Abstract
Patients with high-grade gliomas and glioblastomas (GBMs) have poor survival despite optimal surgical and drug therapy. Minimally invasive diagnostic biomarkers would enable early diagnosis and tumor-specific treatments for ‘personalized targeted’ therapy, and would create the basis for response tracking in patients with GBM. Extracellular vesicles (EVs) isolated from cerebrospinal fluid and blood contain glioma-specific molecules, including tumor-derived EV RNAs that are detectable in small copy numbers in these biofluids. EV RNA mutations or expression changes are also detectable, the analysis of which gives rise to ‘liquid biopsy’ tumor profiling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glioblastoma (GBM) is the most aggressive primary brain tumor. Despite advances in radiation therapy and chemotherapeutic agents, fewer than one in five patients survive two years from diagnosis (Darefsky et al. 2012). This poor survival reflects tumor-induced angiogenesis, cellular invasion of surrounding brain, and tumor-derived immune suppression (Ricci-Vitiani et al. 2010; Wang et al. 2010; Bonavia et al. 2011). GBMs are molecularly heterogeneous, which limits the effectiveness of standardized therapies. In the past few years, GBM heterogeneity has come under scrutiny, beginning with the identification of four molecular subtypes (Verhaak et al. 2010). These subtypes termed “proneural,” “classical,” “mesenchymal,” and “neural” have unique gene signatures based on amplified expression of wild-type genes or mutations in tumor-related genes. Specifically, the “proneural” subtype is defined by focal amplifications in the tyrosine kinase cell surface growth factor receptor platelet-derived growth factor receptor alpha (PDGFRA), and point mutations in the enzyme isocitrate dehydrogenase 1 (IDH1), a catalyst for production of the anti-oxidative molecule NADPH (nicotinamide adenine dinucleotide phosphate). The “classical” subtype is associated with amplifications and mutations in the cell surface tyrosine kinase receptor epidermal growth factor receptor (EGFR), a critical player in cell differentiation and proliferation. The “mesenchymal” subtype possesses high rates of mutations in the gene neurofibromin 1 (NF1), a negative regulator of Ras signaling pathways that promote cell growth and division, and the neural subtype expresses high levels of neuronal markers, including neurofilament light polypeptide (NEFL), the neurotransmitter receptor GABRA1 (gamma-aminobutyric acid A receptor alpha 1), the synaptic vesicle protein synaptotagmin 1 (SYT1), and the solute transporter critical for neuronal chloride equilibrium SLC12A5 (solute carrier family 12 potassium/chloride transporter, member 5) (Verhaak et al. 2010). These GBM molecular subtypes differ in their response to therapy and prognosis (Verhaak et al. 2010). As a result, early identification of subtype conveys obvious clinical utility. In addition, there have been identified other clinically relevant GBM subdivisions based on the expression of overlapping and novel mutations such as the EGFR mutation variant EGFRvIII, IDH1.132, and the mutation-associated CpG island methylator phenotype (G-CIMP; glioma-CpG island methylator phenotype) (Noushmehr et al. 2010; Wong et al. 1992; Heimberger et al. 2005; Bleeker et al. 2010). Such classifications are likely still an oversimplification of GBM complexity, as high-resolution analytics has revealed intra-tumoral heterogeneity of both bulk tissue and individual cells (Patel et al. 2014; Sottoriva et al. 2013), as well as within GBM stem cells (Beier et al. 2007; Beier et al. 2012; Lottaz et al. 2010). These discoveries partially explain the basis for the variable response of many GBM patients to standardized treatments, and may explain the acquired resistance to therapy. Most importantly, they open the door for the creation of therapies tailored to tumor-specific characteristics and for indices which provide a roadmap for changes in therapeutic directions. Unfortunately, these individualized therapies cannot be based upon sequential sampling of brain tumors. The ability to perform sequential longitudinal ‘liquid biopsies’ of minimally invasive biofluids would profoundly alter GBM diagnosis and treatment.
Clinical Rationale for Glioblastoma Diagnostic Biomarkers
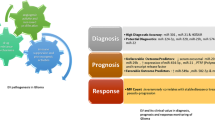
An ideal GBM biomarker would provide a specific early diagnosis, inform the molecular subtyping of the tumor, serve as a risk stratifier for the patient, and guide the clinician to appropriate therapies for downstream targets, as well as provide a template for changes in therapy. Patient care would then include tailored therapies, and provide a novel metric of response to therapy. These biomarkers will improve the sensitivity (proportion of patients with GBM, diagnosed as such) and specificity (proportion of patients without GBM, identified as such) of evaluations of patient care and reduce the cost. Magnetic Resonance Imaging (MRI) is the most commonly used diagnostic technique for GBM. However, MRI can miss early lesions (Chittiboina et al. 2012) and has a specificity ranging from only 50 to 80 % for distinguishing GBM from other intracranial lesions such as low-grade gliomas, lymphomas, and metastases (Weber et al. 2006). MRI is also incapable of providing information on molecular subtype. Biopsies performed after MRI provide tissue upon which histopathologic diagnoses are based. Operations are attended by morbidity, feasible for tumors only in favorable locations, and provide tumor information at a single place in space and time that may not be representative of the evolving and molecularly heterogeneous tumor environment (Patel et al. 2014; Jackson et al. 2001; Nickel et al. 2012). Tissue evaluations, except for methylation status (wherein anti-tumor genes are inactivated by the addition of a methyl group to associated promoter regions) (Thon et al. 2013), have limited ability to predict chemotherapeutic resistance or to differentiate tumor progression from post-treatment necrosis (Sarkaria et al. 2008; Yip et al. 2009; Fischer et al. 2008; Rock et al. 2002).
The cost of treatment for GBM is also prohibitively high. Estimates for the cost of basic treatment for a primary malignant brain tumor are more than $6000 per month (Kutikova et al. 2007), with the bulk of these costs relating to inpatient hospitalizations and surgery. The addition of newer chemotherapeutic agents has demonstrated only modest increases in survival, yet cost approximately $50,000 per life year gained (Messali et al. 2013). Non-invasive GBM biomarkers are sorely needed, as they have the potential to provide inexpensive and molecularly detailed information with high sensitivity and specificity.
RNA EVs as Biomarkers for GBM
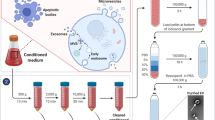
Extracellular nucleic acids are ideal biomarkers that provide detailed information about their cell of origin. They exist in multiple forms such as protein complexes, lipoprotein particles, and EVs, with EVs demonstrating the potential for selective molecular packaging and stability in the presence of degrading enzymes (Witwer et al. 2013). EVs are lipid membrane structures that range from 30 to 1000 nm in size, are released by all cells, and are key to multiple biologic processes including removal of cellular debris, intercellular signaling, and microenvironmental alterations (Gonda et al. 2013; Hochberg et al. 2014). EVs containing brain-derived proteins and lipids, in addition to RNA and DNA, have been isolated from blood and the cerebrospinal fluid, a demonstration that supports the trafficking of these vesicles out of the brain parenchyma. EVs are typically isolated via ultracentrifugation, filtration, or antibody-based aggregation (Gonda et al. 2013), and quantified using electron microscopy or proprietary laser or resistance pulse techniques (NanoSight, qNANO) (Gonda et al. 2013). The EVs from tumor cells contain tumor-specific molecules that are enriched relative to their cells of origin by up to 100-fold. These molecules include small RNAs such as non-coding RNA, microRNA (miRNA), and messenger RNA (mRNA). Early data support a role for tumor-derived EVs in altering tumor genetic stability, niche relations to vasculature and reactive cells, growth rates and predisposition to invasion and metastases, and immune modulation (Bronisz et al. 2014; de Vrij et al. 2015). There appear stem cells with mesenchymal and neural signatures within these tumors the EVs of which may also reflect and influence oncogenic drivers and microenvironmental alterations (Nakano et al. 2015). EV RNA is an especially appealing biomarker, as small copy numbers of key genes can be detected with high sensitivity in the plasma, serum, and cerebrospinal fluid (CSF). These detections involve reverse transcription polymerase chain reaction (RT-PCR) analyses of EV mRNA, which is protected from circulating RNAses by the lipid membrane surrounding EVs (Hochberg et al. 2014).
We and others have identified multiple clinically appealing glioma-specific potential biomarkers (Table 1) (Verhaak et al. 2010; Hochberg et al. 2014; Lechapt-Zalcman et al. 2012; Mellai et al. 2012; Kushwaha et al. 2014; Zhou et al. 2010; Akers et al. 2013; Zhi et al. 2010; McNamara et al. 2013; Hegi et al. 2005; Wang et al. 1997; Towner et al. 2013; Shao et al. 2015), with ongoing validation at the EV level. These EV RNAs are most easily categorized as unique mutations or expression changes. These have been associated with molecular subclassification of GBM, have been correlated with GBM prognosis, and offer the potential for individualized therapeutic targeting based on specific tumor molecular signatures (Verhaak et al. 2010; Heimberger et al. 2005; Bleeker et al. 2010; Masui et al. 2012; Sampson et al. 2010; Pelloski et al. 2007).
GBM-specific gene mutations are not expressed in healthy tissues and are likely specific for their tumor of origin. The multi amino acid mutation (EGFRvIII) in the epidermal growth factor receptor (EGFR) is associated with the “classical” GBM subtype and is targetable with immune therapies and chemotherapy. The downstream pathways for EGFRvIII are different from those for EGFR and thus the mutation opens the possibility of improved prognosis and favorable response to therapy (Verhaak et al. 2010). We have demonstrated serum EV EGFRvIII RNA detection only in blood of patients with GBM (Skog et al. 2008), and in recent work presented at the 2015 International Society for EVs, the CSF of GBM patients with a 50 % sensitivity rate and 98 % specificity. Thus quantitative sampling of EGFRvIII RNA provides real-time assessment of tumor burden and future predictions of therapeutic efficacy (Shao et al. 2012). Similarly, we have demonstrated that EV expression of wild-type EGFR in CSF is linked to GBM chemotherapeutic response, is a marker of drug sensitivity (Sampson et al. 2010), and is a surrogate marker of EGFRvIII mutational status. These approaches demonstrate the feasibility of EV quantification of wild-type genes for GBM characterization and therapeutic tracking. Detecting single point mutations is more challenging, but possible with high-resolution approaches, such as BEAMing (beads, emulsion, amplification, magnetics) PCR and droplet digital PCR (ddPCR). Mutant isocitrate dehydrogenase 1 (IDH1.132) is one such point mutation associated with the “proneural” GBM subtype and a favorable clinical prognosis (Verhaak et al. 2010; Bleeker et al. 2010). Using these high-resolution techniques, we demonstrated that mutant IDH1 EV mRNA was detectable in the CSF of patients with mutant IDH1 gliomas (Chen et al. 2013), establishing its utility in reducing the need for invasive biopsy. This minimally invasive sampling provides a springboard for earlier initiation of aggressive therapies. Characterizations of EV expression of other molecular subtype mutations, such as mutant NF1 associated with the “mesenchymal” subtype, are similarly needed.
It is also possible to bring to patient care the analysis of GBM-related changes in the methylation status. For promoter methylation of the nucleotide repair enzyme O6-methylguanine methyl transferase (MGMT), there are corresponding decreased MGMT mRNA and protein levels, and increased GBM sensitivity to chemotherapeutic agents such as temozolomide (Ramakrishnan et al. 2011). We have shown that MGMT mRNA levels can be detected directly in the serum of patients with GBM using a microfluidic chip-based analysis (Shao et al. 2015). Additionally, the presence of two miRNAs (miR-603 and miR-181d) provides an indirect quantification of MGMT expression (Kushwaha et al. 2014). Other miRs, such as miR-1, have been linked to GBM microenvironmental alterations including tumor cell invasion (Bronisz et al. 2014) and our recent work has identified both overexpression of miR21 in biofluids of high-grade glioma patients as distinct from controls, as well as an EV nine miR signature that offers the same separation for diagnostic purposes. EV expression patterns of GBM molecular subtype defining genetic amplifications, such as the increased PDGFRA expression associated with the “proneural” classification, are obvious areas of further study. Moreover, novel gene expression changes in gliomas are regularly reported as potential GBM biomarkers (Towner et al. 2013; Sreekanthreddy et al. 2010; Reddy et al. 2008; Ruano et al. 2008), providing a rich genetic library for future EV RNA analyses.
Practical Challenges of EV Implementation
Given the clear clinical potential for EV biomarkers for GBM, work is ongoing to optimize the analytical logistics of this technique. These efforts include optimization of biofluid sampling, and increasing the efficiency of sample preparation, processing, and analysis. Blood (plasma/serum) and CSF are the two logical foci of biofluid sampling due to their relative ease of access. Within blood, plasma has traditionally been the preferred EV sampling medium, as serum can be contaminated by platelet-derived EVs released after blood collection during clot formation (Witwer et al. 2013). Sampling of plasma can nonetheless be complicated by the presence of anticoagulants such as heparinoids, which can interfere with reverse transcription/PCR and EV signaling (Witwer et al. 2013). Recent success with serum-derived EVs (Shao et al. 2015; Chen et al. 2013) highlight the need for future studies assessing the differential effects of processing on plasma/serum.
The proximity of CSF to the central nervous system (CNS) makes it appealing for the study of CNS disease given the role of CSF in CNS solute removal (Laterra et al. 1999), as well as avoidance of the restrictive blood–brain barrier that limits molecular trafficking of CNS-derived EVs to the blood. CSF EVs are thus less likely to be diluted by peripheral ‘noise’ EVs which do not arise from the target organ, and also lack potentially confounding platelet-derived particles (Witwer et al. 2013). Amassing samples from enough GBM patients for large-scale correlative studies from either the CSF or blood nonetheless requires a coordinated multi-institutional effort. To address this need, we have developed a biorepository that already contains over 4000 specimens from more than 600 patients, most with brain tumors (Butler et al. 2014), and established biomarker consortia for both high-grade and low-grade gliomas as well as collaborations that further our understanding of the EV populations of CSF under a variety of clinical conditions.
Clinical sample EV isolation and quantification also remains a variable process. Most commonly, samples are subjected to ultracentrifugation, then RNA isolation (with or without pre-amplification), and finally amplification using quantitative RT-PCR. There are, however, multiple commercially available isolation kits and analytic techniques currently in use, as well as a lack of consensus on the fidelity of ‘housekeeping’ reference transcripts using this approach. Reference standards are not defined for this new field and we routinely evaluate biofluid EV concentrations using Nanosight Tracking Analysis based on laser detection, resistive pulse sensing (qNANO), and novel microflow studies based upon multichannel detection on EVs of fluorochrome-labeled antibodies. Normalization studies have only recently begun utilizing absolute EV miRNA expression, normalization to spike-ins or to ‘housekeeping mRNA genes’ (Akers et al. 2013), and similar advances in standardization are needed as the field moves forward.
Future Perspective
EV RNAs have tremendous clinical potential as diagnostic, subtype-defining, and prognostic biomarkers in GBM. The identification of new EV RNA targets and validation of existing EV RNA targets will be accelerated by large-scale biorepositories established for clinic sample warehousing and ongoing standardization studies to streamline sample processing. Parallel efforts to understand EV dynamics in other neurologic diseases are also underway, and include Parkinson’s disease (Kunadt et al. 2015), Alzheimer’s disease (Joshi et al. 2015), neurotrauma (Patz et al. 2013), and low-grade gliomas (Chen et al. 2013). As such, EV RNA may one day replace invasive approaches to diagnose, subtype, and track disease progression in not only GBM, but also a myriad of neuro-pathologies.
References
Akers JC, Ramakrishnan V, Kim R, Skog J, Nakano I, Pingle S, Kalinina J, Hua W, Kesari S, Mao Y, Breakefield XO, Hochberg FH, Van Meir EG, Carter BS, Chen CC (2013) MiR-21 in the extracellular vesicles (EVs) of cerebrospinal fluid (CSF): a platform for glioblastoma biomarker development. PLoS One 8:e78115
Beier D, Hau P, Proescholdt M, Lohmeier A, Wischhusen J, Oefner PJ, Aigner L, Brawanski A, Bogdahn U, Beier CP (2007) CD133(+) and CD133(−) glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res 67:4010–4015
Beier CP, Kumar P, Meyer K, Leukel P, Bruttel V, Aschenbrenner I, Riemenschneider MJ, Fragoulis A, Rummele P, Lamszus K, Schulz JB, Weis J, Bogdahn U, Wischhusen J, Hau P, Spang R, Beier D (2012) The cancer stem cell subtype determines immune infiltration of glioblastoma. Stem Cells Dev 21:2753–2761
Bleeker FE, Atai NA, Lamba S, Jonker A, Rijkeboer D, Bosch KS, Tigchelaar W, Troost D, Vandertop WP, Bardelli A, Van Noorden CJ (2010) The prognostic IDH1(R132) mutation is associated with reduced NADP+-dependent IDH activity in glioblastoma. Acta Neuropathol 119:487–494
Bonavia R, Inda MM, Cavenee WK, Furnari FB (2011) Heterogeneity maintenance in glioblastoma: a social network. Cancer Res 71:4055–4060
Bronisz A, Wang Y, Nowicki MO, Peruzzi P, Ansari KI, Ogawa D, Balaj L, De Rienzo G, Mineo M, Nakano I, Ostrowski MC, Hochberg F, Weissleder R, Lawler SE, Chiocca EA, Godlewski J (2014) Extracellular vesicles modulate the glioblastoma microenvironment via a tumor suppression signaling network directed by miR-1. Cancer Res 74:738–750
Butler WE, Atai N, Carter B, Hochberg F (2014) Informatic system for a global tissue-fluid biorepository with a graph theory-oriented graphical user interface. J Extracell Vesicles. doi:10.3402/jev.v3.24247
Chen WW, Balaj L, Liau LM, Samuels ML, Kotsopoulos SK, Maguire CA, Loguidice L, Soto H, Garrett M, Zhu LD, Sivaraman S, Chen C, Wong ET, Carter BS, Hochberg FH, Breakefield XO, Skog J (2013) BEAMing and droplet digital PCR analysis of mutant IDH1 mRNA in glioma patient serum and cerebrospinal fluid extracellular vesicles. Mol Ther Nucleic Acids. 2:e109
Chittiboina P, Connor DE Jr, Caldito G, Quillin JW, Wilson JD, Nanda A (2012) Occult tumors presenting with negative imaging: analysis of the literature. J Neurosurg 116:1195–1203
Darefsky AS, King JT Jr, Dubrow R (2012) Adult glioblastoma multiforme survival in the temozolomide era: a population-based analysis of Surveillance, Epidemiology, and End Results registries. Cancer 118:2163–2172
de Vrij J, Maas SL, Kwappenberg KM, Schnoor R, Kleijn A, Dekker L, Luider TM, de Witte LD, Litjens M, van Strien ME, Hol EM, Kroonen J, Robe PA, Lamfers ML, Schilham MW, Broekman ML (2015) Glioblastoma-derived extracellular vesicles modify the phenotype of monocytic cells. Int J Cancer. 137:1630–1642
Fischer I, Cunliffe CH, Bollo RJ, Raza S, Monoky D, Chiriboga L, Parker EC, Golfinos JG, Kelly PJ, Knopp EA, Gruber ML, Zagzag D, Narayana A (2008) High-grade glioma before and after treatment with radiation and Avastin: initial observations. Neuro Oncol. 10:700–708
Gonda DD, Akers JC, Kim R, Kalkanis SN, Hochberg FH, Chen CC, Carter BS (2013) Neuro-oncologic applications of exosomes, microvesicles, and other nano-sized extracellular particles. Neurosurgery. 72:501–510
Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L, Bromberg JE, Hau P, Mirimanoff RO, Cairncross JG, Janzer RC, Stupp R (2005) MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352:997–1003
Heimberger AB, Hlatky R, Suki D, Yang D, Weinberg J, Gilbert M, Sawaya R, Aldape K (2005) Prognostic effect of epidermal growth factor receptor and EGFRvIII in glioblastoma multiforme patients. Clin Cancer Res 11:1462–1466
Hochberg FH, Atai NA, Gonda D, Hughes MS, Mawejje B, Balaj L, Carter RS (2014) Glioma diagnostics and biomarkers: an ongoing challenge in the field of medicine and science. Expert Rev Mol Diagn. 14:439–452
Jackson RJ, Fuller GN, Abi-Said D, Lang FF, Gokaslan ZL, Shi WM, Wildrick DM, Sawaya R (2001) Limitations of stereotactic biopsy in the initial management of gliomas. Neuro Oncol 3:193–200
Joshi P, Benussi L, Furlan R, Ghidoni R, Verderio C (2015) Extracellular vesicles in Alzheimer’s disease: friends or foes? Focus on abeta-vesicle interaction. Int J Mol Sci 16:4800–4813
Kunadt M, Eckermann K, Stuendl A, Gong J, Russo B, Strauss K, Rai S, Kugler S, Falomir Lockhart L, Schwalbe M, Krumova P, Oliveira LM, Bahr M, Mobius W, Levin J, Giese A, Kruse N, Mollenhauer B, Geiss-Friedlander R, Ludolph AC, Freischmidt A, Feiler MS, Danzer KM, Zweckstetter M, Jovin TM, Simons M, Weishaupt JH, Schneider A (2015) Extracellular vesicle sorting of alpha-Synuclein is regulated by sumoylation. Acta Neuropathol. 129:695–713
Kushwaha D, Ramakrishnan V, Ng K, Steed T, Nguyen T, Futalan D, Akers JC, Sarkaria J, Jiang T, Chowdhury D, Carter BS, Chen CC (2014) A genome-wide miRNA screen revealed miR-603 as a MGMT-regulating miRNA in glioblastomas. Oncotarget. 5:4026–4039
Kutikova L, Bowman L, Chang S, Long SR, Thornton DE, Crown WH (2007) Utilization and cost of health care services associated with primary malignant brain tumors in the United States. J Neurooncol 81:61–65
Laterra J, Keep R, Betz LA, Goldstein GW (1999) Basic neurochemistry: molecular, cellular and medical aspects, vol 6., Blood-cerebrospinal fluid barrier Lippincott-Raven, Philadelphia
Lechapt-Zalcman E, Levallet G, Dugue AE, Vital A, Diebold MD, Menei P, Colin P, Peruzzy P, Emery E, Bernaudin M, Chapon F, Guillamo JS (2012) O(6)-methylguanine-DNA methyltransferase (MGMT) promoter methylation and low MGMT-encoded protein expression as prognostic markers in glioblastoma patients treated with biodegradable carmustine wafer implants after initial surgery followed by radiotherapy with concomitant and adjuvant temozolomide. Cancer 118:4545–4554
Lottaz C, Beier D, Meyer K, Kumar P, Hermann A, Schwarz J, Junker M, Oefner PJ, Bogdahn U, Wischhusen J, Spang R, Storch A, Beier CP (2010) Transcriptional profiles of CD133+ and CD133− glioblastoma-derived cancer stem cell lines suggest different cells of origin. Cancer Res 70:2030–2040
Masui K, Cloughesy TF, Mischel PS (2012) Review: molecular pathology in adult high-grade gliomas: from molecular diagnostics to target therapies. Neuropathol Appl Neurobiol 38:271–291
McNamara MG, Sahebjam S, Mason WP (2013) Emerging biomarkers in glioblastoma. Cancers. 5:1103–1119
Mellai M, Monzeglio O, Piazzi A, Caldera V, Annovazzi L, Cassoni P, Valente G, Cordera S, Mocellini C, Schiffer D (2012) MGMT promoter hypermethylation and its associations with genetic alterations in a series of 350 brain tumors. J Neurooncol 107:617–631
Messali A, Hay JW, Villacorta R (2013) The cost-effectiveness of temozolomide in the adjuvant treatment of newly diagnosed glioblastoma in the United States. Neuro Oncol. 15:1532–1542
Nakano I, Garnier D, Minata M, Rak J (2015) Extracellular vesicles in the biology of brain tumour stem cells—implications for inter-cellular communication, therapy and biomarker development. Semin Cell Dev Biol. 40:17–26
Nickel GC, Barnholtz-Sloan J, Gould MP, McMahon S, Cohen A, Adams MD, Guda K, Cohen M, Sloan AE, LaFramboise T (2012) Characterizing mutational heterogeneity in a glioblastoma patient with double recurrence. PLoS One 7:e35262
Noushmehr H, Weisenberger DJ, Diefes K, Phillips HS, Pujara K, Berman BP, Pan F, Pelloski CE, Sulman EP, Bhat KP, Verhaak RG, Hoadley KA, Hayes DN, Perou CM, Schmidt HK, Ding L, Wilson RK, Van Den Berg D, Shen H, Bengtsson H, Neuvial P, Cope LM, Buckley J, Herman JG, Baylin SB, Laird PW, Aldape K (2010) Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell 17:510–522
Patel AP, Tirosh I, Trombetta JJ, Shalek AK, Gillespie SM, Wakimoto H, Cahill DP, Nahed BV, Curry WT, Martuza RL, Louis DN, Rozenblatt-Rosen O, Suva ML, Regev A, Bernstein BE (2014) Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science 344:1396–1401
Patz S, Trattnig C, Grunbacher G, Ebner B, Gully C, Novak A, Rinner B, Leitinger G, Absenger M, Tomescu OA, Thallinger GG, Fasching U, Wissa S, Archelos-Garcia J, Schafer U (2013) More than cell dust: microparticles isolated from cerebrospinal fluid of brain injured patients are messengers carrying mRNAs, miRNAs, and proteins. J Neurotrauma 30:1232–1242
Pelloski CE, Ballman KV, Furth AF, Zhang L, Lin E, Sulman EP, Bhat K, McDonald JM, Yung WK, Colman H, Woo SY, Heimberger AB, Suki D, Prados MD, Chang SM, Barker FG 2nd, Buckner JC, James CD, Aldape K (2007) Epidermal growth factor receptor variant III status defines clinically distinct subtypes of glioblastoma. J Clin Oncol 25:2288–2294
Ramakrishnan V, Kushwaha D, Koay DC, Reddy H, Mao Y, Zhou L, Ng K, Zinn P, Carter B, Chen CC (2011) Post-transcriptional regulation of O(6)-methylguanine-DNA methyltransferase MGMT in glioblastomas. Cancer Biomark. 10:185–193
Reddy SP, Britto R, Vinnakota K, Aparna H, Sreepathi HK, Thota B, Kumari A, Shilpa BM, Vrinda M, Umesh S, Samuel C, Shetty M, Tandon A, Pandey P, Hegde S, Hegde AS, Balasubramaniam A, Chandramouli BA, Santosh V, Kondaiah P, Somasundaram K, Rao MR (2008) Novel glioblastoma markers with diagnostic and prognostic value identified through transcriptome analysis. Clin Cancer Res 14:2978–2987
Ricci-Vitiani L, Pallini R, Biffoni M, Todaro M, Invernici G, Cenci T, Maira G, Parati EA, Stassi G, Larocca LM, De Maria R (2010) Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature 468:824–828
Rock JP, Hearshen D, Scarpace L, Croteau D, Gutierrez J, Fisher JL, Rosenblum ML, Mikkelsen T (2002) Correlations between magnetic resonance spectroscopy and image-guided histopathology, with special attention to radiation necrosis. Neurosurgery. 51:912–919 (discussion 919–920)
Ruano Y, Mollejo M, Camacho FI, Rodriguez de Lope A, Fiano C, Ribalta T, Martinez P, Hernandez-Moneo JL, Melendez B (2008) Identification of survival-related genes of the phosphatidylinositol 3′-kinase signaling pathway in glioblastoma multiforme. Cancer 112:1575–1584
Sampson JH, Heimberger AB, Archer GE, Aldape KD, Friedman AH, Friedman HS, Gilbert MR, Herndon JE 2nd, McLendon RE, Mitchell DA, Reardon DA, Sawaya R, Schmittling RJ, Shi W, Vredenburgh JJ, Bigner DD (2010) Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol 28:4722–4729
Sarkaria JN, Kitange GJ, James CD, Plummer R, Calvert H, Weller M, Wick W (2008) Mechanisms of chemoresistance to alkylating agents in malignant glioma. Clin Cancer Res 14:2900–2908
Shao H, Chung J, Balaj L, Charest A, Bigner DD, Carter BS, Hochberg FH, Breakefield XO, Weissleder R, Lee H (2012) Protein typing of circulating microvesicles allows real-time monitoring of glioblastoma therapy. Nat Med 18:1835–1840
Shao H, Chung J, Lee K, Balaj L, Min C, Carter BS, Hochberg FH, Breakefield XO, Lee H, Weissleder R (2015) Chip-based analysis of exosomal mRNA mediating drug resistance in glioblastoma. Nat Commun. 6:6999
Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT Jr, Carter BS, Krichevsky AM, Breakefield XO (2008) Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol 10:1470–1476
Sottoriva A, Spiteri I, Piccirillo SG, Touloumis A, Collins VP, Marioni JC, Curtis C, Watts C, Tavare S (2013) Intratumor heterogeneity in human glioblastoma reflects cancer evolutionary dynamics. Proc Natl Acad Sci USA 110:4009–4014
Sreekanthreddy P, Srinivasan H, Kumar DM, Nijaguna MB, Sridevi S, Vrinda M, Arivazhagan A, Balasubramaniam A, Hegde AS, Chandramouli BA, Santosh V, Rao MR, Kondaiah P, Somasundaram K (2010) Identification of potential serum biomarkers of glioblastoma: serum osteopontin levels correlate with poor prognosis. Cancer Epidemiol Biomarkers Prev. 19:1409–1422
Thon N, Kreth S, Kreth FW (2013) Personalized treatment strategies in glioblastoma: MGMT promoter methylation status. Onco Targets Ther. 6:1363–1372
Towner RA, Jensen RL, Colman H, Vaillant B, Smith N, Casteel R, Saunders D, Gillespie DL, Silasi-Mansat R, Lupu F, Giles CB, Wren JD (2013) ELTD1, a potential new biomarker for gliomas. Neurosurgery. 72:77–90 (discussion 91)
Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, Miller CR, Ding L, Golub T, Mesirov JP, Alexe G, Lawrence M, O’Kelly M, Tamayo P, Weir BA, Gabriel S, Winckler W, Gupta S, Jakkula L, Feiler HS, Hodgson JG, James CD, Sarkaria JN, Brennan C, Kahn A, Spellman PT, Wilson RK, Speed TP, Gray JW, Meyerson M, Getz G, Perou CM, Hayes DN (2010) Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 17:98–110
Wang SI, Puc J, Li J, Bruce JN, Cairns P, Sidransky D, Parsons R (1997) Somatic mutations of PTEN in glioblastoma multiforme. Cancer Res 57:4183–4186
Wang R, Chadalavada K, Wilshire J, Kowalik U, Hovinga KE, Geber A, Fligelman B, Leversha M, Brennan C, Tabar V (2010) Glioblastoma stem-like cells give rise to tumour endothelium. Nature 468:829–833
Weber MA, Zoubaa S, Schlieter M, Juttler E, Huttner HB, Geletneky K, Ittrich C, Lichy MP, Kroll A, Debus J, Giesel FL, Hartmann M, Essig M (2006) Diagnostic performance of spectroscopic and perfusion MRI for distinction of brain tumors. Neurology 66:1899–1906
Witwer KW, Buzas EI, Bemis LT, Bora A, Lasser C, Lotvall J, Nolte-’t Hoen EN, Piper MG, Sivaraman S, Skog J, Thery C, Wauben MH, Hochberg F (2013) Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles. doi:10.3402/jev.v2i0.20360
Wong AJ, Ruppert JM, Bigner SH, Grzeschik CH, Humphrey PA, Bigner DS, Vogelstein B (1992) Structural alterations of the epidermal growth factor receptor gene in human gliomas. Proc Natl Acad Sci USA 89:2965–2969
Yip S, Miao J, Cahill DP, Iafrate AJ, Aldape K, Nutt CL, Louis DN (2009) MSH6 mutations arise in glioblastomas during temozolomide therapy and mediate temozolomide resistance. Clin Cancer Res 15:4622–4629
Zhi F, Chen X, Wang S, Xia X, Shi Y, Guan W, Shao N, Qu H, Yang C, Zhang Y, Wang Q, Wang R, Zen K, Zhang CY, Zhang J, Yang Y (2010) The use of hsa-miR-21, hsa-miR-181b and hsa-miR-106a as prognostic indicators of astrocytoma. Eur J Cancer 46:1640–1649
Zhou X, Ren Y, Moore L, Mei M, You Y, Xu P, Wang B, Wang G, Jia Z, Pu P, Zhang W, Kang C (2010) Downregulation of miR-21 inhibits EGFR pathway and suppresses the growth of human glioblastoma cells independent of PTEN status. Lab Invest 90:144–155
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This manuscript was prepared without external funding. R.C.R., F.H.H., and B.S.C. have no conflicts of interest to report.
Rights and permissions
About this article
Cite this article
Rennert, R.C., Hochberg, F.H. & Carter, B.S. ExRNA in Biofluids as Biomarkers for Brain Tumors. Cell Mol Neurobiol 36, 353–360 (2016). https://doi.org/10.1007/s10571-015-0284-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-015-0284-5