Abstract
FK-506 (Tacrolimus) is a very commonly used immunomodulatory agent that plays important roles in modulating the calcium-dependent phosphoserine–phosphothreonine protein phosphatase calcineurin and thus inhibits calcineurin-mediated secondary neuronal damage. The biological function of FK-506 in the spinal cord has not been fully elucidated. To clarify the anti-inflammatory action of FK-506 in spinal cord injury (SCI), we performed an acute spinal cord contusion injury model in adult rats and hypoxia-treated primary spinal cord microglia cultures. This work studied the activation of NF-κB and proinflammatory cytokine (TNF-a, IL-1b, and IL-6) expression. ELISA and q-PCR analysis revealed that TNF-a, IL-1b, and IL-6 levels significantly increased 3 days after spinal cord contusion and decreased after 14 days, accompanied by the increased activation of NF-κB. This increase was reversed by an FK-506 treatment. Double immunofluorescence labeling suggested that NF-κB activation was especially prominent in microglia. Immunohistochemistry confirmed no alteration in the number of microglia. Moreover, the results in hypoxia-treated primary spinal cord microglia confirmed the effect of FK-506 on TNF-a, IL-1b, and IL-6 expression and NF-κB activation. These findings suggest that FK-506 may be involved in microglial activation after SCI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spinal cord injury (SCI) can result in serious disability, sensory disorder, paralysis, neurologic deficits, and death. Processes occurring after SCI are associated with three phases: The first is the acute phase comprising the initial trauma and affected spinal shock. The second is the secondary phase taking place over a time course of minutes to weeks after the injury and exacerbates the damage inflicted by the primary injury. This phase comprises several interrelated damage processes, including vascular alterations, biochemical disturbances, and cellular responses that lead to an inflammatory response and cell death. The third is the chronic phase, which occurs between days and years after the trauma and is characterized by apoptosis, Wallerian degeneration, and scarring that establishes functional impairment (Bareyre and Schwab 2003; Profyris et al. 2004). Secondary injury, which may cause severe and permanent functional deficits (Carlson and Gorden 2002), is characterized by a key pathophysiological response to SCI. Thus, many clinical and experimental studies have been conducted on potential treatments for secondary injuries of SCI (Thuret et al. 2006).
Inflammation plays an important role in secondary injuries after SCI (Bethea and Dietrich 2002). Injury-induced inflammation can result in neuropathology and secondary necrosis after traumatic SCI (Bartholdi and Schwab 1997; Zhang et al. 1997; Bethea et al. 1998). Proinflammatory cytokines, such as TNF-a, IL-1b, and IL-6, are primarily produced by inflammatory cells that contribute to cell death and lesion expansion (Yang et al. 2005; Smith et al. 2012; Young 1993; Akira et al. 1990). Studies have demonstrated that the activation of NF-κB signaling pathways following SCI is an important step in the inflammatory response and modulates the production of proinflammatory cytokines in the central nervous system (CNS) (Barnes and Karin 1997; Li and Verma 2002).
FK-506 (Tacrolimus) is one of the most commonly used immunomodulatory agent and is reported to have benefit in SCI through various mechanisms, such as neuroprotective and neuroregenerative properties (Saganová et al. 2012), suppression of oxidative injury (Yousuf et al. 2011), and promotion of axonal outgrowth (Pan et al. 2008). Some studies have shown that a treatment with FK506 has the potential to either downregulate a proinflammatory cytokine expression in cerebral ischemia or inhibit the activation of NF-κB in diabetic retinopathy (Zawadzka and Kaminska 2005; Su et al. 2012). We, therefore, speculated that FK-506 might have anti-inflammatory effects on SCI. Therefore, in the present study, we explored the effects of FK-506 on inflammatory responses after SCI.
Materials and Methods
Animal Preparation
Sprague–Dawley male adult rats, weighing 220–250 g each, were obtained from the Animal Center (Nanjing Medical University, Nanjing, China). The rats were raised in a temperature-controlled house under a 12-h light/dark cycle and fed a standard laboratory diet and water ad libitum. The Animal Care and Use Committee of the Nanjing Medical University approved all experiments in accordance with the Declaration of the National Institutes of Health Guide for Care and Use of Laboratory Animals.
Spinal Cord Injury
Spinal cord injury was induced using a weight drop device as previously reported (Zhang et al. 2009). All animals were anesthetized with intraperitoneal injection of 4 % sodium pentobarbital (40 mg/kg, i.p.). An incision was made along the middle of the back, exposing the paravertebral muscles. The exposed dorsal surface of the cord was subjected to weight drop impact using a 10-g metal rod at a height of 25 mm at the T9–T11 level. After the surgical operation, sterile gauze was wrapped around the wound. Animals were injected with penicillin (intramuscularly, 100,000 unit/per animal/day) and buprenorphine (0.03 mg/kg) for 3 days to prevent post-operative infection and to relieve the pain from surgery. A standard diet and water were provided daily to the rats ad libitum. Manual bladder expression was performed twice a day until the rats were able to urinate by themselves. Throughout the process, symptoms of infection, pain, decubitus, dehydration, and autophagia were monitored daily.
Experimental Design
Rats were randomly divided into two groups: the SCI group and SCI/FK-506 group. The SCI group was subjected to SCI, and the SCI/FK-506 group was treated with FK-506 after SCI. FK-506 was purchased from Beijing Novartis Pharma Ltd (Beijing, China), dissolved in 0.9 % sterile saline solution as the concentration of 1 mg/ml and administered (0.5 mg/kg) by gavage 30 min after operation (Zhang et al. 2009). At the indicated time point (3, 7, 14, and 21 days after SCI), the rats were sacrificed, and the spinal cord was immediately exposed from T1 to T12, and the damaged tissue (T9–T10) at the site of injury was cut.
Primary Spinal Cord Microglia Cultures
Glial cultures were prepared from the spinal cords of post-natal day-1 Sprague–Dawley rats (Baskar Jesudasan et al. 2014). The meninges and blood vessels were removed from the spinal cords, and then tissue was finely minced and dissociated enzymatically by 0.25 % Trypsin–EDTA for 20 min at 37 °C. Spinal cord tissues were triturated mechanically in Dulbecco Modified Eagle Medium/Ham’s F12 (DMEM/F12; Gibco) containing 10 % Fetal Bovine Serum (FBS; Gibco) and 2 % Penicillin/Streptomycin (P/S; Gibco) and then plated on poly-l-lysine coated plates. After 21 days of culture, microglia was isolated from glial cultures by mild trypsinization (0.25 % Trypsin–EDTA was diluted in 1:3) for 20–50 min. Then DMEM/F12 containing 2 % P/S was added to the isolated spinal cord microglia (SCM) (Baskar Jesudasan et al. 2014). SCM were further tagged with a PE-conjugated anti-CD11b+ antibody (BD Biosciences, San Jose, CA, USA) followed by an anti-PE antibody conjugated to a magnetic bead. Magnetically tagged CD11b+ cells were isolated using MS columns according to the Miltenyi MACS protocol. As previously reported, this method results in a >97 % pure population of CD11b+cells (Nikodemova and Watters 2012). Isolated CD11b+ cells will subsequently be referred to as “microglia.” Isolate microglia cells were cultured again in DMEM/F12 containing 2 % P/S at 37 °C in 5 % CO2. Cells of passages 3–4 were used for all experiments. For hypoxia treatment, SCM were set in an airtight experimental hypoxia chamber (Billups-Rothenberg, San Diego, CA, USA) containing a gas mixture composed of 95 % N2/5 % CO2 and normoxia (21 % inspired O2) for control. In some case, SCM were pretreated with 100 μM FK506 (according to a pilot study) or ACHP (NF-κB inhibitor, 50 μM) (Zhou et al. 2014) for 30 min and then applied to hypoxia for 6 h.
Cell Viability Assay
Cell viability was determined by the Cell Counting Kit-8 (CCK8) (Dojindo Laboratories, Kumamoto, Japan). Rat spinal cord microglia were cultured and treated in 24-well plates at a density of 2 × 104 cells/well in triplicates. After seeding, the cells were subjected to hypoxia for 0, 2, 4, 6, 12, and 24 h. After hypoxia treatment, 10 μL of CCK-8 solution was added to each well, and the cells were incubated; cell viability was measured at 450 nm by optical density with a microplate absorbance reader (Bio-Tek, Elx800, USA).
LDH Assay
The LDH cytotoxicity detection kit (Roche Diagnostics GmbH, Mannheim, Germany) was used according to the manufacturer’s protocols. The test is based on the determination of LDH activity released from the cytosol of damaged cells into the medium, thus indicating cell damage. Cells were incubated under the same conditions as for the CCK8 assay described above. LDH released into the extracellular medium of cells treated with FK506 was expressed as a percentage of the total LDH activity in the cells [% of LDH released = extracellular LDH/(extracellular LDH+intracellular LDH)].
5-Ethynyl-2-deoxyuridine (EdU) Incorporation Assay
Incorporated EdU was detected using Click-iT1 EdU Assay Kits (Invitrogen, CA, USA) according to the manufacturer’s protocol. Cells were cultured and treated in 6-well plates at a density of 5 × 105 cells/well for 2 h at hypoxia conditions, and then 50 µM of EdU was added to each dish, and cells were cultured for an additional 4, 10, and 22 h, respectively. The cells were fixed with 4 % formaldehyde for 15 min at room temperature and treated with 0.5 % Triton X-100 for 20 min at room temperature for permeabilization. After washing with PBS three times, 100 µl of 1 × Apollo® reaction cocktail was added to each well, and the cells were incubated for 30 min at room temperature. Then, the cells were stained with 100 µl of Hoechst 33342 for 30 min and visualized under a fluorescence microscope (Olympus BX51, Olympus, Japan) and Image J software (NIH). At least 50 cells were randomly selected from a single captured field, and the percentage of positive cells was calculated. The EdU incorporation rate was expressed as the ratio of EdU-positive cells to total Hoechst 33342-positive cells (blue cells). Data points presented in the text are the averages calculated from five different fields. The assay was performed in triplicate.
Enzyme-Linked Immunosorbent Assay (ELISA)
TNF-a, IL-1b, and IL-6 in the spinal cords of separate rat groups and SCM media were quantified by solid-phase sandwich ELISA (R&D systems, Mannheim, Germany), according to the manufacturer’s instructions, and all samples were run in duplicate. Spinal cords were isolated and homogenized in 300 μl PBS. The homogenates were frozen at −20 °C overnight and were centrifuged at 12,000×g at 4 °C. Protein concentration was measured in the supernatants using the BCA method (Pierce, Rockford, IL, USA) and 1 mg proteins of each group were used for ELISA assay. Samples and standards were incubated on plates coated with anti-TNF-a, anti-IL-1b, and anti-IL-6 antibodies (n = 5 per group). Biotinylated antibody was added to mixtures of bound cytokines. To quantify the binding of the secondary antibody, streptavidin-peroxidase conjugate and substrate (tetramethylbenzidine) were added. After stopping the reaction by the addition of citric acid, the absorbance was measured at 450 nm. Concentrations were determined from a standard curve.
mRNA Expression
The tissues of the spinal cord and SCM cells were homogenized under liquid nitrogen, and RNA was isolated using TRIzol reagent (Life Technologies, Carlsbad, CA, USA). RNA was reverse-transcribed into cDNA, and real-time RT-PCR was conducted using SYBR Green PCR Master Mix as per the manufacturer’s instructions (Applied Biosystems, Foster City, CA, USA) as previously described (Livak and Schmittgen 2001). Primer sequences are presented in Table 1. 1 ng cDNA was used for PCR, and the conditions utilized were as follows: 2 min at 50 °C; 10 min at 95 °C; and 40 repetitions of 15 s at 95 °C and 1 min at 60 °C. All mRNA quantification data were normalized to GAPDH as an endogenous control for the mRNA detection. The data were processed using the 2−ΔΔCT method, which compares a single calibrator sample against every unknown sample’s gene expression.
Nuclear Extract Preparation
Nuclear extracts were prepared from cultured SCM using a nuclear and cytoplasmic extraction kit (NE-PER; Thermo Fisher Scientific, Pittsburgh, PA, USA). Cells were mechanically detached and pelleted by centrifugation and then re-suspended in three volumes of solution A [HEPES, 10 mM; MgCl2, 1.5 mM; KCl, 10 mM; dithiothreitol (DTT), 0.5 mM; NP-40, 1 %] with freshly added protease inhibitors (1 μg/mL leupeptin, 5 μg/mL aprotinin, and 1 mM phenylmethylsulfonyl fluoride). The nuclei were collected by centrifugation, and the nuclear pellet was re-suspended in 1 vol. of solution B (HEPES, 20 mM; MgCl2, 1.5 mM; KCl, 300 mM; DTT, 0.5 mM; EDTA, 0.2 mM; 25 % glycerol) with freshly added protease inhibitors as described above and agitated vigorously at 4 °C. Nuclear debris was collected by centrifugation, and the supernatant (nuclear extract) was stored at −80 °C. The protein concentrations were determined using the BCA method (Pierce, Rockford, IL, USA).
Western Blot Analysis
Spinal cords from a separate group of animals and SCM were homogenized in a lysis buffer containing 50 mM Tris–HCl (pH 7.5), 150 mmol/L NaCl, 5 mmol/L EDTA, 10 mmol/L NaF, 1 mmol/L sodium orthovanadate, 1 % Triton X-100, 0.5 % sodium deoxycholate, 1 mmol/L phenylmethylsulfonyl fluoride, and a protease inhibitor cocktail. The lysate was centrifuged at 12,000×g for 25 min at 4 °C. The total protein in each sample was analyzed using the BCA method (Pierce, Rockford, IL, USA). An equal amount of protein samples (50 μg) from each group was boiled in 3 × loading buffer (10 mmol/L Tris–HCl, pH 6.8 including 3 % SDS, 5 % β-mercaptoethanol, 20 % glycerol, and 0.6 % bromophenol blue) for 3 min and separated by SDS–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes. After the transfer, membranes were blocked with 5 % fat-free milk in Tris-buffered saline plus 0.05 % Tween 20 overnight at 4 °C. The membranes were then incubated with a rabbit anti-phosphorylated IkB-α/ß (Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-p-IkB (Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-p65 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-p-p65 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), mouse anti-CD11b (Serotec, USA), or mouse anti-GAPDH (Cell Signaling Technology, USA), for 2 h at room temperature. After washing in TBST three times, the membranes were incubated with a peroxidase-linked anti-rabbit-IgG conjugate for 1 h at room temperature. Finally, blots were washed again in TBST and incubated in enhanced chemiluminescence reagents (Pierce, Rockford, IL, USA). The intensity of the bands was quantified for 2 min and exposed to X-Omat BT film. Signal intensity was quantified using a Bio-Rad image analysis system. The results were normalized using GAPDH as internal control. For background, the primary antibody was omitted. For p65 nuclear translocation assay, the expression of p65 in the nuclei of microglia was also analyzed via western blot. The nuclear extracts were applied to SDS-PAGE and then transferred to nitrocellulose membranes. The membranes were probed by anti-p65 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), and the lamin (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was used as loading control.
Immunohistochemical Study
The spinal cord microglia were detected with its marker CD11b (Su et al. 2012) by immunohistochemical staining. Spinal cords were enucleated and fixed in 4 % PFA and embedded in paraffin. The sections (5 μm thick) were cut and deparaffinized in xylene, dehydrated in gradient ethanol, and then washed with PBS. For heat-induced epitope retrieval, the sections were dipped in 10 mM citrate buffer (pH 6.0) and heated in a microwave oven for 15 min. After pretreatment in 3 % H2O2 in PBS for 15 min and blocking with 5 % BSA at room temperature, sections were incubated with mouse anti-CD11b (Serotec, USA) overnight at 4 °C. After the primary antibody reaction, an immunohistochemical staining kit (DAKO, CA, USA) was used. The sections were washed and stained with 3,3-diaminobenzidine and then counterstained with hematoxylin. The numbers of positive stained cells were counted in each of five randomly selected consecutive fields under a 400-fold magnification. For the simultaneous visualization of p-p65 and CD11b, a double immunofluorescence method was used. Sections were incubated overnight at 4 °C with a mixture of rabbit anti-p-p65 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and mouse anti-CD11b (Serotec, USA), or a mixture of rabbit anti-p-p65 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and mouse anti-GFAP (marker of astrocytes, Santa Cruz Biotechnology, Santa Cruz, CA, USA). The sections were then treated with a mixture of Alexa 568-conjugated goat anti-mouse IgG (for CD11b and GFAP 1:500; Jackson ImmunoResearch Labs, USA) and Alexa 488-conjugated goat anti-rabbit IgG (for p-p65, 1:1000, Jackson ImmunoResearch Labs) for 1 h at room temperature. Imaging was acquired on an Olympus BX51 microscope using an Olympus DP70 digital camera, and photographs of the tissue specimens were taken at 200 × magnification.
Statistical Analysis
All experiments were performed in triplicate. Analysis of the experimental data was carried out using the PDQuest 7.0 software (Bio-Rad Laboratories, Hercules, CA, USA). Data were analyzed with one-way analysis of variance, followed by Bonferroni correction. Data are presented as the mean ± SD. p < 0.05 was considered statistically significant.
Results
FK506 Does Not Affect the Population of Spinal Cord Microglia Cells
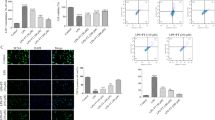
According to Zhang’s report, the dramatically improved functional recovery score in FK-506 treated rats was found 21 days post injury after SCI (Zhang et al. 2009). Thus, the expression of spinal cord microglia (CD11b positive cell) was followed at five time points between 1 and 21 days post injury for both the control group and FK-506-treated rats. The control group and FK-506-treated group were divided into five time-period subgroups: 0 days (sham operation in the control group and sham operation plus the same volume of 0.9 % sterile saline solution), 3, 7, 14, and 21 days. As shown in Fig. 1a, the CD11b positive cells appeared after SCI on days 3 and 7 and then decreased on day 14 after SCI. However, the cell number has no significant difference between post-injury cords treated with or without FK506.
CD 11b immunoreactivity in the post-injury cords treated with or without FK506 rats. The typical microglia cells positively stained by CD 11b were found in the white matter in the spinal cord after SCI. The population of microglia has no significant difference among different groups (a). Stereological quantifications of the positive cells are shown in (b). Data are mean ± SEM. *p < 0.05 vs Sham, n = 3. However, the expression of CD11b, an activated microglia maker, was significantly increased in the spinal cord after SCI days 3 and 7 and attenuated with FK506 treatment in the western blot analysis (c). Relative intensity is shown in (d). Data are mean ± SEM. *p < 0.05 vs Sham, # p < 0.05 VS SCI, n = 3
FK-506 Attenuated the Activation of Spinal Cord Microglia Cells After SCI
CD11b is a marker of activated microglia; as shown in Fig. 1b, the expression of CD11b was significantly increased in the spinal cord after SCI on days 3 and 7 and attenuated with FK506 treatment in the western blot analysis. These results indicated that FK-506 could attenuate the activation of microglia in the spinal cord after SCI. In contrast, FK-506 had no effect on the expression of GFAP, the marker of activated astrocytes.
FK-506 Attenuated the Expression of Proinflammatory Cytokines in the Spinal Cord After SCI
The cytokine expression was also followed at five time points as above (N = 5 each subgroup). As shown in Table 2, the expression of spinal cord TNF-a, IL-1b and IL-6 increased gradually after SCI, peaked on day 7, and then decreased, as measured by ELISA analysis. FK-506 significantly suppressed cytokine expression (p < 0.05 on day 3 and 7). The mRNA expression analyzed by q-PCR confirmed these results (Fig. 2).
FK-506 Attenuated NF-κB Activity in the Spinal Cord After SCI
The activation of NF-κB is a hallmark of inflammation after traumatic SCI (Bethea et al. 1998), which induces the transcription of various inflammatory marker genes, including those of interleukins, cytokines, chemokines, iNOS, and COX-2. To explore the mechanisms of how FK-506 may attenuate the expression of proinflammatory cytokines in spinal cords after SCI, we examined the expression of NF-κB (p-p65) and IkB-α phosphorylation by western blotting. The expression of IκB-α and NF-κB phosphorylation in the spinal cord were increased on days 3 and 7 (Fig. 3a). FK506 treatment significantly prevented the expressions of these markers in spinal cords on both days 3 and 7, indicating the inhibition of activation of NF-κB by FK506 (Fig. 3b).
Western blotting analysis of NF-κB activation in the spinal cord after SCI. a The activation was increased in spinal cords after SCI on day 3 and day 7 (increased expression of phospho-p65 and phosphor-IkB), and FK506 treatment attenuated these increases (b). Relative intensity is shown in c. Data are mean ± SEM. *p < 0.05 vs Sham, # p < 0.05 VS SCI, n = 3
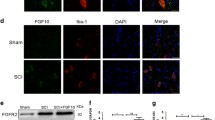
The Expression of Phosphorylated NF-κB (p-p65) in Microglia in the Spinal Cord After SCI
The activity of microglial cells is increased as reflected by their increased expression of CD11b, while the activity of astrocytes is increased with GFAP. NF-κB activation is essential to both astrocyte and microglial activity. Therefore, we examined the expression of the CD11b, GFAP and the p-p65 (activated NF-κB) by immunohistochemistry. As seen in Fig. 4, CD11b expression is co-expressed with p-p65 in the microglia. No p-p65-positive staining was found in GFAP-positive cells (Fig. 4). These results suggested that the decreased release of proinflammatory cytokines is a result of the inactivation of NF-κB in the spinal microglia.
Hypoxia Promoted the Expression of Proinflammatory Cytokines in Cultured SMC
Based on the results of a cell viability and proliferation assay, the decrease of cell viability and proliferation in SMC was not observed up to 24 h after hypoxic exposure (Fig. 5). We, therefore, analyzed the expression of cytokines in the SMC cultured media by ELISA at the time points 0, 2, 4, 6, and 12 h after hypoxic exposure. As shown in Table 3, the levels of TNF-a, IL-1b, and IL-6 in the SMC cultured media increased gradually after hypoxia and peaked at 6 h; mRNA expression analyzed by q-PCR confirmed these results (Fig. 6).
Cytotoxicity and proliferation in microglia subjected to hypoxia at various time points. Cytotoxicity was measured by CCK-8 assay (a) and LDH release (b). Proliferation was measured by EdU incorporation assay (c) and (d). Each bar represents mean ± SD, and experiments were repeated three times. *p < 0.05 VS hypoxia 0 h (or 6 h in EdU incorporation assay, D), n = 3
FK-506 Attenuated the Expression of Proinflammatory Cytokines and NF-κB Activity in Cultured SMC Under Hypoxia
To choose a proper dose, cells were administrated with FK506 at different concentrations: 10, 50,100, and 200 μM. We found that administration of FK506 at the dose of 10 and 50 μM did not exhibit remarkable inhibition in cytokines production by microglia cells under hypoxia conditions, while 100 and 200 μM resulted in significant effects. Thus, we used the dose of 100 μM to analyze the effect of FK-506 on cytokine expression and NF-κB activity. Pretreatment with 100 μM FK-506 for 30 min significantly prevented the increase of TNF-a, IL-1b, and IL-6 6 h after hypoxic exposure in SMC (Table 3) and also significantly attenuated the activation of NF-κB induced by hypoxia treated for 6 h in SMC (Fig. 7a). The inhibitory effect of FK-506 in proinflammatory cytokines and NF-κB activity was confirmed by the NF-κB inhibitor ACHP (50 μM) and its nuclear translocation (Table 3; Fig. 7b).
Western blotting analysis of NF-κB activation in cultured SMC after hypoxia. The activation was increased in hypoxic SMC after 6 h (increased expression of phospho-p65 and phosphor-IkB) and FK506 treatment attenuated these increases (a). These results were confirmed by the p-65 expression in the nuclei (b). Relative intensity is shown in c. Data are mean + SEM. *p < 0.05 vs Control, # p < 0.05 VS hypoxia, n = 3
Discussion
FK-506 is the most commonly used immunomodulatory agent, which inhibits the calcium-dependent phosphoserine–phosphothreonine protein phosphatase and calcineurin, thereby inhibiting the calcineurin-mediated secondary neuronal damage (Diaz-Ruiz et al. 2005). FK-506 is reported to be a potential therapy for SCI through various mechanisms (Saganová et al. 2012; Yousuf et al. 2011; Pan et al. 2008; Zhang et al. 2009). The present study reveals for the first time, to our knowledge, several important findings concerning the role of the FK506 in SCI-induced spinal inflammation.
Many studies have indicated that the inhibition of NF-κB activation protects the spinal cord in spinal cord injury (Goldshmit et al. 2014; Wei and Ma 2014; Wang et al. 2014; Ni et al. 2013; Chengke et al. 2013; Hu et al. 2013; Yin et al. 2012). Thus, NF-κB activation is the key element of the secondary neuronal damage in spinal cord injury. In the present study, the activation of NF-κB increased gradually in a time-dependent manner after SCI and peaked on day 7. NF-κB activity as well as TNF-a, IL-1b, and IL-6 expression was suppressed by FK506 treatment, indicating that NF-κB activation contributes to increased levels of these cytokines. Previous results and data showing the severity of neuronal injury were dependent on the expression of cytokines after a spinal cord injury (Lai and Todd 2008). Thus, from our study as well as others, we conclude that the beneficial effect of FK-506 on SCI therapy is partly due to its inhibitory effect on inflammation and NF-κB activation.
Microglia are a unique population of cells in the CNS and are the resident immune cells of the brain and spinal cord. After trauma or infection, the CD11b expression in the microglia was increased, and thus, microglia were activated (Zhang et al. 2014) and gained the capacity to secrete anti-inflammatory factors, proinflammatory factors, prostaglandins, cytokines, and reactive oxygen species (Lai and Todd 2008; Lai and Todd 2006; Kettenmann et al. 2011). As a major source of TNF-a, IL-1b, IL-6, and trophic factors, including brain-derived neurotrophic factor (BDNF), microglia are major contributors to increased levels of these cytokines after CNS injuries (Lai and Todd 2008; Lambertsen et al. 2009; Yenari et al. 2010; Werry et al. 2011; Green and Nolan 2012; Hua et al. 2012; Du et al. 2009). The present study demonstrates that NF-κB was activated in spinal cord microglia after SCI, whereas activated NF-κB was suppressed by FK-506 treatment as demonstrated in retinal tissue (Su et al. 2012). Several studies have indicated that FK506 inhibits the activation of NF-κB in various cell types by different mechanisms. Du et al. reported that the FK506-mediated suppression of NF-κB activation in macrophages was a result subsequent to the unfolded protein response (Du et al. 2009). Without further examination, we cannot rule out the possibility that the inactivation of NF-κB in FK-506-treated spinal microglia was a result of the increased unfolded protein response, similar to what occurs in macrophages. More studies are needed to delineate how FK506 exhibits similar roles in NF-κB activation in spinal microglia.
The secretory profile of altering the microglial response to NF-κB activation is difficult to predict in vivo, as proinflammatory factors can be produced by other cells, such as neutrophils, after injury. Nonetheless, we have examined the secretory profiles of microglia upon activation to define the effect of NF-κB and FK-506 on microglia functional response to SCI injury using SMC cultures (Yang et al. 2014). Our finding that FK-506 attenuated the expression of proinflammatory cytokines and NF-κB activity in cultured SMC under hypoxia indicates that the effect of FK506 on the reduced production of cytokines is due to the inhibition of NF-κB in microglia.
Similar to microglia, astrocytes are also the main responders to CNS aggravations under various pathological conditions such as injury, infection, hypoxia/ischemia, and neurodegeneration (Farina et al. 2007). In a wide range of these neuroinflammatory conditions, astrocytes undergo a remarkable transition to a reactive state. As reactive astrocytes are believed to mark the beginning of wound healing and repair events after brain injury (Liberto et al. 2004), as well as release inflammatory mediators, it is thought that they perpetuate glia activation by an autocrine or paracrine process, leading to inflammatory amplification, tissue damage, and eventually neuronal loss (Sofroniew 2009; Sticozzi et al. 2013). NF-κB plays a key role in astrocyte reaction (Sofroniew 2009; Sticozzi et al. 2013). In our SCI model, activated NF-κB was not found in spinal astrocytes. These results indicated that the effect of FK506 on the reduced production of cytokines, at least in our model, is not due to NF-κB inhibition in astrocytes. However, a change in activation of astrocytes may deserve a more detailed analysis in a future study. This may, in fact, lead to the disclosure of further neuroendocrine pathways to release cytokines involved in SCI.
In conclusion, we found that FK-506 prevents the activation of NF-κB in microglia, which is consistent with the results obtained from the reduced production of proinflammatory cytokines in the SCI response. There is preliminary evidence supporting an inhibitory effect of FK506 on NF-κB in the microglia in the spinal cord after SCI, which agrees with previous reports in other cells (Vafadari et al. 2013). Thus, FK506 treatment may be useful in the management of patients with SCI.
References
Akira S, Hirano T, Taga T, Kishimoto T (1990) Biology of multifunctional cytokines: IL 6 and related molecules (IL 1 and TNF). FASEB J 4:2860–2867
Bareyre FM, Schwab ME (2003) Inflammation, degeneration and regeneration in the injured spinal cord: insights from DNA microarrays. Trends Neurosci 26:555–563
Barnes PJ, Karin M (1997) Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med 336:1066–1071
Bartholdi D, Schwab ME (1997) Expression of pro-inflammatory cytokine and chemokine mRNA upon experimental spinal cord injury in mouse: an in situ hybridization study. Eur J Neurosci 9:1422–1438
Baskar Jesudasan SJ, Todd KG, Winship IR (2014) Reduced inflammatory phenotype in microglia derived from neonatal rat spinal cord versus brain. PLoS One 9:e99443
Bethea JR, Dietrich WD (2002) Targeting the host inflammatory response in traumatic spinal cord injury. Curr Opin Neurol 15:355–360
Bethea JR, Castro M, Keane RW, Lee TT, Dietrich WD, Yezierski RP (1998) Traumatic spinal cord injury induces nuclear factor-kappaB activation. J Neurosci 18:3251–3260
Carlson GD, Gorden C (2002) Current developments in spinal cord injury research. Spine J 2:116–128
Chengke L, Weiwei L, Xiyang W, Ping W, Xiaoyang P, Zhengquan X, Hao Z, Penghui Z, Wei P (2013) Effect of infliximab combined with methylprednisolone on expressions of NF-κB, TRADD, and FADD in rat acute spinal cord injury. Spine 3814:E861–E869
Diaz-Ruiz A, Vergara P, Perez-Severiano F, Segovia J, Guizar-Sahagun G, Ibarra A, Rios C (2005) Cyclosporin-A inhibits constitutive nitric oxide synthase activity and neuronal and endothelial nitric oxide synthase expressions after spinal cord injury in rats. Neurochem Res 30:245–251
Du S, Hiramatsu N, Hayakawa K, Kasai A, Okamura M, Huang T, Yao J, Takeda M, Araki I, Sawada N, Paton AW, Paton JC, Kitamura M (2009) Suppression of NF-kappaB by cyclosporin a and tacrolimus (FK506) via induction of the C/EBP family: implication for unfolded protein response. J Immunol 182:7201–7211
Farina C, Aloisi F, Meinl E (2007) Astrocytes are active players in cerebral innate immunity. Trends Immunol 28:138–145
Goldshmit Y, Frisca F, Pinto AR, Pébay A, Tang JK, Siegel AL, Kaslin J, Currie PD (2014) Fgf2 improves functional recovery-decreasing gliosis and increasing radial glia and neural progenitor cells after spinal cord injury. Brain Behav 4:187–200
Green HF, Nolan YM (2012) GSK-3 mediates the release of IL-1beta, TNFalpha and IL-10 from cortical glia. Neurochem Int 61:666–671
Hu JZ, Huang JH, Xiao ZM, Li JH, Li XM, Lu HB (2013) Tetramethylpyrazine accelerates the function recovery of traumatic spinal cord in rat model by attenuating inflammation. J Neurol Sci 324:94–99
Hua K, Schindler MK, McQuail JA, Forbes ME, Riddle DR (2012) Regionally distinct responses of microglia and glial progenitor cells to whole brain irradiation in adult and aging rats. PLoS One 7:e52728
Kettenmann H, Hanisch UK, Noda M, Verkhratsky A (2011) Physiology of microglia. Physiol Rev 91:461–553
Lai AY, Todd KG (2006) Hypoxia-activated microglial mediators of neuronal survival are differentially regulated by tetracyclines. Glia 53:809–816
Lai AY, Todd KG (2008) Differential regulation of trophic and proinflammatory microglial effectors is dependent on severity of neuronal injury. Glia 56:259–270
Lambertsen KL, Clausen BH, Babcock AA, Gregersen R, Fenger C, Nielsen HH, Haugaard LS, Wirenfeldt M, Nielsen M, Dagnaes-Hansen F, Bluethmann H, Faergeman NJ, Meldgaard M, Deierborg T, Finsen B (2009) Microglia protect neurons against ischemia by synthesis of tumor necrosis factor. J Neurosci 29:131913–131930
Li Q, Verma IM (2002) NF-kappaB regulation in the immune system. Nat Rev Immunol 2:725–734
Liberto CM, Albrecht PJ, Herx LM, Yong VW, Levison SW (2004) Pro-regenerative properties of cytokine-activated astrocytes. J Neurochem 89:1092–1100
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C (T)) Method. Methods 25:402–408
Ni B, Cao Z, Liu Y (2013) Glycyrrhizin protects spinal cord and reduces inflammation in spinal cord ischemia-reperfusion injury. Int J Neurosci 123:745–751
Nikodemova M, Watters JJ (2012) Efficient isolation of live microglia with preserved phenotypes from adult mouse brain. J Neuroinflammation 9:147
Pan F, Chen A, Guo F, Zhu C, Tao F (2008) Effect of FK506 on expression of hepatocyte growth factor in murine spinal cord following peripheral nerve injury. J Huazhong Univ Sci Technolog Med Sci 28:159–162
Profyris C, Cheema SS, Zang D, Azari MF, Boyle K, Petratos S (2004) Degenerative and regenerative mechanisms governing spinal cord injury. Neurobiol Dis 15:415–436
Saganová K, Gálik J, Blaško J, Korimová A, Račeková E, Vanický I (2012) Immunosuppressant FK506: focusing on neuroprotective effects following brain and spinal cord injury. Life Sci 91:77–82
Smith PD, Puskas F, Meng X, Lee JH, Cleveland JC Jr, Weyant MJ, Fullerton DA, Reece TB (2012) The evolution of chemokine release supports a bimodal mechanism of spinal cord ischemia and reperfusion injury. Circulation 126(11 Suppl 1):S110–S117
Sofroniew MV (2009) Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci 32:638–647
Sticozzi C, Belmonte G, Meini A, Carbotti P, Grasso G, Palmi M (2013) IL-1β induces GFAP expression in vitro and in vivo and protects neurons from traumatic injury-associated apoptosis in rat brain striatum via NFκB/Ca2+-calmodulin/ERK mitogen-activated protein kinase signaling pathway. Neuroscience 252:367–383
Su L, Ji J, Bian J, Fu Y, Ge Y, Yuan Z (2012) Tacrolimus (FK506) prevents early retinal neovascularization in streptozotocin-induced diabetic mice. Int Immunopharmacol 14:606–612
Thuret S, Moon LD, Gage FH (2006) Therapeutic interventions after spinal cord injury. Nat Rev Neurosci 7:628–643
Vafadari R, Kraaijeveld R, Weimar W, Baan CC (2013) Tacrolimus inhibits NF-κB activation in peripheral human T cells. PLoS One. 8(4):e60784
Wang YF, Zu JN, Li J, Chen C, Xi CY, Yan JL (2014) Curcumin promotes the spinal cord repair via inhibition of glial scar formation and inflammation. Neurosci Lett 560:51–56
Wei HY, Ma X (2014) Tamoxifen reduces infiltration of inflammatory cells, apoptosis and inhibits IKK/NF-κB pathway after spinal cord injury in rats. Neurol Sci 35(11):1763–1768. doi:10.1007/s10072-014-1828-z
Werry EL, Liu GJ, Lovelace MD, Nagarajah R, Hickie IB, Bennett MR (2011) Lipopolysaccharide-stimulated interleukin-10 release from neonatal spinal cord microglia is potentiated by glutamate. Neuroscience 175:93–103
Yang L, Jones NR, Blumbergs PC, Van Den Heuvel C, Moore EJ, Manavis J, Sarvestani GT, Ghabriel MN (2005) Severity-dependent expression of pro-inflammatory cytokines in traumatic spinal cord injury in the rat. J Clin Neurosci 12:276–284
Yang Z, Zhao TZ, Zou YJ, Zhang JH, Feng H (2014) Hypoxia induces autophagic cell death through hypoxia-inducible factor 1α in microglia. PLoS One 9:e96509
Yenari MA, Kauppinen TM, Swanson RA (2010) Microglial activation in stroke: therapeutic targets. Neurother J Am Soc Exp Neuro Ther 7:378–391
Yin X, Yin Y, Cao FL, Chen YF, Peng Y, Hou WG, Sun SK, Luo ZJ (2012) Tanshinone IIA attenuates the inflammatory response and apoptosis after traumatic injury of the spinal cord in adult rats. PLoS One 7:e38381
Young W (1993) Secondary injury mechanisms in acute spinal cord injury. J Emerg Med 11(Suppl 1):13–22
Yousuf S, Atif F, Kesherwani V, Agrawal SK (2011) Neuroprotective effects of Tacrolimus (FK-506) and Cyclosporin (CsA) in oxidative injury. Brain Behav 1:87–94
Zawadzka M, Kaminska B (2005) A novel mechanism of FK506-mediated neuroprotection: downregulation of cytokine expression in glial cells. Glia 49:36–51
Zhang Z, Krebs CJ, Guth L (1997) Experimental analysis of progressive necrosis after spinal cord trauma in the rat: etiological role of the inflammatory response. Exp Neurol 143:141–152
Zhang J, Zhang A, Sun Y, Cao X, Zhang N (2009) Treatment with immunosuppressants FTY720 and tacrolimus promotes functional recovery after spinal cord injury in rats. Tohoku J Exp Med 219:295–302
Zhang X, Zeng L, Yu T, Xu Y, Pu S, Du D, Jiang W (2014) Positive feedback loop of autocrine BDNF from microglia causes prolonged microglia activation. Cell Physiol Biochem 34:715–723
Zhou N, Fu Y, Wang Y, Chen P, Meng H, Guo S, Zhang M, Yang Z, Ge Y (2014) p27 kip1 haplo-insufficiency improves cardiac function in early-stages of myocardial infarction by protecting myocardium and increasing angiogenesis by promoting IKK activation. Sci Rep 7(4):5978
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Liu, G., Fan, G., Guo, G. et al. FK506 Attenuates the Inflammation in Rat Spinal Cord Injury by Inhibiting the Activation of NF-κB in Microglia Cells. Cell Mol Neurobiol 37, 843–855 (2017). https://doi.org/10.1007/s10571-016-0422-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-016-0422-8