Abstract
Ginsenosides are the major active components of ginseng, which have been proven to be effective in therapies for neurodegenerative diseases. Ginsenoside Rb1 (GS-Rb1) is the most abundant among all the identified ginsenosides and has been shown to exert neuroprotective effects, although the underlying molecular mechanisms remain unclear. Connexins are a family of transmembrane proteins that form gap junctions, which are important for diffusion of cytosolic factors such as ions and second messenger signaling molecules. Previous studies have shown that a subset of connexin proteins is involved in neuroprotection. We investigated the protective effects of GS-Rb1 against traumatic brain injury (TBI) and the potential mechanism using TBI mouse model. We discovered that TBI-induced brain injury and up-regulation of connexin40 (Cx40) protein expression as early as 6 h post-TBI, which was reversed by administration of GS-Rb1. In addition, we found that the protective effects of GS-Rb1 are dose and time dependent and are partially mediated through phosphorylation of ERK1/2 signaling pathway, as evidenced by the abolishment of GS-Rb1-mediated elevation of p-ERK1/2 expression and inhibition of Cx40 expressions when ERK inhibitor U0126 was used. Our study provides evidence that Cx40 is implicated in TBI-induced brain injuries, and GS-Rb1 exerts neuroprotective activity against TBI involving down-regulation of Cx40 expression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ginseng, the root of Panax ginseng C.A. Meyer, has been widely used for over 2000 years in east Asia and has been gaining popularity in the west during the past decades (Cho 2012; Park et al. 2012). Ginsenosides, the major active components of ginseng, have been demonstrated to provide therapeutic value for treatment of neurodegenerative diseases (Cheng et al. 2005, 2013; Cho 2012). To date, more than 40 different ginsenosides have been identified and isolated from different species of ginseng (Cheng et al. 2005). Ginsenosides are generally categorized as protopanaxadiol (PPD), protopanaxatriol (PPT), and oleanane saponins, based on the chemical structure (Christensen 2009). Ginsenoside Rb1 (GS-Rb1), a member of PPT sub-family, is the most abundant among all the identified ginsenosides and has received wide attention due to its diverse biological activities (Cheng et al. 2013). It has been reported that GS-Rb1 exhibits neuroprotective effects on cortical neurons and dopaminergic neurons against glutamate toxicity (Chen et al. 2010; Radad et al. 2004), possesses suppressive effect on local inflammation in rats with cerebral ischemia as well as in a rat model of Alzheimer’s disease (Wang et al. 2011; Zhu et al. 2012), and protects against cerebral ischemia in rats by promoting neurogenesis (Gao et al. 2010). Other studies have provided evidence showing that GS-Rb1 can enhance nerve growth factor (NGF)-mediated neurite outgrowth of cultured chick embryonic dorsal root ganglia (Nishiyama et al. 1994), prevent MPP+-induced apoptosis in PC12 cells (Hashimoto et al. 2012), and improve spatial learning and increases hippocampal synaptophysin level in mice (Mook-Jung et al. 2001). All these studies suggest the potential therapeutic value of GS-Rb1 for treatment of neurological disorders. However, the mechanism by which GS-RB1 exerts its neuroprotective effects is not clear.
Gap junctions are composed of thousands of intercellular channels permeable to cytosolic factors, such as ions and second messenger signaling molecules (Smyth et al. 2014; Jara et al. 1995). Connexins (Cx) are widely distributed proteins that oligomerize into hexameric transmembrane channels termed connexons, which forms gap junction when coupled with connexons on the surface of neighboring cells (Smyth et al. 2014). Several pieces of evidence suggest that gap junction proteins play important roles in neuroprotection using different pharmacological as well as genetic approaches. However, the conclusions remain controversial (Contreras et al. 2004). There are several types of connexin proteins, such as Cx23, Cx43, and Cx40, among which Cx43 is the most widely studied (Smyth et al. 2014; Contreras et al. 2004). Cx43 has been reported to be involved in neuroprotection against reperfusion-induced injury, after global cerebral ischemia, etc. (Li et al. 2005; Davidson et al. 2013). As a close family member of Cx43, Cx40 has been shown to be necessary for recovery of ischemic hindlimb perfusion, and both Cx40 and Cx43 have been reported to be involved in atherosclerosis and ischemia–reperfusion injury (Morel and Kwak 2012; Fang et al. 2012). In addition, the absence of Cx40 gene polymorphism has been found in patients with unexplained cerebral ischemic events (Chaldoupi et al. 2009). However, the neuroprotective effects of Cx40 have not been investigated in detail.
In our study, we investigated the neuroprotective mechanism of GS-Rb1 post-traumatic brain injury (TBI) and the potential implication of Cx40 gap junction protein in this process.
Materials and Methods
Animals
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Committee on the Ethics of Animal Experiments of The People’s Hospital of Pu Dong New Area. All surgery was performed under sodium pentobarbital anesthesia, and all efforts were made to minimize suffering. Wistar rats were purchased from SLAC laboratory animal (Shanghai, China). TBI model was generated via CCI as described before with some modifications (Chen et al. 2003). Briefly, wistar rats were anesthetized with 4 % isoflurane in oxygen and placed in the stereotaxic frame. A 3.5-mm-diameter craniotomy was performed over the right parietal cortex between bregma and lambda, 1 mm lateral to the midline. A pneumatic piston impactor device (3 mm diameter, rounded tip) was used to induce injury in the brain at the center of the craniotomy at a depth of 1 mm (velocity 4.5 m/s). Rats were randomly divided into three groups: sham operation (Sham), vehicle injection (veh), and GS-Rb1 injection (GS-Rb1). Sham-operated mice underwent identical surgical procedures but did not receive a CCI. Immediately after TBI, rats were randomly subjected to receiving vehicle or GS-Rb1 via intraperitoneal injection. For each single experiment at every time point, six animals were used.
Brain Infarction Measurement
Rats from each group were subjected local infarct volume measurements, as previously described (Swanson et al. 1990). Briefly, after reperfusion, brains were removed and immediately frozen in isopentane cooled with dry ice, and serial sections from anterior to posterior were prepared as 2 mm slices. Samples were stained in 2 % 2,3,5-triphenyltetrazolium chloride (TTC, Sigma, St. Louis, MO, USA) for 20 min at 37 °C and then fixed in 10 % buffered formaldehyde for 24 h. After TTC staining, the infarcted brains were visualized as an area of unstained (white) tissue and measured using Image-Pro Plus 6.0 software. The data represent the percentage of infarct volume per ipsilateral hemisphere volume in the coronal slices.
Brain Water Content
Brain water content was measured using Hatashita’s wet–dry method (Hatashita et al. 1988). Briefly, rats were killed by cervical dislocation, and their brains were immediately removed and placed onto a frozen plate and then weighed to determine wet weight. Next, brains were dried in a desiccating oven at 110 °C for 24 h and weighed again to determine dry weight. Brain water content was calculated using the following formula: brain water content (%) = (wet weight − dry weight) × 100/wet weight.
Neurological Score
Neurological deficit was evaluated using the Neurological Severity Score (NSS) on a 10-point scale according to Chen’s method (Chen et al. 1996) by a researcher blinded to treatment. On this scale, one point represents failure of one of 10 tasks, such that the maximum score of 10 points indicates severe neurological dysfunction, whereas 0 point indicates normal function.
Western Blot Analysis
Western blot analysis on brain tissue was performed as previously described (Deng et al. 2014). Briefly, whole cell lysate from the tissues was extracted using RIPA lysis buffer (Santa Cruz) containing 1 % protease inhibitor cocktail. For electrophoresis, a total of 30 µg of protein were loaded onto a 12 % SDS-PAGE gel. After transfer, membranes were blocked in 5 % non-fat milk in Tris-buffered saline (TBS)/Tween-20 (0.2 %) overnight at 4 °C and then incubated with rabbit polyclonal antibodies against Cx40 (1:500, Santa Cruz), p-ERK1/2 (Y204, 1:500, Santa Cruz), ERK1/2 (H197, 1:500, Santa Cruz), and GAPDH (1:2000, Santa Cruz) diluted in TBS/T for 1 h at 37 °C. After three washes in TBS/T, the membranes were incubated with anti-rabbit IgG conjugated to HRP (Zhongshan Golden Bridge Biotechnology) at a dilution of 1:2000 in TBS/T for 40 min at 37 °C. The immunoreactive bands were visualized with an enhanced chemiluminescence kit (Zhongshan Golden Bridge Biotechnology).
RT-PCR
Non-fixed brains from injured or sham animals were removed after cervical dislocation 6, 12, 24, 48, and 72 h following injury or sham surgery. A 3-mm coronal section was taken from the injured area or the contralateral hemisphere over the parietal cortex, snap-frozen in liquid nitrogen, and stored at −70 °C until use. RT-PCR was performed as previously described (Hung et al. 2006). The total was extracted using RNeasy Mini Kit (Qiagen, CA, USA). Then 1 µg RNA was converted to cDNA using a First Strand cDNA Synthesis Kit (Roche). Real-time quantitative PCR reactions were set up in triplicate with Ssofast Master Mix (Biorad) and run on a LightCycler® 480 (Roche). The following primers were used in the current study: Cx40: (Fwd: TTT GGC AAG TCA CGG CAG GG, Rev: TTG TCA CTG TGG TAG CCC TGA GG); GAPDH: (Fwd: GGC ACA GTC AAG GCT GAG AAT G, Rev: ATG GTG GTG AAG ACG CCA GTA).
Statistical Analysis
Statistical analysis was performed using SPSS 16.0 software. Data are presented as mean ± standard error of the mean (SEM). Two-way analysis of variance (ANOVA) followed by Tukey’s post hoc test was used to determine between-group differences. Statistical difference was considered to be significant only if p < 0.05.
Results
TBI-Induced Brain Injuries and Up-Regulated Cx40 Expression
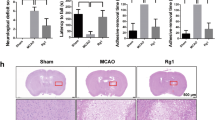
Consistent with previous reports, we found that TBI-induced brain injuries gradually within 24 h after TBI induction as demonstrated by increased brain infarction volume, brain water content, and neurological deficiency (Fig. 1a–c). These effects leveled off with moderate recovery between 24 and 72 h. Importantly, we observed concomitant up-regulation of Cx40 in both mRNA and protein levels as early as 6 h after TBI induction (Fig. 1d–f) and slight decrease between 24 and 72 h post injury, suggesting that Cx40 is involved in TBI-induced brain injuries.
Brain injuries and Cx40 mRNA and protein expression in cortex at indicated time after TBI (6, 12, 24, 48, and 72 h). Brain injuries are characterized by brain infarction volume (a), brain water content (b), and neurological severity score (c). The mRNA expressions of Cx40 in the cortex of different experimental groups were analyzed by RT-PCR (d). Cx40 protein expression was analyzed by Western blotting (e), while the relative optical densities of Cx40 normalized to GAPDH were shown (f). Six animals were used for each time point. Data are presented as mean ± SEM, *p < 0.05 and # p < 0.01 versus sham control
Dose-Dependent Protective Effects of GS-Rb1 Against TBI-Induced Brain Injuries
Previous studies reported that GS-Rb1 exhibited dose-dependent effects on angiogenesis, neural plasticity and apoptosis, etc. (Cheng et al. 2005, 2013; Kimura et al. 2012). We set out to evaluate the potential dose-dependent protective effects of GS-Rb1 at four different doses. As shown in Fig. 2, 20 and 40 mg/kg of GS-Rb1 showed maximum recovery from TBI-induced brain injuries, while 5 mg/kg GS-Rb1 treatment failed to induce significant differences compared to vehicle administration, as evidenced by brain infarction volume, brain water content and neurological severity scores. Therefore, we used 20 mg/kg for the following study.
Dose-dependent protective effects of GS-Rb1 against TBI-induced brain injuries. Animals were tested and samples were collected at 24 h post-TBI. Brain infarction volume (a), brain water content (b), and neurological severity score (c) were analyzed in the rats treated by saline (veh) or different dose of GS-Rb1 (5, 10, 20 and 40 mg/kg). Six animals were used for each experimental group. Data are presented as mean ± SEM. *p < 0.05 and # p < 0.01 versus sham control; + p < 0.05 and ^ p < 0.01 versus veh control
Cx40 was Implicated in GS-Rb1-Mediated Neuroprotective Effects Against TBI-Induced Brain Injury
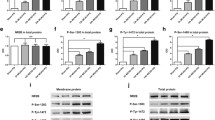
We next investigated time-dependent protective effects of GS-Rb1 on TBI-induced brain injury. As shown in Fig. 3a–c, 20 mg/kg of GS-RB1 significantly reduced brain infarction volume, brain water content, and neurological deficit as early as 6 h post-TBI, and these protective effects persisted till 72 h post-TBI. Concomitantly, we observed a decrease of mRNA as well as protein levels of Cx40 in the same time-dependent manner (Fig. 3d–f), suggesting that Cx40 is implicated in GS-Rb1-mediated neuroprotective effects against TBI-induced brain injury. Furthermore, to investigate the possible correlation between Cx40 expression and brain injury, Cx40 expression after TBI to brain infarction volume was compared by using Spearman’s correlation coefficient (R), and the result (Fig. 3g, R = 0.8623) suggested Cx40 expression was indeed positively correlated with the brain infarction volume.
GS-Rb1 (20 mg/kg) significantly attenuated TBI-induced brain injuries and Cx40 mRNA and protein expression in the cortex at different time points post-TBI (6, 12, 24, 48, and 72 h). Brain infarction volume (a), brain water content (b), and neurological severity score (c) were examined in veh and 20 mg/kg GS-Rb1 treated groups at indicated time. The mRNA expressions of Cx40 in the cortex of veh and 20 mg/kg GS-Rb1 groups were analyzed by RT-PCR (d). Cx40 protein expression was analyzed by Western blotting (e), while the relative optical densities of Cx40 normalized to GAPDH were shown in (f). g Correlation study of Cx40 expression and brain infarction volume by using Spearman’s correlation coefficient. Six animals were used for each single experiment at every time point. Data are presented as mean ± SEM, + p < 0.05 and ^ p < 0.01 versus veh group
The ERK1/2 Signaling Pathway was Involved in the Protective Effects of GS-Rb1 on TBI-Induced Brain Injuries
Phospho-ERK has been reported to be involved in neuroprotective activity of leptin against brain ischemic injury (Deng et al. 2014). To elucidate the mechanism of neuroprotective effects of GS-Rb1, the expressions of p-ERK1/2 and Cx40 were assessed in the presence and absence of ERK inhibitor, U0126 (500 μg/kg, intravenous injection). p-ERK1/2 expression was notably decreased in brains compared with that of sham group 6 h post-TBI, which was partially reversed by GS-Rb1 administration (Fig. 4a). Cx40 expression was significantly elevated in brains after TBI, and GS-Rb1 treatment was able to reverse this elevation (Fig. 4b). U0126 treatment immediately after TBI greatly abolished the GS-Rb1-induced elevation of p-ERK1/2 expression and largely blocked the GS-Rb1-mediated inhibition of Cx40 expression, suggesting the activation of ERK1/2 is essential for GS-Rb1-mediated neuroprotection against brain injuries.
The ERK1/2 signaling pathway was involved in the protective effects of GS-Rb1 on TBI-induced brain injuries. The animals were treated by 20 mg/kg GS-Rb1 and sacrificed for Western blot analysis at 6 h post-TBI. U0126 was used as inhibitor for ERK signaling pathway. a Representative Western blot bands of pERK1 and total ERK1/2 in sham-, veh-, 20 mg/kg GS-Rb1-treated groups and co-treatment of 20 mg/kg GS-Rb1 and U0126 group, and relative optical density of pERK1/2 to total ERK1/2. b Western blot analysis and relative optical density of Cx40 expression in the experimental groups. GAPDH was used as control. Data are presented as mean ± SEM, *p < 0.05 and # p < 0.01 versus sham control, ^ p < 0.01 versus veh group < 0.01 versus GS-Rb1-treated group
Discussion
TBI represents brain dysfunction resulting from external mechanical forces of shearing, tearing or stretching (Wang et al. 2015). It induces contusion, hemorrhage, and a series of complex inflammatory responses that lead to secondary injuries (Wang et al. 2015; Chen et al. 2007). Neural inflammation is associated with activation of microglia, which can release various neurotoxic substances that may contribute to neuronal death after TBI (Kreutzberg 1996). TBI-induced inflammatory cascade also results in the release of various pro- and anti-inflammatory cytokines, among which interleukin-1 beta (IL-1β) and tumor necrosis factor alpha (TNF-α) are the two well-characterized pro-inflammatory cytokines that elevate after TBI (Taupin et al. 1993). Inhibition of TBI-induced expression of IL-1β and TNF-α has been shown to exhibit neuroprotective effects in mice and rats (Wang et al. 2015).
GS-Rb1 is the major active component of ginseng extracts, which exerts multiple biological functions including anti-inflammatory, anti-apoptosis, and neuroprotective activities (Cheng et al. 2013). It has been reported that GS-Rb1 can prevent IL-1β-induced inflammation and apoptosis in human articular chondrocytes, and reduce inflammation in obese mice fed on high-fat diet through regulation of inflammatory and apoptotic genes (Cheng et al. 2013; Wu et al. 2014). It has also been reported to protect neural progenitor cells against oxidative injury in rats by activating Nrf2 pathway (Ni et al. 2014). More importantly, GS-Rb1 has been shown to exert neuroprotective activities in cortical neurons and dopaminergic neurons and in rats with cerebral ischemia, partially through regulation of local inflammatory responses, apoptotic machinery, and promotion of neurogenesis (Chen et al. 2010; Radad et al. 2004; Wang et al. 2011; Zhu et al. 2012; Gao et al. 2010). In our present study, we demonstrated that administration of GS-Rb1 decreased TBI-induced brain infarct volume in rats and attenuated TBI-induced brain edema and neuronal deficit. These results suggest that GS-Rb1 exerts neuroprotective activities against TBI.
It has been reported that GS-Rb1 in orally administered ginseng is metabolized to bioactive compound K before being absorbed into the blood stream (Kim et al. 2013). These metabolizing activities are significantly different between individuals depending on the composition of gut microbiota (Kim et al. 2013). Other reports also showed that different concentrations of GS-Rb1 exerted differential effects on wound healing or angiogenesis after administration (Cho et al. 2015; Kimura et al. 2012). These reports suggest that the biological actions of GS-Rb1 are dose dependent. In agreement with previous reports, our data showed that the neuroprotective effects of GS-Rb1 against TBI were also indeed dose dependent, with 20 and 40 mg/kg being the optimal dosages in our animal model.
Connexin proteins are integral components that form hemichannels or gap junction complexes which allow for exchange of ions and small molecules (Smyth et al. 2014). Gap junction channels play important roles in brain damage in vitro as well in vivo (Contreras et al. 2004). It is proposed that detrimental molecules can spread from more injured to less-injured cells to harm the latter when coupled cells are subjected to stress or injury; similarly, healthy molecules can spread the other way around. The ultimate consequences depend on the balance of these two actions (Contreras et al. 2004). A growing body of evidence showed that gap junction channels contribute to ischemic brain injury, although the conclusions remain controversial (Contreras et al. 2004). The first evidence supporting the role of gap junctions in mediating spread of damage in CNS was that octanol, a gap junction blocker, reduced infarct volume after focal ischemia (Rawanduzy et al. 1997). A recent study showed that blockage of connexin hemichannel was neuroprotective after global cerebral ischemia in near-term fetal sheep (Davidson et al. 2013). In the rat brain, down-regulation of specific connexins reduced neuronal cell death in a global ischemia model (Frantseva et al. 2002a). There are several family members of connexin proteins, among which Cx43 is one of the best characterized. It has been shown that Cx43 exerted detrimental effects in models of focal ischemia and other neurodegenerative conditions (Schulz et al. 2015). Brain slices from Cx43 deficient mice exhibited markedly reduced neuronal cell death (Frantseva et al. 2002b). Inhibition of Cx43 has been shown to be involved in leptin mediated neuroprotection against brain ischemic injury (Deng et al. 2014). Although being a close family member of Cx43, the potential involvement of Cx40 in neuroprotection is poorly investigated. In our study, we observed an up-regulation of Cx40 as early as 6 h post-TBI which persisted till 72 h post-TBI. Administration of GS-Rb1 reversed these effects, suggesting that Cx40 is involved in GS-Rb1 mediated neuroprotection. This is the first evidence that Cx40 may be implicated in neuroprotection. However, it remains to be determined how Cx40 expression is up-regulated in response to GS-Rb1 treatment and how this up-regulation confers neuroprotective activities. Of note, although our study does not provide direct evidence showing whether GS-Rb1 blocks hemichannels or gap junction channels, we think GS-Rb1 is almost impossible to block this type of channels. First of all, hemichannels are transported to the surface membrane after assembly, and they remain closed until docking with a hemichannel in an apposed membrane. Second, gap junction channels connect cells via channels not open to the extracellular space.
Many connexin proteins, including Cx40, have been demonstrated to be phosphoproteins (Solan and Lampe 2005). Previous reports demonstrated that activation of MEK/ERK signaling pathways may be involved in leptin mediated neuroprotection by regulation of Cx43 expression (Deng et al. 2014; Weng et al. 2007). In addition, Cx40 has been shown to be the major target involved in lipopolysaccharide-induced decrease in electrical coupling in microvascular endothelial cells in an ERK1/2-dependent manner (Bolon et al. 2007). Our results showed that TBI induced a decrease in phosphorylation of ERK1/2, whereas GS-Rb1 significantly up-regulated ERK1/2 phosphorylation post-TBI, supporting an important role of ERK1/2 signaling pathway in GS-Rb1 mediated neuroprotection against TBI, potentially through regulation of Cx40 expression. Meanwhile, when U0126, an ERK pathway inhibitor, was administrated immediately after TBI, it was able to greatly abolish the GS-Rb1-induced elevation of p-ERK1/2 expression and largely block the GS-Rb1-mediated inhibition of Cx40 expression, strongly indicating that the activation of ERK1/2 is essential for GS-Rb1-mediated neuroprotection against TBI. Further research is needed to unravel the detailed link between activation of ERK signaling pathway and Cx40 expression.
In conclusion, our study shows a positive relationship between TBI-induced brain injuries and Cx40 expression. Also, we demonstrated neuroprotective effects of GS-Rb1 against TBI and provided the first evidences that expression of Cx40 may be involved in GS-Rb1 mediated neuroprotection. GS-Rb1 may represent a valuable therapeutic reagent for clinical treatment of TBI.
References
Bolon ML, Kidder GM, Simon AM, Tyml K (2007) Lipopolysaccharide reduces electrical coupling in microvascular endothelial cells by targeting connexin40 in a tyrosine-, ERK1/2-, PKA-, and PKC-dependent manner. J Cell Physiol 211(1):159–166. doi:10.1002/jcp.20928
Chaldoupi SM, Soedamah-Muthu SS, Regieli J, Werf C, Nelen M, van der Smagt JJ, Algra A, Hauer RN, Doevendans PA, Loh P (2009) Absence of connexin 40 gene polymorphism, as a marker of undetected atrial fibrillation in patients with unexplained cerebral ischemic events. Eur J Cardiovasc Prev Rehabil 16(5):616–620. doi:10.1097/HJR.0b013e32832da03a
Chen Y, Constantini S, Trembovler V, Weinstock M, Shohami E (1996) An experimental model of closed head injury in mice: pathophysiology, histopathology, and cognitive deficits. J Neurotrauma 13(10):557–568
Chen S, Pickard JD, Harris NG (2003) Time course of cellular pathology after controlled cortical impact injury. Exp Neurol 182(1):87–102
Chen SF, Hung TH, Chen CC, Lin KH, Huang YN, Tsai HC, Wang JY (2007) Lovastatin improves histological and functional outcomes and reduces inflammation after experimental traumatic brain injury. Life Sci 81(4):288–298. doi:10.1016/j.lfs.2007.05.023
Chen Z, Lu T, Yue X, Wei N, Jiang Y, Chen M, Ni G, Liu X, Xu G (2010) Neuroprotective effect of ginsenoside Rb1 on glutamate-induced neurotoxicity: with emphasis on autophagy. Neurosci Lett 482(3):264–268. doi:10.1016/j.neulet.2010.07.052
Cheng Y, Shen LH, Zhang JT (2005) Anti-amnestic and anti-aging effects of ginsenoside Rg1 and Rb1 and its mechanism of action. Acta Pharmacol Sin 26(2):143–149. doi:10.1111/j.1745-7254.2005.00034.x
Cheng W, Wu D, Zuo Q, Wang Z, Fan W (2013) Ginsenoside Rb1 prevents interleukin-1 beta induced inflammation and apoptosis in human articular chondrocytes. Int Orthop 37(10):2065–2070. doi:10.1007/s00264-013-1990-6
Cho IH (2012) Effects of Panax ginseng in Neurodegenerative Diseases. J Ginseng Res 36(4):342–353. doi:10.5142/jgr.2012.36.4.342
Cho YL, Hur SM, Kim JY, Kim JH, Lee DK, Choe J, Won MH, Ha KS, Jeoung D, Han S, Ryoo S, Lee H, Min JK, Kwon YG, Kim DH, Kim YM (2015) Specific activation of insulin-like growth factor-1 receptor by ginsenoside Rg5 promotes angiogenesis and vasorelaxation. J Biol Chem 290(1):467–477. doi:10.1074/jbc.M114.603142
Christensen LP (2009) Ginsenosides chemistry, biosynthesis, analysis, and potential health effects. Adv Food Nutr Res 55:1–99. doi:10.1016/S1043-4526(08)00401-4
Contreras JE, Sanchez HA, Veliz LP, Bukauskas FF, Bennett MV, Saez JC (2004) Role of connexin-based gap junction channels and hemichannels in ischemia-induced cell death in nervous tissue. Brain Res Brain Res Rev 47(1–3):290–303. doi:10.1016/j.brainresrev.2004.08.002
Davidson JO, Green CR, Nicholson LF, Bennet L, Gunn AJ (2013) Connexin hemichannel blockade is neuroprotective after, but not during, global cerebral ischemia in near-term fetal sheep. Exp Neurol 248:301–308. doi:10.1016/j.expneurol.2013.06.026
Deng ZH, Liao J, Zhang JY, Liang C, Song CH, Han M, Wang LH, Xue H, Zhang K, Zabeau L, Tavernier J, Yan GT (2014) Inhibition of the connexin 43 elevation may be involved in the neuroprotective activity of leptin against brain ischemic injury. Cell Mol Neurobiol 34(6):871–879. doi:10.1007/s10571-014-0066-5
Fang JS, Angelov SN, Simon AM, Burt JM (2012) Cx40 is required for, and cx37 limits, postischemic hindlimb perfusion, survival and recovery. J Vasc Res 49(1):2–12. doi:10.1159/000329616
Frantseva MV, Kokarovtseva L, Naus CG, Carlen PL, MacFabe D, Perez Velazquez JL (2002a) Specific gap junctions enhance the neuronal vulnerability to brain traumatic injury. J Neurosci 22(3):644–653
Frantseva MV, Kokarovtseva L, Perez Velazquez JL (2002b) Ischemia-induced brain damage depends on specific gap-junctional coupling. J Cereb Blood Flow Metab 22(4):453–462. doi:10.1097/00004647-200204000-00009
Gao XQ, Yang CX, Chen GJ, Wang GY, Chen B, Tan SK, Liu J, Yuan QL (2010) Ginsenoside Rb1 regulates the expressions of brain-derived neurotrophic factor and caspase-3 and induces neurogenesis in rats with experimental cerebral ischemia. J Ethnopharmacol 132(2):393–399. doi:10.1016/j.jep.2010.07.033
Hashimoto R, Yu J, Koizumi H, Ouchi Y, Okabe T (2012) Ginsenoside Rb1 prevents MPP(+)-induced apoptosis in PC12 cells by stimulating estrogen receptors with consequent activation of ERK1/2, Akt and Inhibition of SAPK/JNK, p38 MAPK. Evid Based Complement Alternat Med 2012:693717. doi:10.1155/2012/693717
Hatashita S, Hoff JT, Salamat SM (1988) Ischemic brain edema and the osmotic gradient between blood and brain. J Cereb Blood Flow Metab 8(4):552–559. doi:10.1038/jcbfm.1988.96
Hung TH, Chen SF, Hsu JJ, Hsieh CC, Hsueh S, Hsieh TT (2006) Tumour necrosis factor-alpha converting enzyme in human gestational tissues from pregnancies complicated by chorioamnionitis. Placenta 27(9–10):996–1006. doi:10.1016/j.placenta.2005.11.002
Jara PI, Boric MP, Saez JC (1995) Leukocytes express connexin 43 after activation with lipopolysaccharide and appear to form gap junctions with endothelial cells after ischemia-reperfusion. Proc Natl Acad Sci USA 92(15):7011–7015
Kim KA, Jung IH, Park SH, Ahn YT, Huh CS, Kim DH (2013) Comparative analysis of the gut microbiota in people with different levels of ginsenoside Rb1 degradation to compound K. PLoS One 8(4):e62409. doi:10.1371/journal.pone.0062409
Kimura Y, Sumiyoshi M, Sakanaka M (2012) Effects of ginsenoside Rb(1) on skin changes. J Biomed Biotechnol 2012:946242. doi:10.1155/2012/946242
Kreutzberg GW (1996) Microglia: a sensor for pathological events in the CNS. Trends Neurosci 19(8):312–318
Li Z, Lin XM, Gong PL, Zeng FD, Du GH (2005) Effects of Gingko biloba extract on gap junction changes induced by reperfusion/reoxygenation after ischemia/hypoxia in rat brain. Am J Chin Med 33(6):923–934. doi:10.1142/S0192415X05003430
Mook-Jung I, Hong HS, Boo JH, Lee KH, Yun SH, Cheong MY, Joo I, Huh K, Jung MW (2001) Ginsenoside Rb1 and Rg1 improve spatial learning and increase hippocampal synaptophysin level in mice. J Neurosci Res 63(6):509–515
Morel S, Kwak BR (2012) Roles of connexins in atherosclerosis and ischemia-reperfusion injury. Curr Pharm Biotechnol 13(1):17–26
Ni N, Liu Q, Ren H, Wu D, Luo C, Li P, Wan JB, Su H (2014) Ginsenoside Rb1 protects rat neural progenitor cells against oxidative injury. Molecules 19(3):3012–3024. doi:10.3390/molecules19033012
Nishiyama N, Cho SI, Kitagawa I, Saito H (1994) Malonylginsenoside Rb1 potentiates nerve growth factor (NGF)-induced neurite outgrowth of cultured chick embryonic dorsal root ganglia. Biol Pharm Bull 17(4):509–513
Park HJ, Kim DH, Park SJ, Kim JM, Ryu JH (2012) Ginseng in traditional herbal prescriptions. J Ginseng Res 36(3):225–241. doi:10.5142/jgr.2012.36.3.225
Radad K, Gille G, Moldzio R, Saito H, Rausch WD (2004) Ginsenosides Rb1 and Rg1 effects on mesencephalic dopaminergic cells stressed with glutamate. Brain Res 1:41–53. doi:10.1016/j.brainres.2004.06.030
Rawanduzy A, Hansen A, Hansen TW, Nedergaard M (1997) Effective reduction of infarct volume by gap junction blockade in a rodent model of stroke. J Neurosurg 87(6):916–920. doi:10.3171/jns.1997.87.6.0916
Schulz R, Gorge PM, Gorbe A, Ferdinandy P, Lampe PD, Leybaert L (2015) Connexin 43 is an emerging therapeutic target in ischemia/reperfusion injury, cardioprotection and neuroprotection. Pharmacol Ther. doi:10.1016/j.pharmthera.2015.06.005
Smyth JW, Zhang SS, Sanchez JM, Lamouille S, Vogan JM, Hesketh GG, Hong T, Tomaselli GF, Shaw RM (2014) A 14-3-3 mode-1 binding motif initiates gap junction internalization during acute cardiac ischemia. Traffic 15(6):684–699. doi:10.1111/tra.12169
Solan JL, Lampe PD (2005) Connexin phosphorylation as a regulatory event linked to gap junction channel assembly. Biochim Biophys Acta 1711(2):154–163. doi:10.1016/j.bbamem.2004.09.013
Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR (1990) A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab 10(2):290–293. doi:10.1038/jcbfm.1990.47
Taupin V, Toulmond S, Serrano A, Benavides J, Zavala F (1993) Increase in IL-6, IL-1 and TNF levels in rat brain following traumatic lesion. Influence of pre- and post-traumatic treatment with Ro5 4864, a peripheral-type (p site) benzodiazepine ligand. J Neuroimmunol 42(2):177–185
Wang Y, Liu J, Zhang Z, Bi P, Qi Z, Zhang C (2011) Anti-neuroinflammation effect of ginsenoside Rbl in a rat model of Alzheimer disease. Neurosci Lett 487(1):70–72. doi:10.1016/j.neulet.2010.09.076
Wang K, Zhang L, Rao W, Su N, Hui H, Wang L, Peng C, Tu Y, Zhang S, Fei Z (2015) Neuroprotective effects of crocin against traumatic brain injury in mice: involvement of notch signaling pathway. Neurosci Lett 591:53–58. doi:10.1016/j.neulet.2015.02.016
Weng Z, Signore AP, Gao Y, Wang S, Zhang F, Hastings T, Yin XM, Chen J (2007) Leptin protects against 6-hydroxydopamine-induced dopaminergic cell death via mitogen-activated protein kinase signaling. J Biol Chem 282(47):34479–34491. doi:10.1074/jbc.M705426200
Wu Y, Yu Y, Szabo A, Han M, Huang XF (2014) Central inflammation and leptin resistance are attenuated by ginsenoside Rb1 treatment in obese mice fed a high-fat diet. PLoS One 9(3):e92618. doi:10.1371/journal.pone.0092618
Zhu J, Jiang Y, Wu L, Lu T, Xu G, Liu X (2012) Suppression of local inflammation contributes to the neuroprotective effect of ginsenoside Rb1 in rats with cerebral ischemia. Neuroscience 202:342–351. doi:10.1016/j.neuroscience.2011.11.070
Acknowledgments
This work was supported by the Health Planning Commission Project of Pu Dong New Area, Shanghai (No. PKJ2012-Y3) and the Health Planning Commission Project of Pu Dong New Area (No. PWZz-2013-13).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors (Wei Chen, Yijun Guo, Wenjin Yang, Ping Zheng, Jinsong Zeng and Wusong Tong) declare there is no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Chen, W., Guo, Y., Yang, W. et al. Involvement of Connexin40 in the Protective Effects of Ginsenoside Rb1 Against Traumatic Brain Injury. Cell Mol Neurobiol 36, 1057–1065 (2016). https://doi.org/10.1007/s10571-015-0299-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-015-0299-y