Abstract
The brain in Alzheimer’s disease is under increased oxidative stress, and this may have a role in the pathogenesis and neural death in this disorder. It has been verified that numerous signaling pathways involved in neurodegenerative disorders are activated in response to reactive oxygen species (ROS). EUK134, a synthetic salen–manganese antioxidant complex, has been found to possess many interesting pharmacological activities awaiting exploration. The present study is to characterize the role of Notch signaling in apoptotic cell death of SK-N-MC cells. The cells were treated with hydrogen peroxide (H2O2) or menadione to induce oxidative stress. The free-radical scavenging capabilities of EUK134 were studied through the MTT assay, glutathione peroxidase (GPx) enzyme activity assay, and glutathione (GSH) Levels. The extents of lipid peroxidation, protein carbonyl formation, and intracellular ROS levels, as markers of oxidative stress, were also studied. Our results showed that H2O2/menadione reduced GSH levels and GPx activity. However, EUK134 protected cells against ROS-induced cell death by down-regulation of lipid peroxidation and protein carbonyl formation as well as restoration of antioxidant enzymes activity. ROS induced apoptosis and increased NICD and HES1 expression. Inhibition of NICD production proved that Notch signaling is involved in apoptosis through p53 activation. Moreover, H2O2/menadione led to Numb protein down-regulation which upon EUK134 pretreatment, its level increased and subsequently prevented Notch pathway activation. We indicated that EUK134 can be a promising candidate in designing natural-based drugs for ROS-induced neurodegenerative diseases. Collectively, ROS activated Notch signaling in SK-N-MC cells leading to cell apoptosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oxidative stress has been implicated to play a crucial role in the pathogenesis of Alzheimer’s disease, the most common type of dementia (Barnham et al. 2004; Markesbery 1997; Miranda et al. 2000). Reactive oxygen species (ROS), mainly superoxide anions (\({\text{O}}_{2}^{ - }\)) and hydrogen peroxide (H2O2), are generated by metabolic reactions in all aerobic organisms (Cadenas and Davies 2000). Their relentless production coupled with their damaging nature has led to a harmful function. However, the cells have obviously evolved multiple intracellular defenses for their elimination. It would, therefore, seem that antioxidant molecules including manganese–salen complexes like EUK134 [manganese 3-methoxy N,N′ bis (salicylidene) ethylene-diamine chloride] having combined superoxide dismutase (SOD) and catalase mimetic functions should be beneficial for blocking these detrimental effects (Doctrow et al. 1997). Accordingly, the target for ROS action has been studied mainly in relation to these disorders leading to the identification of various signal transduction pathways activated in response to changes in the intracellular levels of ROS (Rhee 1999). ROS-induced activation of signaling pathways such as Notch signaling may amplify the toxicity of these species. The Notch signaling pathway is a well-conserved signaling pathway with probable roles in regulating stem cell maintenance, differentiation, and apoptosis. Notch genes encode highly conserved cell-surface receptors which are then processed by γ-secretase to release NICD (Notch Intracellular Domain) (Bray 1998). NICD, in the nucleus, activates transcription of target genes including c-Myc, cyclinD1, p21/Waf1, NFkB2, Ifi-202, Ifi-204, Ifi-D3, ADAM19, Notch1, Notch3, and bcl-2 (Borggrefe and Oswald 2009). Notch signaling is modified by a number of additional factors including Numb, a cytoplasmic negative regulatory protein which regulates the ubiquitin-dependent degradation of NICD with ultimate cell’s fate determination (Lu et al. 1998; Spana and Doe 1996). Since in AD the cells are exposed to various ROS, it is of clinical significance to elucidate the mode and mechanism of cell death in AD. The possibility of Notch1 involvement in AD pathophysiology via activation by oxidants led us to compare the patterns of NICD expression in response to H2O2 and menadione (superoxide anion producer) in the SK-N-MC neuroblastoma cells, with the aim of finding anti-apoptotic clues to attenuate cell death in AD. Moreover, we investigated the relationship between Notch and p53 pathways to find out whether Notch signaling pathway could induce cell death through p53 activation.
Materials and Methods
Materials
The cell culture medium (RPMI-1640), fetal bovine serum (FBS), and penicillin–streptomycin were purchased from Gibco BRL (Life technology, Paisley, Scotland). Hydrogen peroxide (H2O2) and dimethyl sulfoxide (DMSO) were obtained from Merck (Darmstadt, Germany). The SK-N-MC cell line was obtained from Pasteur Institute of Iran (Tehran, Iran). MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide], phenylmethylsulphonyl fluoride (PMSF), leupeptin, pepstatin, aprotinin, dithionitrobenzoic acid (DTNB), glutathione (GSH), and (γ-secretase inhibitor) DAPT were purchased from Sigma Chem. Co (Germany). 2′,7′-Dichlorofluorescein diacetate (DCFH2-DA) was purchased from Molecular Probe (Eugene, OR, USA). Ethidium bromide (EtBr) and acridine orange (AO) were obtained from Pharmacia LKB Biotechnology AB Uppsala (Sweden). EUK134 was prepared by first synthesizing the ligand and then complexing the ligand to manganese using reported methods (Boucher and Farrell 1973). Anti-bcl-2, anti-Bax, anti-cleaved caspase-9, anti-p53, anti-p21, and anti-tubulin antibodies were purchased from Biosource (Nivelles, Belgium). Anti-NICD was obtained from cell signaling (MA, USA). Anti-HES1, anti-MDM2, and anti-Numb were purchased from Santa Cruz Biotechnology (Germany). Chemiluminescence detection system was purchased from Amersham-Pharmacia (Piscataway, NJ, USA).
Cell Culture
Human SK-N-MC cell line was cultured in RPMI-1640 medium supplemented with FBS (10 %, v/v), streptomycin (100 μg/ml), and penicillin (100 u/ml). The cells were incubated in a 5 % CO2 humidified atmosphere at 37 °C. To examine the effect of γ-secretase inhibitor DAPT on H2O2-induced apoptosis, SK-N-MC cells were pretreated with 20 μmol/l DAPT for 1 h. After 1 h, the medium was discarded, and the cells were treated with H2O2 (150 µM) for additional 24 h.
Cell Viability Determination
Cell viability was evaluated using the MTT assay which is dependent on the reduction of MTT to formazan by viable cells. The resulting intracellular purple formazan is quantified spectrophotometrically. The cells were seeded in 96-well plates at a concentration of 5 × 104/well for 24 h, and then pretreated with different concentrations of EUK134. Two hours later, the medium of each well was replaced with fresh medium containing H2O2 (150 µM) or menadione (20 µM) and incubated at 37 °C. After 24 h, the medium was discarded, and 10 μl MTT (5 mg/ml) was added to each well. After 4 h of incubation, the supernatants were removed. The formazan crystals in each well were dissolved in 100 μl of DMSO, and the absorbance was measured via ELISA reader (Exert 96, Asys Hitch, Ec Austria) at a wavelength of 570 nm.
Detection of Intracellular ROS
The generation of reactive oxygen radicals was monitored using 2′,7′-dichlorodihydro-fluorescein diacetate (DCFH2-DA). The oxidation of DCFH2 by intracellular ROS, mainly H2O2, results in fluorescent DCF which stains the cells (Gomes et al. 2005). Thus, the DCF fluorescence intensity correlates with the amount of peroxide produced by the cells (Lebel et al. 1992). To do the test, 10 µM DCFH2-DA in PBS was added to the cells treated with H2O2 (150 µM) or menadione (20 µM). Then, the fluorescence intensity was evaluated using a varian spectrofluorometer (model Cary Eclipse) with excitation and emission at 485 and 530 nm, respectively.
Glutathione Peroxidase Assay
Glutathione peroxidase (GPx) activity was monitored in a 1-ml cuvette containing 0.890 ml of 100 mM potassium phosphate buffer (pH 7.0), 1 mM EDTA, 1 mM NaN3, 0.2 mM NADPH, 1 u/ml GSH reductase, and 1 mM GSH. Cell lysate was added to make a total volume of 0.9 ml. The reaction was initiated by the addition of 100 µl of 2.5 mM H2O2, and the conversion of NADPH to NADP+ was monitored with a spectrophotometer at 340 nm for 3 min. GPx activity was expressed as nmoles of NADPH oxidized to NADP+/min/mg protein, using a molar extinction coefficient of 6.22 × 106 (cm/M) for NADPH (Flohe and Gunzler 1984).
Determination of Lipid Peroxidation
Lipid peroxidation was measured via thiobarbituric acid reactive substance (TBARS) according to the double-heating method (Drapper and Hadley 1990). Malondialdehyde (MDA) level was assayed by absorbance of the purple (MDA)-TBA complex at 532 nm. After drug treatment, cells were exposed to 150 µM H2O2 or 20 µM menadione for 24 h. Then, the cell lysates were mixed with 0.5 ml of 10 % trichloroacetic acid and heated at 95 °C for 15 min. After cooling, the samples were centrifuged at 1,500×g for 10 min, and 2 ml of each sample supernatant was mixed with TBA solution (0.67 % w/v). Each tube was then placed in a boiling water bath for 15 min. After cooling to room temperature, the absorbance was determined at 532 nm. The concentration of MDA was calculated based on the extinction coefficient of the TBA–MDA complex [ε = 1.56 × 105 (cm/M)], and it was expressed as nmol/mg protein.
Determination of Protein Oxidation
For the determination of PCO levels, 1 ml of 10 mM 2,4-dinitrophenylhydrazine (DNPH) in 2 M HCl was added to the cell lysates. Samples were incubated for 1 h at room temperature. Then, 1 ml of trichloroacetic acid (TCA 10 % w/v) was added to each mixture and centrifuged at 3,000×g for 10 min. Each protein pellet was washed three times with 2 ml of ethanol/ethyl acetate (1:1, v/v) and dissolved in 1 ml of guanidine hydrochloride (6 M, pH 2.3). The carbonyl content was calculated based on the molar extinction coefficient of DNPH [ε = 2.2 × 104 (cm/M)] and was expressed as nmol/mg protein.
Reduced Glutathione Evaluation
Intracellular GSH level was evaluated using dithionitrobenzoic acid (DTNB) method. After drug treatment, the concentration of GSH was determined spectrophotometrically at 412 nm in the whole cell lysate using DTNB, and the reduced GSH level was expressed as µg/mg protein (Jollow et al. 1974).
Immunoblotting
The treated cells were harvested and lysed using lysis buffer containing 1 % Triton X-100, 1 % SDS, 10 mM Tris (pH 7.4), 100 mM NaCl, 1 mM EGTA, 1 mM EDTA, 20 mM sodium pyrophosphate, 2 mM Na3VO4, 1 mM NaF, 0.5 % sodium deoxycholate, 10 % glycerol, 1 mM phenylmethylsulphonyl fluoride (PMSF), 10 µg/ml leupeptin, 1 µg/ml pepstatin, and 60 µg/ml aprotinin. Protein concentration of each sample was determined using Lowry’s method. Equal quantities of protein (50 µg) were subjected to SDS–polyacrylamide gel electrophoresis (PAGE) and electrically transferred to nitrocellulose membranes. The filter membranes were blocked in Tris-buffered saline (pH 7.4) containing 0.1 % Tween-20 and 5 % BSA (bovine serum albumin) overnight at 4 °C. The blocked blots were incubated with primary antibodies for 1 h at room temperature using antibody dilutions as recommended by the manufacturer in Tris-buffered saline (pH 7.4), 0.1 % Tween-20 following 1 h incubation with anti-rabbit, or anti-mouse horseradish peroxidase-conjugated secondary antibodies (Biosource, Belgium). The proteins bands were detected by an enhanced chemiluminescence (ECL) detection system (Amersham-Pharmacia, Piscataway, NJ), and the bands were quantified using Scion Image software.
Statistical Analysis
Data were expressed as percent of values of untreated control cells, and each value represents the mean ± SD (n = 3). The significant differences between the means of the treated and untreated groups were calculated by unpaired Student’s t test, and P values less than 0.05 were considered significant.
Results
EUK134 Enhanced the Viability of H2O2/Menadione-Treated Cells
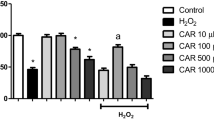
Many studies have investigated the role of ROS on cell death in neuroblastoma cells. The SK-N-MC cells were treated separately with hydrogen peroxide (H2O2) and/or menadione (superoxide anion radical producer) to induce oxidative stress. To find the concentration of ROS where 50 % of cells are viable, we examined a range of concentrations of H2O2 (100, 150, and 300 µM) and/or menadione (10, 20, and 30 µM). Our data indicated that H2O2 and menadione reduced the cell viability to 50.3 and 51 % at 150 µM H2O2 and 20 µM menadione, respectively (Fig. 1a). Regarding these data, the rest of experiments have been carried out based on obtained doses. Pretreatment of the cells with EUK134 for 2 h (10–30 μM) and subsequent treatment with 150 µM H2O2 or 20 µM menadione for 24 h enhanced the extent of viability in a concentration-dependent manner. EUK134 (20 μM) restored the cell viability by 33.2 and 26 % among H2O2 and menadione-treated cells, respectively (Fig. 1b).
Effects of H2O2 and menadione on viability of SK-N-MC cells. SK-N-MC cells were treated with different concentrations of H2O2 (100, 150, and 300 µM) or menadione (10, 20, and 30 µM) for 24 h (a), SK-N-MC cells were pretreated with EUK134 (10, 20, and 30 µM) and exposed to H2O2 (150 µM) or menadione (20 µM) for 24 h (b), and then cell viability was examined by MTT assay. The data are the means of three independent measurements ± SD. *,**significantly different from control cells (P < 0.05); # , ##significantly different from ROS-treated cells (P < 0.05). (*, #represents H2O2-treated cells and **, ##represents menadione-treated cells)
EUK-134 Decreased H2O2/Menadione-Induced ROS Generation
To evaluate the effects of EUK134 on intracellular reactive oxygen species levels induced by H2O2/menadione, we measured ROS levels using DCFH2-DA staining. As Fig. 2 indicates, ROS production increased by a factor of 4.35 and 5.34 in response to H2O2 or menadione, respectively. However, EUK134 pretreatment of SK-N-MC cells attenuated H2O2/menadione-induced ROS generation.
Effect of different concentrations of EUK134 on intracellular ROS, lipid peroxidation, protein carbonyl, and GSH levels and glutathione peroxidase activity in H2O2 or menadione-treated cells. SK-N-MC cells were pretreated with EUK134 (10 and 20 µM) for 2 h before treatment with 150 µM H2O2 or 20 µM menadione for 24 h (a). ROS levels were evaluated using 2′,7′-dichlorofluorescein diacetate (DCFH2-DA), and the fluorescence intensity of 10,000 cells was measured. Lipid and protein oxidation were measured by analysis of MDA and PCO, respectively. Reduced glutathione level was monitored by DTNB assay (b). The glutathione peroxidase activity was monitored with a spectrophotometer at 340 nm. The data are the means of three independent measurements ± SD. *,**significantly different from control cells (P < 0.05); # , ##significantly different from ROS-treated cells (P < 0.05)
Restoration of GPx Activity by EUK134
To study whether the intracellular antioxidant status is altered by EUK134, the activity of GPx was determined at different concentrations of EUK134 among the H2O2/menadione-treated cells. H2O2 (150 μM) and/or menadione (20 μM) reduced GPx activity to 1.14 and 0.98 nmol/min/mg protein, respectively (compared to 2.14 nmol/min/mg protein in untreated control cells). As shown in Fig. 2b, pretreatment of the cells with EUK134 at concentrations of 10 and 20 µΜ increased the enzyme activity to 1.47 and 1.68 nmol/min/mg protein among H2O2-treated cells and 1.13 and 1.49 nmol/min/mg protein in menadione-treated cells.
Effect of EUK134 on H2O2/Menadione-Induced Lipid Peroxidation
Oxidative stress leads to oxidation of biomolecules including lipids. One of the final products of the lipid peroxidation process is MDA. MDA levels are measured as a marker of H2O2/menadione-Induced oxidative stress. As Fig. 2a shows, H2O2 (150 µM) or menadione (20 µM) treatment increased the extent of MDA formation (2.25 nmol/mg protein in H2O2-treated cells and 2.45 nmol/mg protein in menadione-treated cells) relative to control cells (0.57 nmol/mg protein). However, pretreatment of cells with EUK134 (20 µM) followed by H2O2 or menadione treatment reduced MDA generation to 0.79 and 1.61 nmol/mg protein.
Effect of EUK134 on H2O2/Menadione-Induced PCO Formation
Protein carbonyls are stable and also are widely used as markers to assess the levels of protein oxidation. The extent of protein carbonyl formation can be determined experimentally by derivatization of the carbonyl groups with 2,4-dinitrophenylhydrazine (DNPH), followed by spectroscopic or immunochemical detection of the resulting hydrazone product. After treatment with H2O2 (150 µM) or menadione (20 µM), the PCO formation increased significantly (3.24 nmol/mg protein in H2O2-treated cells and 3.66 nmol/mg protein in menadione-treated cells versus 0.49 nmol/mg protein in control cells). However, pretreatment with EUK134 reduced protein carbonyl levels to 1.16 and 2.35 nmol/mg protein, respectively (Fig. 2a).
EUK134 Prevented H2O2/Menadione-Induced GSH Depletion
Glutathione is critical for protecting the brain from oxidative stress which participates in the detoxification of hydrogen peroxide by various GPxs (Hermes-Lima 2004). Based on DTNB assay, the intracellular GSH concentration of the cells reduced to 4.2 and 3.6 µg/mg protein when the cells were treated with 150 µM H2O2 or 20 µM menadione for 24 h, respectively (Fig. 2a). However, the cellular GSH contents of the cells pretreated with 20 µM EUK134 followed by H2O2 and/or menadione treatments were 8.5 and 7.4 µg/mg protein, respectively, relative to the control cells (9.16 µg/mg protein).
EUK134 Decreased Bax/bcl2 ratio and Procaspase-9 Activation in H2O2/Menadione-Treated Cells
Previous findings have suggested that oxidative stress-induced apoptosis correlates with the level of expression of apoptosis regulatory proteins, Bax, and bcl-2, in the cells. Pro-apoptotic Bax protein promotes apoptosis by binding to and antagonizing the bcl-2 (Oltvai et al. 1993). In response to a variety of apoptotic stimuli, activated Bax forms an oligomeric pore in the mitochondrial membrane by which cytochrome c is released to cytosol to promote apoptosis by activating caspases (Verhagen et al. 2000). Only caspase-9 bound to the apoptosome is able to efficiently cleave and activate down-stream effector caspases such as caspase-3 (Rodriguez and Lazebnik, 1999). Pretreatment of cells with EUK134, prior to H2O2 or menadione treatments, reduced procaspase-9 activation (Fig. 3a). Our data suggested that Bax/bcl-2 ratio, as an index of apoptosis, increased in response to oxidative stress. However, EUK134 reduced Bax/bcl-2 ratio and procaspase-9 activation.
Effects of H2O2 and menadione on Bax, bcl2, cleaved caspase-9, MDM2, p53, p21, NICD, HES1, and Numb expression levels in H2O2/menadione-treated SK-N-MC cells. Cells were pretreated with 20 μM EUK134 followed by incubation with 150 μM H2O2 or 20 µM menadione for 24 h. Bax, bcl-2, and cleaved caspase-9 (a) NICD, HES1, and Numb (b) MDM2, p53, and p21 (c) protein expression was measured by western blot analysis using monoclonal antibodies against each factor. Intensity of each band was quantified based on densitometry analysis. All data are representative of three independent experiments ± SD (P < 0.05). *,**significantly different from control cells (P < 0.05); # , ##significantly different from ROS-treated cells (P < 0.05)
Effect of EUK134 on H2O2/Menadione-Induced Expression of NICD, HES1, and Numb
Numb is a cell fate determinant with Notch receptors antagonizing activity (Roegiers and Jan 2004). Thus, we hypothesized that Notch signaling might be impressed by Numb to lead to H2O2/menadione-induced apoptosis. To disclose this hypothesis, we first investigated the effect of H2O2 and superoxide anion radicals on the expression of the Notch intracellular domain (NICD). In addition, HES1 expression was followed as a Notch down-stream target gene. Our data showed that H2O2 or menadione treatment significantly increases the NICD and HES1 expression supporting the idea that oxidative stress could activate Notch signaling (Fig. 3b). On the other hand, H2O2/menadione treatment resulted in down-regulation of Numb expression in a time-dependent manner. Pretreatment with EUK134, however, decreased NICD and HES1 levels. Treatment of SK-N-MC cells with DAPT (20 µM) completely blocked NICD production leading to HES1 down-regulation as well as up-regulation of Numb (Fig. 4). The present research provides the evidence that EUK134 could protect SK-N-MC cells from oxidative stress-induced apoptosis by Numb up-regulation leading to the suppression of Notch signaling activity.
The effect of γ-secretase inhibitor DAPT on NICD, HES1, Numb, MDM2, and p53 expression. Cells were pretreated with 20 µM γ-secretase inhibitor DAPT for 1 h followed by incubation with 150 μM H2O2 for 24 h. NICD, HES1 and Numb (a) MDM2 and p53 (b) proteins expression was measured by western blot analysis using monoclonal antibodies against each factor. Intensity of each band was quantified based on densitometry analysis. All data are representative of three independent experiments ± SD (P < 0.05). *Significantly different from control cells (P < 0.05); #significantly different from ROS-treated cells (P < 0.05)
Effect of EUK134 on H2O2/Menadione-Induced Expression of P53
P53, a major tumor suppressor protein, responds to diverse cellular stresses. MDM2 protein binds to p53 to prevent its transcriptional activity. In addition, MDM2 regulates p53 degradation via its E3 ubiquitin-ligase activity (Kubbutat et al. 1997). We analysed cyclin-dependent kinase inhibitor p21 expression level as a p53 down-stream gene expressed in response to oxidative stress (El-Deiry et al. 1993). To evaluate the effects of EUK134 on p53 expression and cell survival, the cells were pretreated with EUK134 for 2 h followed by H2O2/menadione treatment. Studies have shown that H2O2 and menadione have been implicated in regulating p53 expression levels. Treatment with either of the oxidants resulted in up-regulation of p53 and p21 expression parallel to down-regulation of MDM2. In contrast, EUK134 attenuated the effects of oxidative stress on p53, p21, and MDM2 levels (Fig. 3c). Inhibition of Notch signaling activation with DAPT led to decreased p53 and also increased MDM2 levels, which is consistent with p53 ubiquitination by MDM2 (Fig. 4).
Discussion
Oxidative stress has been implicated in a variety of neurodegenerative disorders including AD (Halliwell and Gutteridge 1990). Currently, it is known that numerous signaling pathways are activated in response to oxidative stress which might modulate cell death (Rhee 1999). Some of the relevant studies have focused on the mode of action of different types of reactive oxygen species (ROS) including superoxide anion radicals and hydrogen peroxide involved in AD induction and progression (Conde de la Rosa et al. 2006; Dunning et al. 2009). Menadione is a strong superoxide anion generating agent, while hydrogen peroxide is believed to produce hydroxyl radicals (Criddle et al. 2006). It is of clinical importance to know how SK-N-MC cells would react toward these two types of intracellular oxidants and what would be the effects on the Notch signaling pathways.
Notch signaling, classically associated with cell differentiation, has also been shown to direct cells into proliferative or apoptotic states in a cell type-specific manner. Notch signaling activation can promote proliferation in mammalian astrocytes (Furukawa et al. 2000). It has been reported that Notch activation is involved in the regulation of apoptotic cell death during retina development in Drosophila and mammalian neural development (Scheer et al. 2001). A recent study has also provided the evidence that NICD induces extensive apoptosis in early neural progenitor cells via a p53-dependent pathway activated in these cells (Yang et al. 2004). In other words, Notch activation in neural progenitor cells leads to elevated levels of nuclear p53 and transcriptional up-regulation of Bax, a major gene mediating p53-dependent apoptotic cell death, demonstrating a crucial role for Notch in the regulation of apoptosis in early neural progenitors (Yang et al. 2004). Taken together, these studies suggest an apoptotic role for Notch. Thus, removal of excess reactive oxygen species or suppression of their generation may be effective in preventing Notch activation and oxidative cell death. This work is strengthening an important role of ROS on Notch signaling pathway activation, the relationship between this pathway and p53-dependent apoptosis, and utilization of antioxidant therapy for preventing or delaying reactive oxygen species-induced apoptosis. The present research verifies that EUK134 is efficacious to protect cells against H2O2/menadione-mediated injury as showed significant neuroprotection via inhibition of lipid peroxidation, enhancement of endogenous antioxidant defense enzymes, attenuation of ROS levels, and reduction in protein carbonyl formation. Our data provided the evidence that H2O2-scavenging potential of EUK134 was more effective than its scavenging potential on menadione. Exposure of cells to H2O2/menadione enhanced Bax/bcl2 ratio and procaspase-9 activation. To scrutinise the mechanism underlying oxidative stress-induced apoptosis, we analysed p53, p21 and MDM2 expression levels. The relatively high levels of p53 expressed in oxidant-treated cells suggest that H2O2/menadione-induced apoptosis is dependent on p53 tumor suppressor protein. P21 protein was also up-regulated in response to both oxidants. Exposure of SK-N-MC cells to oxidative stress increased also NICD and HES1 expression. Our results also confirmed that NICD increased HES1 level and subsequently induced apoptosis in SK-N-MC cells through p53 up-regulation. This fact is consistent with previous documents by Huang et al. who showed that HES1 modulates p53 signaling by inhibiting MDM2 function. Knowing that HES1 is under the influence of Notch1, supports a model for cell type-specific crosstalk between the Notch and p53 signal transduction pathways in which Notch leads to p53 activation by up-regulating target genes such as HES1. Based on our data, Notch inhibition decreased HES1 level resulting in MDM2 activation and subsequent p53 ubiquitination. This finding provides an explanation for the apoptotic effect of Notch in some cell types (Chin et al. 1996; Huang et al. 2003).
It has been demonstrated that Numb promotes NICD ubiquitination and degradation (McGill and McGlade 2003). Our present data showed that exposure of SK-N-MC cells to H2O2/menadione decreased Numb content in a time-dependent manner. However, EUK134 remarkably raised the Numb protein level leading to inhibition of Notch signaling activity (Fig. 5). Based on the present data, we hypothesized that Numb could act as a survival factor capable of regulating the blockade of ROS-induced apoptosis by modulating the activity of Notch signaling. Regarding the significant effect on potentiation of Numb expression with subsequent NICD inactivation, EUK134 acts as an excellent antioxidant and highly valuable for clinical evaluation. In addition, our data indicated that Notch signaling pathway is activated in response to either H2O2 or menadione, meaning that Notch signaling activation is independent of at least these two types of ROS (OH∙ and \({\text{O}}_{2}^{ - }\)).
Abbreviations
- MTT:
-
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- GSH:
-
Glutathione
- DTNB:
-
Dithionitrobenzoic acid
- MDA:
-
Malondialdehyde
- PBS:
-
Phosphate buffer saline
- PCO:
-
Protein carbonyl
References
Barnham KJ, Masters CL, Bush AI (2004) Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov 3:205–214. doi:10.1038/nrd1330
Borggrefe T, Oswald F (2009) The Notch signaling pathway: transcriptional regulation at Notch target genes. Cell Mol Life Sci 66:1631–1646. doi:10.1007/s00018-009-8668-7
Boucher LJ, Farrell MO (1973) Manganese schiff’s bases complexes-Ι: synthesize and spectroscopy of some anion complexes (4-sec butylsalicylaldehydeethylenediiminato) manganese (ш). J Inorg Nucl Chem 35:3731–3738
Bray S (1998) Notch signaling in Drosophila: three ways to use a pathway. Cell Dev Biol 9:591–597. doi:10.1006/scdb.1998.0262
Cadenas E, Davies KJ (2000) Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med 29:222–230. doi:10.1016/S0891-5849(00)00317-8
Chin YE, Kitagawa M, Su WC, You ZH, Iwamoto Y, Fu XY (1996) Cell growth arrest and induction of cyclin-dependent kinase inhibitor p21 WAF1/CIP1 mediated by STAT1. Science 272:719–722. doi:10.1126/science.272.5262.719
Conde de la Rosa L, Schoemaker MH, Vrenken TE, Buist-Homan M, Havinga R, Jansen PL et al (2006) Superoxide anions and hydrogen peroxide induce hepatocytes death by different mechanisms: involvement of JNK and ERK MAP kinases. J Hepatol 44:918–929. doi:10.1016/j.jhep.2005.07.034
Criddle DN, Gillies S, Baumgartner-Wilson HK, Jaffar M, Chinje E, Passmore S et al (2006) Menadione-induced reactive oxygen species generation via redox cycling promotes apoptosis of murine pancreatic acinar cells. J Biol Chem 281:40485–40492. doi:10.1074/jbc.M607704200
Doctrow SR, Huffman K, Marcus CB, Musleh W, Bruce A, Baudry M et al (1997) Salen-manganese complexes: combined superoxide dismutase/catalase mimics with broad pharmacological efficacy. Adv Pharmacol 38:247–269. doi:10.1016/S1054-3589(08)60987-4
Drapper HH, Hadley M (1990) Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol 186:421–431. doi:10.1016/0076-6879(90)86135-I
Dunning S, Hannivoort RA, Boer JF, Buist-Homan M, Faber KN, Moshage H (2009) Superoxide anions and hydrogen peroxide inhibit proliferation of activated rat stellate cells and induce different modes of cell death. Liver Int 29:922–932. doi:10.1111/j.1478-3231.2009.02004.x
El-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM et al (1993) WAF1, a potential mediator of p53 tumor suppression. Cell 75:817–825. doi:10.1016/0092-8674(93)90500-P
Flohe L, Gunzler WA (1984) Assays of glutathione peroxidase. Methods Enzymol 105:114–121
Furukawa T, Mukherjee S, Bao Z, Morrow E, Rax CC (2000) Hes1, and Notch1 promote the formation of Muller glia by postnatal retinal progenitor cells. Neuron 26:383–394
Gomes A, Fernandes E, Lima JLFC (2005) Fluorescence probes used for detection of reactive oxygen species. J Biochem Biophys Methods 65:45–80
Halliwell B, Gutteridge JMC (1990) Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol 186:1–85. doi:10.2165/00002512-200118090-00004
Hermes-Lima M (2004) Oxygen in biology and biochemistry: role of free radicals Functional. In: Storey KB (ed) Metabolism: regulation and adaptation. Wiley, Hoboken, pp 319–368. doi:10.1002/047167558X.ch12
Huang Q, Raya A, DeJesus P, Chao SH, Quon KC, Caldwell JS et al (2003) Identification of p53 regulators by genome-wide functional analysis. Cell Biol 101:3456–3461. doi:10.1073/pnas.0308562100
Jollow DJ, Mitchell JR, Zampaglione N, Gillete JR (1974) Bromobenzene induced liver necrosis: protective role of glutathione and evidence for 3,4 bromobenzeneoxide as the hepatotoxic intermediate. Pharmacology 11:151–169. doi:10.1159/000136485
Kubbutat MH, Jones SN, Vousden KH (1997) Regulation of p53 stability by Mdm2. Nature 387:299–303. doi:10.1038/387299a0
Lebel CP, Ischiropoulos H, Bondy SC (1992) Evaluation of the probe 2′,7′-dichlorofluorecein as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol 5:227–231. doi:10.1021/tx00026a012
Lu B, Rothenberg M, Jan LY, Jan YN (1998) Partner of Numb colocalizes with Numb during mitosis and directs Numb asymmetric localization in Drosophila neural and muscle progenitors. Cell 95:225–235. doi:10.1016/S0092-8674(00)81753-5
Markesbery WR (1997) Oxidative stress hypothesis in Alzheimer’s disease. Biol Med 23:134–147. doi:10.1016/S0891-5849(96)00629-6
McGill MA, McGlade CJ (2003) Mammalian Numb proteins promote Notch1 receptor ubiquitination and degradation of the Notch1 intracellular domain. J Biol Chem 278:23196–23203. doi:10.1074/jbc.M302827200
Miranda MI, Ramirez-Lugo L, Bermudez-Rattoni F (2000) Cortical cholinergic activity is related to the novelty of the stimulus. Brain Res 882:230–235. doi:10.1016/S0926-6410(00)00050-1
Oltvai ZN, Milliman CL, Korsmeyer SJ (1993) Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 74:609–619. doi:10.1016/0092-8674(93)90509-0
Rhee SG (1999) Redox signaling: hydrogen peroxide as intracellular messenger. Exp Mol Med 31:53–59. doi:10.1038/emm.1999.9
Rodriguez J, Lazebnik Y (1999) Caspase-9 and APAF-1 form an active holoenzyme. Genes Dev 13:3179–3184. doi:10.1101/gad.13.24.3179
Roegiers F, Jan YN (2004) Asymmetric cell division. Curr Opin Cell Biol 16:195–205. doi:10.1016/j.ceb.2004.02.010
Scheer N, Groth A, Hans S, Campos-Ortega JA (2001) An instructive function for Notch in promoting gliogenes is in the zebra fish retina. Development 128:1099–1107
Spana EP, Doe CQ (1996) Numb antagonizes Notch signaling to specify sibling neuron cell fates. Neuron 17:21–26. doi:10.1016/S0896-6273(00)80277-9
Verhagen AM, Ekert PG, Pakusch M, Silke J, Connolly LM, Reid GE et al (2000) Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell 102:43–53
Yang X, Klein R, Tian X, Cheng HT, Kopan R, Shen J (2004) Notch activation induces apoptosis in neural progenitor cells through a p53-dependent pathway. Dev Biol 269:81–94. doi:10.1016/j.ydbio.2004.01.014
Acknowledgments
The author appreciates the financial support of this investigation by the Research Council of University of Tehran.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kamarehei, M., Yazdanparast, R. Modulation of Notch Signaling Pathway to Prevent H2O2/Menadione-Induced SK-N-MC Cells Death by EUK134. Cell Mol Neurobiol 34, 1037–1045 (2014). https://doi.org/10.1007/s10571-014-0079-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-014-0079-0