC), and pro-caspase-1 (Jin and Flavell 2010). The inflammasome can regulate the activation of caspase-1 and the subsequent proteolytic maturation and secretion of interleukin-1β (IL-1β) and interleukin-18 (IL-18) (Sadatomi et al. 2017). A previous study showed that the activation of NLRP3 required two different mechanisms. One mechanism is driven by toll-like receptor (TLR)/nuclear factor-κB (NF-κB) at the transcriptional level (Hornung and Latz 2010). Another mechanism affects the activation of NLRP3 at the posttranscriptional level (Rubartelli 2012).
The role of MAMs in the activation of the NLRP3 inflammasome is still unclear, but more studies have suggested that MAMs are critical in the regulation of inflammation. With the exception of the ER and peroxisomes, mitochondria are the main source of ROS (Dostert et al. 2008). Recent studies indicated that ROS could promote the activation of the NLRP3 inflammasome (Yin et al. 2017). VDAC1 is a critical regulator of mitochondrial metabolic activity through the uptake of Ca2+ into the mitochondria from MAMs. VDAC1 is essential for the production of mitochondrial ROS. When the activity of the OMM channel VDAC was inhibited, the formation of the NLRP3 inflammasome was selectively abrogated (Zhou et al. 2011). Thioredoxin-interacting protein (TXNIP) is another bridge between oxidative stress and NLRP3. During mitochondrial oxidative stress, TXNIP can mediate the activation of NLPR3 in primary rat hepatocytes and in THP1 macrophage cells (Zhang et al. 2015; Zhou et al. 2011). During ER stress, TXNIP could be induced by PERK and inositol-requiring enzyme 1 (IRE1) pathways and then induce IL-1β production by the NLRP3 inflammasome (Oslowski et al. 2012). When TXNIP was silenced, the activation of the NLRP3 inflammasome was blocked, which indicated that TXNIP expression is essential for NLRP3 inflammasome activation (Zhang et al. 2015).
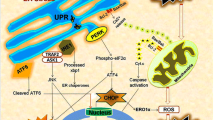
PERK
PERK is a protein kinase that belongs to the eukaryotic translation initiation factor 2α (eIF2α) kinase subfamily. The PERK protein is particularly enriched at MAMs and appears to be crucial for tethering the ER to the mitochondria and thus for MAM integrity (Verfaillie et al. 2012). Under normal physiological conditions, PERK is bound by the chaperone glucose-regulated protein 78 (Grp78) to keep PERK in an inactive state (Bertolotti et al. 2000). ER stress could promote the disassociation of Grp78 from the cytoplasmic domain and activate PERK to regulate ER stress-related inflammation and apoptosis (Walter and Ron 2011). A recent study indicated that PERK not only plays an important role in ER stress but also affects MAMs to maintain the ER–mitochondria juxtapositions (Verfaillie et al. 2012). The activation of the PERK/JAK1/STAT3 signaling pathway could elicit a feed-forward inflammatory loop that involves astrocytes and microglia to drive neuroinflammation. However, the activation of microglia and the subsequent production of IL-6 and oncostatin M (OSM) could be abolished when the PERK was silenced via siRNA (Guthrie et al. 2016; Meares et al. 2014). Moreover, knockdown of PERK could result in the activation of NF-κB and ROS generation in astrocytes under OGD conditions, which indicated that PERK is required for ROS generation and is involved in the activation of NF-κB in astrocytes (Liu and Du 2015).
Sig1-R
Sig-1R, as a chaperone of the MAMs, is a single 25 kD polypeptide that interacts with several protein targets. Sig-1R could form a complex at MAMs with Grp78 and bind to the IP3Rs to be involved in the regulation of Ca2+ mobilization from ER stores (Hayashi and Su 2003; Hayashi and Su 2007). The neuroprotective effects of Sig-1R are attributed to anti-inflammatory actions in various disease models. In a stroke model, Allahtavakoli et al. found that PRE-084, a Sig-1R agonist, could elevate the expression of proinflammatory cytokines and decrease the expression of anti-inflammatory cytokines (Allahtavakoli and Jarrott 2011). In another study, scientists found that PRE-084 could significantly reduce the number of active microglial cells. However, when Sig-1R expression was knocked out, treatment with PRE-084 did not have any restorative effects on mice, which indicated that Sig-1R regulated inflammation (Francardo et al. 2014). Consistent with previous studies, Dong et al. found that PRE-084 could reduce microglial activation and nitrosative and oxidative stress to proteins after TBI (Dong et al. 2016). SKF83959 (3-methyl-6-chloro-7,8-hydroxy-1-[3-methylphenyl]-2,3,4,5-tetrahydro-1H-3-benzazepine), an atypical dopamine receptor-1 agonist, can enhance the activity of endogenous dehydroepiandrosterone (DHEA) in a synergistic manner and inhibit the activation of BV2 microglia and the expression/release of proinflammatory cytokines (Wu et al. 2015). Sig-1R activation could affect the expression of ionized calcium-binding adaptor molecule-1 (Iba1) in microglia/macrophages of the ischemic hemisphere after experimental stroke. However, Sig-1R has no influence on post-stroke inflammatory mediators (Ruscher et al. 2012). All of these results suggest that MAMs may play an important role in initiating the inflammatory response to external stimulus.
Mitochondria-Associated ER Membranes and Apoptosis
Apoptosis is a process of major biomedical interest; its deregulation plays an important role in the pathogenesis of central nervous system diseases. Ca2+ homeostasis is crucial in the control of cell fate. Recent data highlight the important role of MAMs in the regulation of Ca2+ homeostasis and suggest that MAMs are critical hubs for apoptosis (Danese et al. 2017; Giorgi et al. 2011).
IP3Rs
IP3Rs are important Ca2+ channels that regulate the release of Ca2+ from ER to mitochondria, and IP3Rs are highly concentrated in MAMs. Recent studies revealed that IP3Rs, as cellular hubs, could integrate many signaling pathways and control cell fate (Ivanova et al. 2014). Glycogen synthase kinase-3β (GSK3β) is a multifunctional kinase that can aggravate myocardial ischemia–reperfusion injury. GSK3β can interact with the IP3R Ca2+ channeling complex in MAMs. When GSK3β was inhibited, both cytosolic and mitochondrial Ca2+ overload and subsequent cell death was limited (Gomez et al. 2016). Members of the Bcl-2-family, including Bcl-2, Bcl-Xl, and Mcl-1, have been reported to play an important role in the regulation of IP3R channels (Distelhorst and Bootman 2011). They could inhibit Ca2+ release from the ER via interacting with the IP3R Ca2+ channel (Rong et al. 2008). Bcl-2-related ovarian killer (Bok) is a proapoptotic Bcl-2 family member, and cellular overexpression of Bok could induce apoptosis (Hsu et al. 1997). Recent evidence has indicated that Bok interacts strongly with IP3Rs and may contribute to the structural integrity or stability of IP3R tetramers (Schulman et al. 2013). In return, Bok is dramatically stabilized by binding to IP3Rs and the proapoptotic effects of overexpressed Bok could be limited (Schulman et al. 2016). PKB/Akt, which is a well-known prosurvival factor, exerts critical neuroprotective effects by phosphorylating downstream targets after TBI. PKB/Akt could be activated by IP3Rs and then inhibit Ca2+ release from IP3Rs after Ca2+-dependent apoptosis was stimulated (Stephens et al. 1998; Szado et al. 2008). In addition, phosphatase and tensin homolog (PTEN), a well-known negative regulator of PKB/Akt signaling, have been reported to be located at the MAMs. PTEN can directly reduce IP3Rs phosphorylation and enhance Ca2+ transfer to the mitochondria (Bononi et al. 2013). Previous observations indicated that mTORc2 resides at the MAMs and could interact with both the ER and the mitochondria. Moreover, mTORC2 controlled MAM integrity and mitochondrial function via Akt-mediated phosphorylation of the MAM-associated proteins, which include IP3Rs, Hexokinase 2, and PACS-2 (Betz et al. 2013). Cytochrome c could be released from the mitochondria, and it is a critical factor for apoptosis induction. A recent study indicated that cytochrome c could bind to IP3R channels and translocate to the ER upon apoptosis induction to promote apoptotic Ca2+ release (Boehning et al. 2003).
PACS-2
PACS-2 is a novel sorting protein that links the ER–mitochondria axis to ER homeostasis and plays an important role in the control of cell fate. When stimulated by apoptotic inducers, PACS-2 could translocate Bid to mitochondria to initiate the formation of mitochondrial truncated Bid, the release of cytochrome c, the activation of caspase-3, and eventually cause cell death (Simmen et al. 2005). Aslan JE and colleagues found that PACS-2, as an essential tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) effector, is required for the killing of tumor cells in vitro and virally infected hepatocytes in vivo (Aslan et al. 2009). A recent study demonstrated that cellular inhibitor of apoptosis (cIAP) could negatively regulate TRAIL cytotoxicity by mediating the ubiquitination of PACS-2 (Guicciardi et al. 2014). It is well known that NF-κB can promote cell survival by inducing the expression of anti-apoptotic proteins, including Bcl-Xl, and can protect mitochondria from stress-induced mitochondrial outer membrane permeabilization (MOMP). A recent study suggested that PACS-2 is required for Bcl-xL induction following DNA damage in primary mouse thymocytes. When PACS-2 was knocked down, thymocytes exhibited a blunted induction of Bcl-xL, increased MOMP, and accelerated apoptosis (Barroso-Gonzalez et al. 2016).
PERK
As described before, PERK is crucial for tethering the ER to the mitochondria and is present in MAMs. The pharmacological inhibition of PERK could attenuate brain injury in a subarachnoid hemorrhage model via the activation of the Akt signal pathway, which suggests that PERK is crucial in regulating cell fate (Yan et al. 2016). In addition, PERK is also the central regulator of ER stress and can determine cell fate by interacting with its downstream molecules (Liu et al. 2015). Among the large number of downstream signaling pathways, eIF2α could be considered part of the critical PERK-mediated signaling pathway. A recent study indicated that eIF2α could be dephosphorylated by GADD34 and contributes to survival by suppressing the ATF4/CHOP signaling pathway (Chambers et al. 2015). Nuclear factor-erythroid 2-related factor 2 (Nrf2), a nuclear transcription factor, is known as a critically important mechanism for cellular protection and cell survival following TBI (Zhang and Teng 2016). The activation of Nrf2 can negatively regulate ER stress-induced apoptosis, which suggested that Nrf2 is an anti-survival factor (Zhang et al. 2014). A recent study demonstrated that Nrf2 was one of the direct substrate molecules of PERK that could initiate the separation of NRF2 from Keap1 with the assistance of Nrf1 (Digaleh et al. 2013). Indeed, PERK is required for the regulation of inter-organellar communication during ROS-induced cell death. The loss of PERK may cause defects in cell death sensitivity and decreased mitochondrial Ca2+ uptake. Furthermore, PERK deficiency could reduce ER stress-induced apoptosis by reducing caspase activation and cytochrome c release (Verfaillie et al. 2012). A recent study indicated that PERK physically interacts with MFN2 and that the inhibition of PERK could reduce ROS production, normalize mitochondrial Ca2+, and improve mitochondrial morphology (Munoz et al. 2013).
Sig1-R
Previous studies demonstrated that Sig-1R, which is implicated in neuroprotection, carcinogenesis, and neuroplasticity, is a Ca2+-sensitive ligand-operated receptor chaperone at MAMs. The major mechanism of its neuroprotective function is the regulation of intracellular Ca2+ homeostasis. Sig1-R can promote Ca2+ entry into mitochondria through the stabilization of IP3R3 at the MAMs and could decrease apoptosis (Hayashi and Su 2007). In an in vitro model of ischemia, Katnik C indicated that 1,3-di-o-tolyl-guanidine (DTG), a sigma receptor agonist, can attenuate intracellular Ca2+ elevations in response to ischemia induced by sodium azide and glucose deprivation (Katnik et al. 2006). Another mechanism of its neuroprotective function is possibly through the modulation of ROS-neutralizing proteins. The overexpression of Sig1-R can activate the antioxidant response element (ARE) to upregulate NAD(P)H quinone oxidoreductase 1 (NQO1) and superoxide dismutase 1 (SOD1) expression in COS cells to reduce oxidative stress. However, the anti-oxidative stress function was abolished when Sig1-R was knocked out (Pal et al. 2012). Sig1-R can also regulate the production of ROS through the ER–mitochondrial Rac1 system, and this system could promote a mild pro-oxidant milieu for cellular signaling and induce plastic transformation in neurons (Natsvlishvili et al. 2015). 4-Phenyl-1-(4-phenylbutyl) piperidine (PPBP), a Sig1-R agonist, could protect neurons via a mechanism that involves the anti-apoptotic protein Bcl-2 (Yang et al. 2007). The mechanism of this protection may include NF-κB and/or extracellular signal-regulated kinase (ERK) pathways (Ha et al. 2014; Meunier and Hayashi 2010). Another study demonstrated that Sig1-R may promote cell survival via the regulation of ER stress, p38 MAPK activation, ROS production, and proteins involved in apoptosis (Caspases-3, Bax) in breast cancer cells (Happy et al. 2015). In a TBI model, Dong et al. found that PRE-084 can significantly reduce lesion volume, lessen brain edema, and accelerate the recovery of nerve function and body weight after TBI, which indicated that MAMs may play an important role in cell fate and neural function following TBI (Dong et al. 2016).
Mitochondria-Associated ER Membranes and TBI
TBI is mainly divided into primary brain injury and secondary brain injury. The primary brain injury occurs immediately after trauma and is inevitable. Secondary brain injury occurs hours to days after the primary brain injury and is another blow to the central nervous system (Gao et al. 2016; Park et al. 2008). Secondary brain injury is related to numerous interrelated biochemical pathways that mainly include a cerebral inflammatory response and apoptosis, which are induced by mitochondrial dysfunction, autophagy, the disruption of Ca2+ homeostasis, oxidative stress, excitotoxicity, and free radical generation (Faridar et al. 2011; Pearn et al. 2016). Mitochondrial dysfunction, ER stress, autophagy dysfunction, the dysregulation of Ca2+ homeostasis, and oxidative stress were closely related to MAMs, which suggested that MAM dysfunction may play an important role in TBI (Arruda et al. 2014; Verfaillie et al. 2012; Wang et al. 2015; Yu et al. 2015).
Mitochondria are the only energy-producing organelles in the cell. Following TBI, the body goes through a state of metabolic crisis, and mitochondrial dysfunction becomes apparent (Yonutas et al. 2016). Mitochondrial dysfunction plays an important role in proinflammatory signaling and cell apoptosis, which are closely related to mitochondrial oxidative stress, the inflammatory cycle, and inflammasome formation (Lopez-Armada et al. 2013). An experimental study indicated that MAMs could regulate PACS-2, IP3R1, and Ca2+ transport and improve mitochondrial function, which indicated that MAMs may be a potential therapeutic target for the inflammatory response and cell apoptosis after TBI (Arruda et al. 2014).
The endoplasmic reticulum is an important organelle that can regulate protein synthesis, processing, transport, and calcium homeostasis. Following TBI, misfolded and unfolded proteins in the endoplasmic reticulum will aggregate, which can cause ER stress (Harvey et al. 2015). PERK activation is the first indicator of ER stress and aggravates inflammation and apoptosis following TBI (Dash et al. 2015; Nakka et al. 2014). Therefore, PERK, which is particularly enriched at the MAMs and is essential for MAM integrity, builds the bridge between MAMs and TBI.
Autophagy is a lysosomal degradation pathway that degrades damaged organelles into basic biomolecules and can be induced by TBI (Zhang et al. 2016). During autophagy, double membrane-bound organelles, which are called autophagosomes, are formed. Previous studies have indicated that the formation of autophagosomes requires the presence of MAM–mitochondria contacts (Hailey et al. 2010). A recent study indicated that autophagy, especially mitophagy, is a negative regulator of NLPP3 inflammasome activation (Kim et al. 2016). The activation of the NLRP3-inflammasome could cause the processing and release of IL-1β and IL-18 and enhance the progression of the inflammatory response after TBI, which links the MAMs and the inflammatory response after TBI (Liu et al. 2013). In addition, autophagy can increase cell survival and improve functional recovery following injury, which suggests that MAM-regulated autophagy may be a potential therapeutic target for TBI (Lipinski et al. 2015).
Ca2+ homeostasis is thought to be one of the fundamental pathological mechanisms of cell death induced by TBI. Mitochondria are involved in the regulation of cellular Ca2+ signaling mainly through the mitochondrial Ca2+ uniporter (MCU). TBI causes a disruption in ion homeostasis and an uncontrolled influx of Ca2+ into neurons. Ca2+ overload induced by mitochondria through MCU can aggravate mitochondrial dysfunction and cell death following TBI (Cheng et al. 2013). In addition, Ca2+ could activate lipid peroxidases, proteases, and phospholipases, which could increase the intracellular concentration of free fatty acids and free radicals (Sande and West 2010). The disruption of Ca2+ homeostasis can lead to cell injury and apoptosis, which could be mediated by IP3Rs located in MAMs (Werner and Engelhard 2007).
Moreover, excessive cytosolic Ca2+ could induce the degradation of the cytoskeleton and extracellular matrix proteins and then enhance ROS production (Dirnagl et al. 1999). Evidence demonstrates that ROS are generated by mitochondria and contribute to the pathophysiology of TBI (Marklund et al. 2001). TBI could induce structural and functional damage in mitochondria during an early event, which in turn could contribute to the production of ROS and eventually lead to cell death and poor cognitive outcome (Fischer et al. 2016). Evidence indicated that the propagation of ROS signals between the ER and mitochondria could be modulated by PERK, which is a MAM component that plays a key role in the regulation of ER–mitochondria juxtapositions and mitochondrial apoptosis following TBI (Verfaillie et al. 2012). Nrf2, an anti-oxidative stress factor that is a direct substrate of PERK, could be affected by MAMs and protect against TBI by regulating microglial function (Digaleh et al. 2013; Wu and Liu 2016). Furthermore, as a chaperone at the MAMs, Sig-1R could reduce microglial activation and oxidative stress and accelerate the recovery of nerve function after TBI (Dong et al. 2016).
Conclusions and Future Directions
In a word, MAMs play a critical role in many cellular processes and signaling pathways. The physical interaction between these two organelles could regulate lipid transport, mitochondrial dynamics, Ca2+ transfer, and the inflammatory response and could uniquely reflect cell health. For instance, when the number of ER–mitochondria contact sites was increased, Ca2+ transfer to the mitochondria could be enhanced and could ultimately induce cell death. When the expression of PERK was elevated, ROS generation and ER stress could also be enhanced and eventually cause cell death. All of these findings indicate that MAMs are not only structures between the ER and mitochondria but also a structural platform that accommodates several regulatory or effector proteins to regulate biological processes. However, studies on the role of MAMs in TBI are still in their infancy, and many questions remained to be solved. Have we already identified all proteins that constitute MAMs? Can TBI change the protein composition or stability of MAMs? What are the mechanisms underlying the regulation of MAMs? If we answer these questions, we may find potential treatments for TBI.
References
Allahtavakoli M, Jarrott B (2011) Sigma-1 receptor ligand PRE-084 reduced infarct volume, neurological deficits, pro-inflammatory cytokines and enhanced anti-inflammatory cytokines after embolic stroke in rats. Brain Res Bull 85(3–4):219–224
Arruda AP, Pers BM, Parlakgul G, Guney E, Inouye K, Hotamisligil GS (2014) Chronic enrichment of hepatic endoplasmic reticulum-mitochondria contact leads to mitochondrial dysfunction in obesity. Nat Med 20(12):1427–1435
Aslan JE, You H, Williamson DM, Endig J, Youker RT, Thomas L, Shu H, Du Y, Milewski RL, Brush MH, Possemato A, Sprott K, Fu H, Greis KD, Runckel DN, Vogel A, Thomas G (2009) Akt and 14-3-3 control a PACS-2 homeostatic switch that integrates membrane traffic with TRAIL-induced apoptosis. Mol Cell 34(4):497–509
Barroso-Gonzalez J, Auclair S, Luan S, Thomas L, Atkins KM, Aslan JE, Thomas LL, Zhao J, Zhao Y, Thomas G (2016) PACS-2 mediates the ATM and NF-kappaB-dependent induction of anti-apoptotic Bcl-xL in response to DNA damage. Cell Death Differ 23(9):1448–1457
Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D (2000) Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol 2(6):326–332
Betz C, Stracka D, Prescianotto-Baschong C, Frieden M, Demaurex N, Hall MN (2013) mTOR complex 2-Akt signaling at mitochondria-associated endoplasmic reticulum membranes (MAM) regulates mitochondrial physiology. Proc Natl Acad Sci USA 110(31):12526–12534
Boehning D, Patterson RL, Sedaghat L, Glebova NO, Kurosaki T, Snyder SH (2003) Cytochrome c binds to inositol (1,4,5) trisphosphate receptors, amplifying calcium-dependent apoptosis. Nat Cell Biol 5(12):1051–1061
Bononi A, Bonora M, Marchi S, Missiroli S, Poletti F, Giorgi C, Pandolfi PP, Pinton P (2013) Identification of PTEN at the ER and MAMs and its regulation of Ca(2+) signaling and apoptosis in a protein phosphatase-dependent manner. Cell Death Differ 20(12):1631–1643
Chambers JE, Dalton LE, Clarke HJ, Malzer E, Dominicus CS, Patel V, Moorhead G, Ron D, Marciniak SJ (2015) Actin dynamics tune the integrated stress response by regulating eukaryotic initiation factor 2alpha dephosphorylation. Elife. doi:10.7554/eLife.04872
Cheng G, Fu L, Zhang HY, Wang YM, Zhang LM, Zhang JN (2013) The role of mitochondrial calcium uniporter in neuroprotection in traumatic brain injury. Med Hypotheses 80(2):115–117
Csordas G, Renken C, Varnai P, Walter L, Weaver D, Buttle KF, Balla T, Mannella CA, Hajnoczky G (2006) Structural and functional features and significance of the physical linkage between ER and mitochondria. J Cell Biol 174(7):915–921
Danese A, Patergnani S, Bonora M, Wieckowski MR, Previati M, Giorgi C, Pinton P (2017) Calcium regulates cell death in cancer: Roles of the mitochondria and mitochondria-associated membranes (MAMs). Biochim Biophys Acta. doi:10.1016/j.bbabio.2017.01.003
Dash PK, Hylin MJ, Hood KN, Orsi SA, Zhao J, Redell JB, Tsvetkov AS, Moore AN (2015) Inhibition of eukaryotic initiation factor 2 alpha phosphatase reduces tissue damage and improves learning and memory after experimental traumatic brain injury. J Neurotrauma 32(20):1608–1620
de Brito OM, Scorrano L (2008) Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 456(7222):605–610
Digaleh H, Kiaei M, Khodagholi F (2013) Nrf2 and Nrf1 signaling and ER stress crosstalk: implication for proteasomal degradation and autophagy. Cell Mol Life Sci 70(24):4681–4694
Dirnagl U, Iadecola C, Moskowitz MA (1999) Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci 22(9):391–397
Distelhorst CW, Bootman MD (2011) Bcl-2 interaction with the inositol 1,4,5-trisphosphate receptor: role in Ca(2+) signaling and disease. Cell Calcium 50(3):234–241
Dong H, Ma Y, Ren Z, Xu B, Zhang Y, Chen J, Yang B (2016) Sigma-1 receptor modulates neuroinflammation after traumatic brain injury. Cell Mol Neurobiol 36(5):639–645
Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J (2008) Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 320(5876):674–677
Faridar A, Bershad EM, Emiru T, Iaizzo PA, Suarez JI, Divani AA (2011) Therapeutic hypothermia in stroke and traumatic brain injury. Front Neurol 2:80
Filadi R, Greotti E, Turacchio G, Luini A, Pozzan T, Pizzo P (2015) Mitofusin 2 ablation increases endoplasmic reticulum-mitochondria coupling. Proc Natl Acad Sci USA 112(17):E2174–E2181
Fischer TD, Hylin MJ, Zhao J, Moore AN, Waxham MN, Dash PK (2016) Altered mitochondrial dynamics and TBI pathophysiology. Front Syst Neurosci 10:29
Francardo V, Bez F, Wieloch T, Nissbrandt H, Ruscher K, Cenci MA (2014) Pharmacological stimulation of sigma-1 receptors has neurorestorative effects in experimental parkinsonism. Brain 137(7):1998–2014
Gao C, Qian Y, Huang J, Wang D, Su W, Wang P, Guo L, Quan W, An S, Zhang J, Jiang R (2016) A three-day consecutive fingolimod administration improves neurological functions and modulates multiple immune responses of CCI mice. Mol Neurobiol. doi:10.1007/s12035-016-0318-0
Giorgi C, Wieckowski MR, Pandolfi PP, Pinton P (2011) Mitochondria associated membranes (MAMs) as critical hubs for apoptosis. Commun Integr Biol 4(3):334–335
Gomez L, Thiebaut PA, Paillard M, Ducreux S, Abrial M, Crola DSC, Durand A, Alam MR, Van Coppenolle F, Sheu SS, Ovize M (2016) The SR/ER-mitochondria calcium crosstalk is regulated by GSK3beta during reperfusion injury. Cell Death Differ 23(2):313–322
Grimm S (2012) The ER-mitochondria interface: the social network of cell death. Biochim Biophys Acta 1823(2):327–334
Guicciardi ME, Werneburg NW, Bronk SF, Franke A, Yagita H, Thomas G, Gores GJ (2014) Cellular inhibitor of apoptosis (cIAP)-mediated ubiquitination of phosphofurin acidic cluster sorting protein 2 (PACS-2) negatively regulates tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) cytotoxicity. PLoS ONE 9(3):e92124
Guthrie LN, Abiraman K, Plyler ES, Sprenkle NT, Gibson SA, McFarland BC, Rajbhandari R, Rowse AL, Benveniste EN, Meares GP (2016) Attenuation of PKR-like ER kinase (PERK) signaling selectively controls endoplasmic reticulum stress-induced inflammation without compromising immunological responses. J Biol Chem 291(30):15830–15840
Ha Y, Shanmugam AK, Markand S, Zorrilla E, Ganapathy V, Smith SB (2014) Sigma receptor 1 modulates ER stress and Bcl2 in murine retina. Cell Tissue Res 356(1):15–27
Hailey DW, Rambold AS, Satpute-Krishnan P, Mitra K, Sougrat R, Kim PK, Lippincott-Schwartz J (2010) Mitochondria supply membranes for autophagosome biogenesis during starvation. Cell 141(4):656–667
Happy M, Dejoie J, Zajac CK, Cortez B, Chakraborty K, Aderemi J, Sauane M (2015) Sigma 1 receptor antagonist potentiates the anti-cancer effect of p53 by regulating ER stress, ROS production, Bax levels, and caspase-3 activation. Biochem Biophys Res Commun 456(2):683–688
Harvey LD, Yin Y, Attarwala IY, Begum G, Deng J, Yan HQ, Dixon CE, Sun D (2015) Administration of DHA reduces endoplasmic reticulum stress-associated inflammation and alters microglial or macrophage activation in traumatic brain injury. ASN Neuro. doi:10.1177/1759091415618969
Hayashi T, Su TP (2003) Intracellular dynamics of sigma-1 receptors (sigma(1) binding sites) in NG108-15 cells. J Pharmacol Exp Ther 306(2):726–733
Hayashi T, Su TP (2007) Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell 131(3):596–610
Hornung V, Latz E (2010) Critical functions of priming and lysosomal damage for NLRP3 activation. Eur J Immunol 40(3):620–623
Hsu SY, Kaipia A, McGee E, Lomeli M, Hsueh AJ (1997) Bok is a pro-apoptotic Bcl-2 protein with restricted expression in reproductive tissues and heterodimerizes with selective anti-apoptotic Bcl-2 family members. Proc Natl Acad Sci USA 94(23):12401–12406
Ivanova H, Vervliet T, Missiaen L, Parys JB, De Smedt H, Bultynck G (2014) Inositol 1,4,5-trisphosphate receptor-isoform diversity in cell death and survival. Biochim Biophys Acta (BBA) 1843(10):2164–2183
Jin C, Flavell RA (2010) Molecular mechanism of NLRP3 inflammasome activation. J Clin Immunol 30(5):628–631
Katnik C, Guerrero WR, Pennypacker KR, Herrera Y, Cuevas J (2006) Sigma-1 receptor activation prevents intracellular calcium dysregulation in cortical neurons during in vitro ischemia. J Pharmacol Exp Ther 319(3):1355–1365
Kim MJ, Yoon JH, Ryu JH (2016) Mitophagy: a balance regulator of NLRP3 inflammasome activation. BMB Rep 49(10):529–535
Lipinski MM, Wu J, Faden AI, Sarkar C (2015) function and mechanisms of autophagy in brain and spinal cord trauma. Antioxid Redox Signal 23(6):565–577
Liu J, Du L (2015) PERK pathway is involved in oxygen-glucose-serum deprivation-induced NF-kB activation via ROS generation in spinal cord astrocytes. Biochem Biophys Res Commun 467(2):197–203
Liu HD, Li W, Chen ZR, Hu YC, Zhang DD, Shen W, Zhou ML, Zhu L, Hang CH (2013) Expression of the NLRP3 inflammasome in cerebral cortex after traumatic brain injury in a rat model. Neurochem Res 38(10):2072–2083
Liu Z, Lv Y, Zhao N, Guan G, Wang J (2015) Protein kinase R-like ER kinase and its role in endoplasmic reticulum stress-decided cell fate. Cell Death Dis 6(7):e1822
Lopez-Armada MJ, Riveiro-Naveira RR, Vaamonde-Garcia C, Valcarcel-Ares MN (2013) Mitochondrial dysfunction and the inflammatory response. Mitochondrion 13(2):106–118
Marchi S, Patergnani S, Pinton P (2014) The endoplasmic reticulum–mitochondria connection: one touch, multiple functions. Biochim Biophys Acta (BBA) 1837(4):461–469
Marklund N, Clausen F, Lewander T, Hillered L (2001) Monitoring of reactive oxygen species production after traumatic brain injury in rats with microdialysis and the 4-hydroxybenzoic acid trapping method. J Neurotrauma 18(11):1217–1227
Meares GP, Liu Y, Rajbhandari R, Qin H, Nozell SE, Mobley JA, Corbett JA, Benveniste EN (2014) PERK-dependent activation of JAK1 and STAT3 contributes to endoplasmic reticulum stress-induced inflammation. Mol Cell Biol 34(20):3911–3925
Meunier J, Hayashi T (2010) Sigma-1 receptors regulate Bcl-2 expression by reactive oxygen species-dependent transcriptional regulation of nuclear factor kappaB. J Pharmacol Exp Ther 332(2):388–397
Morre DJ, Merritt WD, Lembi CA (1971) Connections between mitochondria and endoplasmic reticulum in rat liver and onion stem. Protoplasma 73(1):43–49
Munoz JP, Ivanova S, Sanchez-Wandelmer J, Martinez-Cristobal P, Noguera E, Sancho A, Diaz-Ramos A, Hernandez-Alvarez MI, Sebastian D, Mauvezin C, Palacin M, Zorzano A (2013) Mfn2 modulates the UPR and mitochondrial function via repression of PERK. EMBO J 32(17):2348–2361
Nakka VP, Prakash-babu P, Vemuganti R (2014) Crosstalk between endoplasmic reticulum stress, oxidative stress, and autophagy: potential therapeutic targets for acute CNS injuries. Mol Neurobiol 53(1):532–544
Natsvlishvili N, Goguadze N, Zhuravliova E, Mikeladze D (2015) Sigma-1 receptor directly interacts with Rac1-GTPase in the brain mitochondria. BMC Biochem 16:11
Oslowski CM, Hara T, O’Sullivan-Murphy B, Kanekura K, Lu S, Hara M, Ishigaki S, Zhu LJ, Hayashi E, Hui ST, Greiner D, Kaufman RJ, Bortell R, Urano F (2012) Thioredoxin-interacting protein mediates ER stress-induced beta cell death through initiation of the inflammasome. Cell Metab 16(2):265–273
Pal A, Fontanilla D, Gopalakrishnan A, Chae YK, Markley JL, Ruoho AE (2012) The sigma-1 receptor protects against cellular oxidative stress and activates antioxidant response elements. Eur J Pharmacol 682(1–3):12–20
Park E, Bell JD, Baker AJ (2008) Traumatic brain injury: can the consequences be stopped? CMAJ 178(9):1163–1170
Pearn ML, Niesman IR, Egawa J, Sawada A, Almenar-Queralt A, Shah SB, Duckworth JL, Head BP (2016) Pathophysiology associated with traumatic brain injury: current treatments and potential novel therapeutics. Cell Mol Neurobiol. doi:10.1007/s10571-016-0400-1
Pizzo P, Pozzan T (2007) Mitochondria-endoplasmic reticulum choreography: structure and signaling dynamics. Trends Cell Biol 17(10):511–517
Rizzuto R, Brini M, Murgia M, Pozzan T (1993) Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science 262(5134):744–747
Rodríguez-Arribas M, Yakhine-Diop SMS, Pedro JMB, Gómez-Suaga P, Gómez-Sánchez R, Martínez-Chacón G, Fuentes JM, González-Polo RA, Niso-Santano M (2016) Mitochondria-associated membranes (MAMs): overview and its role in Parkinson’s disease. Mol Neurobiol. doi:10.1007/s12035-016-0140-8
Rong YP, Aromolaran AS, Bultynck G, Zhong F, Li X, McColl K, Matsuyama S, Herlitze S, Roderick HL, Bootman MD, Mignery GA, Parys JB, De Smedt H, Distelhorst CW (2008) Targeting Bcl-2-IP3 receptor interaction to reverse Bcl-2’s inhibition of apoptotic calcium signals. Mol Cell 31(2):255–265
Rowland AA, Voeltz GK (2012) Endoplasmic reticulum–mitochondria contacts: function of the junction. Nat Rev Mol Cell Biol 13(10):607–625
Rubartelli A (2012) Redox control of NLRP3 inflammasome activation in health and disease. J Leukoc Biol 92(5):951–958
Ruscher K, Inacio AR, Valind K, Rowshan RA, Kuric E, Wieloch T (2012) Effects of the sigma-1 receptor agonist 1-(3,4-dimethoxyphenethyl)-4-(3-phenylpropyl)-piperazine dihydro-chloride on inflammation after stroke. PLoS ONE 7(9):e45118
Sadatomi D, Nakashioya K, Mamiya S, Honda S, Kameyama Y, Yamamura Y, Tanimura S, Takeda K (2017) Mitochondrial function is required for extracellular ATP-induced NLRP3 inflammasome activation. J Biochem. doi:10.1093/jb/mvw098
Sande A, West C (2010) Traumatic brain injury: a review of pathophysiology and management. J Vet Emerg Crit Care 20(2):177–190
Schroder K, Tschopp J (2010) The inflammasomes. Cell 140(6):821–832
Schulman JJ, Wright FA, Kaufmann T, Wojcikiewicz RJ (2013) The Bcl-2 protein family member Bok binds to the coupling domain of inositol 1,4,5-trisphosphate receptors and protects them from proteolytic cleavage. J Biol Chem 288(35):25340–25349
Schulman JJ, Wright FA, Han X, Zluhan EJ, Szczesniak LM, Wojcikiewicz RJ (2016) The stability and expression level of Bok are governed by binding to inositol 1,4,5-trisphosphate receptors. J Biol Chem 291(22):11820–11828
Simmen T, Aslan JE, Blagoveshchenskaya AD, Thomas L, Wan L, Xiang Y, Feliciangeli SF, Hung CH, Crump CM, Thomas G (2005) PACS-2 controls endoplasmic reticulum-mitochondria communication and Bid-mediated apoptosis. EMBO J 24(4):717–729
Soltys BJ, Gupta RS (1992) Interrelationships of endoplasmic reticulum, mitochondria, intermediate filaments, and microtubules—a quadruple fluorescence labeling study. Biochem Cell Biol 70(10–11):1174–1186
Stephens L, Anderson K, Stokoe D, Erdjument-Bromage H, Painter GF, Holmes AB, Gaffney PR, Reese CB, McCormick F, Tempst P, Coadwell J, Hawkins PT (1998) Protein kinase B kinases that mediate phosphatidylinositol 3,4,5-trisphosphate-dependent activation of protein kinase B. Science 279(5351):710–714
Szabadkai G, Bianchi K, Varnai P, De Stefani D, Wieckowski MR, Cavagna D, Nagy AI, Balla T, Rizzuto R (2006) Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J Cell Biol 175(6):901–911
Szado T, Vanderheyden V, Parys JB, De Smedt H, Rietdorf K, Kotelevets L, Chastre E, Khan F, Landegren U, Soderberg O, Bootman MD, Roderick HL (2008) Phosphorylation of inositol 1,4,5-trisphosphate receptors by protein kinase B/Akt inhibits Ca2+ release and apoptosis. Proc Natl Acad Sci USA 105(7):2427–2432
van Vliet AR, Verfaillie T, Agostinis P (2014) New functions of mitochondria associated membranes in cellular signaling. Biochim Biophys Acta 1843(10):2253–2262
Vance JE (1990) Phospholipid synthesis in a membrane fraction associated with mitochondria. J Biol Chem 265(13):7248–7256
Vance JE (2014) MAM (mitochondria-associated membranes) in mammalian cells: lipids and beyond. Biochim Biophys Acta (BBA) 1841(4):595–609
Verfaillie T, Rubio N, Garg AD, Bultynck G, Rizzuto R, Decuypere JP, Piette J, Linehan C, Gupta S, Samali A, Agostinis P (2012) PERK is required at the ER-mitochondrial contact sites to convey apoptosis after ROS-based ER stress. Cell Death Differ 19(11):1880–1891
Walter P, Ron D (2011) The unfolded protein response: from stress pathway to homeostatic regulation. Science 334(6059):1081–1086
Wang CH, Tsai TF, Wei YH (2015) Role of mitochondrial dysfunction and dysregulation of Ca(2+) homeostasis in insulin insensitivity of mammalian cells. Ann N Y Acad Sci 1350:66–76
Werner C, Engelhard K (2007) Pathophysiology of traumatic brain injury. Br J Anaesth 99(1):4–9
Wu G, Liu Z (2016) Nuclear factor erythroid 2-related factor 2 (Nrf2) mediates neuroprotection in traumatic brain injury at least in part by inactivating microglia. Med Sci Monit 22:2161–2166
Wu Z, Li L, Zheng LT, Xu Z, Guo L, Zhen X (2015) Allosteric modulation of sigma-1 receptors by SKF83959 inhibits microglia-mediated inflammation. J Neurochem 134(5):904–914
Yan F, Cao S, Li J, Dixon B, Yu X, Chen J, Gu C, Lin W, Chen G (2016) Pharmacological inhibition of PERK attenuates early brain injury after subarachnoid hemorrhage in rats through the activation of Akt. Mol Neurobiol. doi:10.1007/s12035-016-9790-9
Yang S, Bhardwaj A, Cheng J, Alkayed NJ, Hurn PD, Kirsch JR (2007) Sigma receptor agonists provide neuroprotection in vitro by preserving bcl-2. Anesth Analg 104(5):1179–1184
Yin Y, Zhou Z, Liu W, Chang Q, Sun G, Dai Y (2017) Vascular endothelial cells senescence is associated with NOD-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome activation via reactive oxygen species (ROS)/thioredoxin-interacting protein (TXNIP) pathway. Int J Biochem Cell Biol 84:22–34
Yonutas HM, Vekaria HJ, Sullivan PG (2016) Mitochondrial specific therapeutic targets following brain injury. Brain Res 1640(Pt A):77–93
Yu KN, Chang SH, Park SJ, Lim J, Lee J, Yoon TJ, Kim JS, Cho MH (2015) Titanium dioxide nanoparticles induce endoplasmic reticulum stress-mediated autophagic cell death via mitochondria-associated endoplasmic reticulum membrane disruption in normal lung cells. PLoS ONE 10(6):e131208
Zhang D, Teng J (2016) Nrf2 knockout: the effect on neurological dysfunction and the activation of glial cells of mice after brain injury. Pak J Pharm Sci 29(4 Suppl):1365–1369
Zhang B, Wang XQ, Chen HY, Liu BH (2014) Involvement of the Nrf2 pathway in the regulation of pterostilbene-induced apoptosis in HeLa cells via ER stress. J Pharmacol Sci 126(3):216–229
Zhang X, Zhang JH, Chen XY, Hu QH, Wang MX, Jin R, Zhang QY, Wang W, Wang R, Kang LL, Li JS, Li M, Pan Y, Huang JJ, Kong LD (2015) Reactive oxygen species-induced TXNIP drives fructose-mediated hepatic inflammation and lipid accumulation through NLRP3 inflammasome activation. Antioxid Redox Signal 22(10):848–870
Zhang L, Ding K, Wang H, Wu Y, Xu J (2016) Traumatic brain injury-induced neuronal apoptosis is reduced through modulation of PI3K and autophagy pathways in mouse by FTY720. Cell Mol Neurobiol 36(1):131–142
Zhou R, Yazdi AS, Menu P, Tschopp J (2011) A role for mitochondria in NLRP3 inflammasome activation. Nature 469(7329):221–225
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (Grant Nos. 813300151, 81501704, 81671902, and 81370029) and the Tianjin Research Program of Application Foundation and Advanced Technology (Grant No. 13JCQNJCl0500). We are grateful to Guoqiang Chang, Guili Yang, Weiyun Cui, Lei Zhou, Li Liu, and Yan Chai from the Tianjin Neurological Institute for their technical support.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Sun, D., Chen, X., Gu, G. et al. Potential Roles of Mitochondria-Associated ER Membranes (MAMs) in Traumatic Brain Injury. Cell Mol Neurobiol 37, 1349–1357 (2017). https://doi.org/10.1007/s10571-017-0484-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-017-0484-2