Abstract
Two novel derivatives of carnosine—(S)-trolox-l-carnosine (STC) and (R)-trolox-l-carnosine (RTC) are characterized in terms of their antioxidant and membrane-stabilizing activities as well as their resistance to serum carnosinase. STC and RTC were synthesized by N-acylation of l-carnosine with (S)- and (R)-trolox, respectively. STC and RTC were found to react more efficiently with 2,2-diphenyl-1-picrylhydrazyl radical (DPPH) and protect serum lipoproteins from Fe2+-induced oxidation more successfully than carnosine and trolox. At the same time, STC, RTC and trolox suppressed oxidative hemolysis of red blood cells (RBC) less efficiently than carnosine taken in the same concentration. When oxidative stress was induced in suspension of cerebellum granule cells by their incubation with N-methyl-d-aspartate (NMDA), or hydrogen peroxide (H2O2), both STC and RTC more efficiently decreased accumulation of reactive oxygen species (ROS) than carnosine and trolox. Both STC and RTC were resistant toward hydrolytic degradation by human serum carnosinase. STC and RTC were concluded to demonstrate higher antioxidant capacity and better ability to prevent cerebellar neurons from ROS accumulation than their precursors, carnosine and trolox.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oxidative modification of lipids and proteins caused by reactive oxygen species (ROS) is thought to be one of the main factors declining the viability of living cells through neurodegeneration or aging processes. In order to protect cells from ROS attack, various antioxidants are used in modern medicine. Carnosine (β-alanyl-l-histidine) is a naturally occurring antioxidant and cell protector, which is accumulated in large quantities in excitable tissues (skeletal muscles, heart, some parts of brain) (Boldyrev and Severin 1990; Boldyrev and Johnson 2002) where it plays an important role in protecting of lipids, proteins and nucleic acids from oxidative damage (Boldyrev 2007; Boldyrev et al. 2004; Leinsoo et al. 2006). Therefore, carnosine itself is used as cosmetics or dietary supplements, (Prokopieva et al. 2000; Rona et al. 2004; Babizhayev 2006) or as an antiulcer drug in a form of a Zn ion complex (“Polaprezinc”, Hamari Chemicals, Ltd, Japan) (Matsukura and Tanaka 2000; Mahmood et al. 2007).
One of the reasons for limited use of carnosine as a drug is considered to be its fast metabolism by means of serum and kidney carnosinases, which quickly decreases serum level of carnosine thus preventing its long-lasting action (Lenny et al. 1982; Pegova et al. 2000). In our work, we have combined carnosine with a vitamin E derivative, trolox, which is known to demonstrate an antioxidant capacity in several models both in vitro and in vivo (Grasborn-Frodl et al. 1996; Sharma and Kaundal 2007). This resulted in stabilization of the molecule against carnosinase attack with the same or even higher antioxidant and membrane-stabilizing capacity compared to carnosine or trolox.
Methods and Materials
Carnosine Derivatives
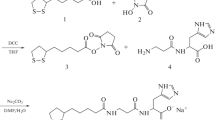
The following synthetic carnosine derivatives were used: (S)-6-hydroxy-2,5,7,8-tetramethylchroman-2-carbonyl-β-alanyl-l-histidine, (S)-trolox-l-carnosine (STC), and (R)-6-hydroxy-2,5,7,8-tetramethylchroman-2-carbonyl-β-alanyl-l-histidine, (R)-trolox-l-carnosine (RTC), being both water and ethanol soluble. Structure of the compounds is presented in Fig. 1.
The derivatives were originally synthesized in “Hamari Chemicals, Ltd” (Patent JP2008-19188), and their structure was identified using 1H- and 13C-NMR spectroscopy (200 and 50.3 MHz, respectively) and mass-spectrometry (ESI-MS).
Preparation of the N-acyl l-Carnosine Derivatives
Carnosine Benzyl Ester Bis(p-Toluenesulfonate)
p-Toluenesulfonic acid monohydrate (TsOH·H2O) (39.9 g, 0.21 mmol) was dissolved in a solution of l-carnosine (22.6 g, 0.1 mmol) in distilled water (100 ml), and the whole solution was concentrated in vacuo. To the resulting residue were added TsOH·H2O (5.0 g, 0.026 mmol), benzyl alcohol (75 ml) and chloroform (80 ml), and the whole suspension was refluxed with azeotropic removal of water for 12 h. After evaporation of chloroform in vacuo, ethyl acetate (AcOEt) (300 ml) was added to the residue, and the whole was stirred for 0.5 h, then the supernatant was taken off by decantation. The oily residue was further washed with AcOEt (4 × 300 ml) in the same manner, and the resulting viscous oil was dried in vacuo to give carnosine benzyl ester bis(p-toluenesulfonate) (52.5 g, 79.7%) as an amorphous solid: 1H-NMR (200 MHz, DMSO-d6) δ 2.30 (6H, s, 2 × Me), 2.45–2.56 (2H, m, CH2CO of β-Ala, overlapped with a signal of DMSO-d5), 2.88–3.23 (4H, m, NCH2 of β-Ala and β-H2 of His), 4.60–4.74 (1H, m, α-H), 5.12 (2H, s, CH2-Ph), 7.08–7.16 (4H, br d-like, ArH of TsOH), 7.28–7.53 (10H, m, ArH of CH2Ph and TsOH and 5-H of the imidazole ring), 7.68 (3H, br s), 8.99 (1H, d, J = 1.2 Hz, 2-H of the imidazole ring).
(R)-Trolox-l-Carnosine Benzyl Ester (RTC Benzyl Ester)
(R)-Trolox® (1.70 g, 6.8 mmol) was dissolved in a solution of carnosine benzyl ester bis(p-toluenesulfonate) (5.81 g, 8.8 mmol) in pyridine (5 ml) at 16–18°C. To this solution was added 1-ethyl-3-[3-(dimethylamino)propyl]carbodiimide hydrochloride (1.70 g, 9.8 mmol) at 3–5°C, and the whole was stirred at 16–8°C for 7 h. Pyridine was evaporated off in vacuo, and the residue was dissolved in AcOEt (30 ml). The resulting solution was washed with 10% aq. NaCl, and the aqueous phase was extracted with AcOEt (20 ml). The combined organic phase was washed with 10% aq. NaCl (20 ml), dried (MgSO4) and concentrated in vacuo. The oily residue thus obtained was flash chromatographed on silica gel (45 g, elution with chloroform, then 15:1 chloroform/methanol) to give RTC benzyl ester (1.79 g, 51.1%) as a white amorphous solid: 1H-NMR (200 MHz, CDCl3) δ 1.50 (3H, s, 2-Me), 1.69–1.88 (1H, m), 2.05–2.67 (5H, m, CH2CO of β-Ala and 3H of trolox), 2.07 (3H, s, Me), 2.15 (6H, s, 2 × Me), 2.90 and 2.97 (1H each, ABX system, J AB = 15.6 Hz, J AX = 6.1 Hz, β-H2 of His), 3.21–3.38 (1H, m, one of NCH2 of β-Ala), 3.49–3.67 (1H, m, one of NCH2 of β-Ala), 4.46 (1H, dt, J = 6.8, 6.1 Hz, α-H of His), 5.04 and 5.10 (1H each, ABq, J = 12.3 Hz, CH 2Ph), 6.33 (2H, br, ArOH and NH of the imidazole ring), 6.49 (1H, d, J = 1.0 Hz, 5-H of the imidazole ring), 6.54 (1H, d, J = 6.8 Hz, NH of His), 7.16 (1H, dd, J = 7.0, 5.2 Hz, NH of β-Ala), 7.22–7.37 (5H, m, ArH), 7.49 (1H, d, J = 1.0 Hz, 2-H of the imidazole ring).
(S)-Trolox-l-Carnosine Benzyl Ester (STC Benzyl Ester)
According to the same procedure described earlier, STC benzyl ester was obtained from carnosine benzyl ester bis(p-toluenesulfonate) and (S)-trolox® in the form of a white amorphous solid (81%): 1H-NMR (200 MHz, CDCl3) δ 1.47 (3H, s, 2-Me), 1.77–1.94 (1H, m), 2.06 (3H, s, Me), 2.13 (6H, s, 2 × Me), 2.18–2.68 (5H, m, CH2CO of β-Ala and 3H of trolox), 2.99 and 3.05 (1H each, ABX system, J AB = 15.6 Hz, J AX = 5.7 Hz, β-H2 of His), 3.37–3.56 (2H, m, NCH2 of β-Ala), 4.73 (1H, dt, J = 7.5, 5.7 Hz, α-H of His), 5.03 and 5.09 (1H each, ABq, J = 12.3 Hz, CH 2Ph), 5.90 (2H, br, ArOH and NH of the imidazole ring), 6.55 (1H, d, J = 0.9 Hz, 5-H of the imidazole ring), 7.11 (1H, d, J = 7.5 Hz, NH of His), 7.18–7.35 (6H, m, ArH and NH of β-Ala), 7.47 (1H, d, J = 0.9 Hz, 2-H of the imidazole ring).
(R)-Trolox-l-Carnosine (RTC)
The reaction vessel was charged sequentially with RTC benzyl ester (700 mg, 1.27 mmol), methanol (3 ml) and 10% Pd–C (containing 52.7% of water, 100 mg) and then evacuated and refilled with hydrogen four times followed by vigorous stirring under hydrogen atmosphere at 16–18°C for 5 h. The catalyst was filtered off, and the filtrate was concentrated in vacuo. The residual amorphous solid was washed as a slurry with AcOEt (5 ml), then chloroform (3 ml), dissolved in methanol (3 ml) and concentrated in vacuo to give RTC (564 mg, 97%) as a pale-yellow amorphous solid, whose HPLC assay showed 99.7 area %: 1H-NMR (200 MHz, CD3OD) δ 1.44 (3H, s, 2-Me), 1.81 (1H, ddd, J = 13.4, 8.2, 6.2 Hz), 2.05 (3H, s, Me), 2.13 (3H, s, Me), 2.14 (3H, s, Me), 2.17–2.70 (5H, m, CH2CO of β-Ala and 3H of trolox), 3.06 (1H, A part of ABX system, J AB = 15.3 Hz, J AX = 7.8 Hz, one of β-H2 of His), 3.20 (1H, B part of ABX system, J AB = 15.3 Hz, J BX = 5.6 Hz, one of β-H2 of His), 3.30–3.48 (2H, m, NCH2 of β-Ala), 4.66 (1H, dd, J = 7.8, 5.6 Hz, α-H of His), 7.27 (1H, d, J = 1.5 Hz, 5-H of the imidazole ring), 8.70 (1H, d, J = 1.5 Hz, 2-H of the imidazole ring); 13C-NMR (50.3 MHz, CD3OD) δ 12.02, 12.35, 12.97, 21.56, 24.72, 28.22, 30.95, 36.04, 36.59, 52.89, 79.10, 118.48, 118.62, 122.19, 123.28, 124.85, 131.47, 135.04, 145.67 (4-C of the imidazole ring), 147.27 (2-C of the imidazole ring), 173.44 (C=O), 173.80 (C=O), 177.08 (C=O); ESI-MS m/z (M + H)+ 459.1 for C23H31N4O6 (459.2 for isotope model MH).
(S)-Trolox-l-Carnosine (STC)
According to the same procedure described for the preparation of RTC, STC was obtained from STC benzyl ester in the form of a pale-yellow amorphous solid (quant.), whose HPLC assay showed 99.3 area %: 1H-NMR (200 MHz, CD3OD) δ 1.43 (3H, s, 2-Me), 1.79 (1H, ddd, J = 13.4, 8.2, 6.4 Hz), 2.04 (3H, s, Me), 2.12 (3H, s, Me), 2.13 (3H, s, Me), 2.18–2.68 (5H, m, CH2CO of β-Ala and 3H of Trolox), 3.02 (1H, A part of ABX system, J AB = 15.3 Hz, J AX = 7.0 Hz, one of β-H2 of His), 3.16 (1 H, B part of ABX system, J AB = 15.3 Hz, J BX = 5.3 Hz, one of β-H2 of His), 3.33–3.46 (2H, m, NCH2 of β-Ala), 4.46 (1H, dd, J = 7.0, 5.3 Hz, α-H of His), 7.16 (1H, d, J = 1.1 Hz, 5-H of the imidazole ring), 8.47 (1H, d, J = 1.1 Hz, 2-H of the imidazole ring); 13C-NMR (50.3 MHz, CD3OD) δ 11.99, 12.39, 12.97, 21.59, 24.83, 29.46, 31.02, 36.23, 36.66, 54.93, 79.10, 118.30, 118.62, 122.34, 123.17, 124.96, 132.75, 134.89, 145.70 (4-C of the imidazole ring), 147.27 (2-C of the imidazole ring), 173.29 (C=O), 176.06 (C=O), 176.97 (C=O); ESI-MS m/z (M + H)+ 459.1 for C23H31N4O6.
The purity of the compounds synthesized was determined by using HPLC.
HPLC System for l-Carnosine and its Derivatives
The ≪ Luna 5 μ, C18(2) ≫ column (Phenomenex), 250 mm × 4.6 mm size was used. Mobile phase for carnosine assay included the following: heptanesulfonic acid (0.5%), NaH2PO4 (0.1 M, pH 2.7), acetonitrile/water (5/95, v/v); flow rate was 1 ml/min under isocratic regime (28°C); sample volume 20 μl and UV registration at 210 nm (O’Dowd et al. 1990). Retention time (tR) for l-carnosine was 7.3 min.
For analysis of carnosine derivatives, the same column was used but mobile phase contained 0.2% trifluoroacetic acid, acetonitrile/water ratio was 25/75 (v/v); other conditions were the same as for l-carnosine. tR for both of the compounds tested was 9.7 min.
Direct Oxidation of Stable DPPH Radical (DPPH Test)
Direct antioxidant activity of the compounds used was tested by measuring their ability to neutralize the stable 2,2-diphenyl-1-picrylhydrazyl radical (DPPH) (Schlesier et al. 2002; Friaa and Brault 2006). The antioxidant activity was measured as follows. To 3 ml DPPH (final concentration of 10 μM in ethanol), 5–60 μl of stock solution of compounds tested (trolox, STC and RTC were solubilized in 96% ethanol, and carnosine was solubilized in water, all in 30 mM concentration) to the final concentrations in the range of 0.015–0.600 mM were added. Decrease in optical density (measured at 519 nm) with time corresponding to the rate of DPPH neutralization was used to calculate antioxidant activity of the compounds, which was expressed in nmol/min using molar DPPH extinction coefficient equal to 9690 M−1cm−1.
Oxidative Hemolysis of Human Red Blood Cells (RBC)
Hemolysis of RBC isolated from healthy donors by standard procedure (Prokopieva et al. 2000) was initiated by adding of 0.4 mM NaClO to the sample tube containing 5 × 106 cells/ml and measured spectrophotometrically at 630 nm. Reaction medium contained 10 mM NaH2PO4, 10 mM Na4P2O7 (pH 9.0) and 125 mM NaCl. The reaction was stopped by adding 1 N HCl. The difference between initial and final values of optical density was taken up 100% hemolysis, and T0.5 values (calculated as half a period of total hemolysis) were used for comparison of the antihemolytic efficiency. The stock solutions of carnosine, STC and RTC (50 mM) and trolox (5 mM) were prepared in deionized water with adjustment of pH to 9.0 and added to the samples at final concentration in the range of 0.025–0.400 mM.
Fe2+-Induced in Vitro Lipoprotein Oxidation (Chemiluminescence Test, ChL Test)
The method used is based on the measurement of ChL in a suspension of lipoproteins being oxidized by Fe2+ ions added in excess to reaction medium (Fedorova et al. 1999). Lipoproteins were prepared from serum of healthy donors and placed into measuring unit of 1 ml of reaction medium (60 mM KH2PO4, 105 mM KCl, pH 7.45) in amount corresponding to that containing in 200 μl of serum. The stock solutions of carnosine, STC, RTC (50 mM) and trolox (5 mM) were prepared using reaction medium. Reaction was started by addition of 5 mM FeSO4 (final concentration), and ChL signal was monitored using LKB 1251 Chemiluminometer (Sweden) and was expressed in mV.
The typical experimental curve is presented in Fig. 2 where the parameters measured are also noted. These are “h”, the height of the initial ChL burst (reflects the stationary level of lipid hydroperoxides); “τ”, the lag period of Fe2+-induced oxidation (characterizes the resistance of the sample against oxidation being dependent on intrinsic antioxidant capacity of lipoproteins) and ν defining the initial rate of oxidation (measured as increment of ChL signal, mV/s) (Dobrota et al. 2005).
Cell-Protecting Ability of Carnosine, Trolox and its Derivatives, SRC and RTC
Primary (non-differentiated) culture of cerebellum granule cells prepared from 8- to 10-day-old rats was used in the experiments as described previously (Boldyrev et al. 2000). Concerning the protocol, cell suspension contains rather heterogeneous population of cells contaminated by some glial cells (Oyama et al. 1996). During flow cytometric analysis, however, from the total cell population, the area was gated with the size of 10 μm corresponded to neurons. This approach allows to discriminate neurons from other cells and to analyze neuronal response to oxidative stress independently of the other cells in the suspension (Sureda et al. 1998). Oxidative stress was induced by incubation of cell suspension with either specific ligand, N-methyl-d-aspartic acid (NMDA, 0, 25–1 mM during 30 min), or non-specific agent, hydrogen peroxide (H2O2, 20 mM, during 30 min). Percentage of dead cells was calculated using propidium iodide (PI), intracellular ROS level was measured with 2,7-dichlorodihydrofluorescein diacetate (DCF-DA) as a label (Boldyrev et al. 2004). Flow cytometric measurements were performed using « FACStar » (« Becton–Dickinson » , USA). In all cases, amount of dead cells was in the range of 15–25% and did not increase statistically significant during experimental procedure.
STC, RTC and carnosine were dissolved in Tyrode’s solution, initial concentration of all compounds being 125 mM (pH 7.4). Trolox was dissolved in DMSO at initial concentration of 250 mM. The compounds studied were added in a final concentration of 0.25 mM to cell suspension 60 min before induction of oxidative stress (in such a case, an amount of DMSO in trolox samples was small enough and did not damage the neuronal cells). Each portion of cells was divided into three groups to measure the parameters characteristic of intact cells, and those portions were exposed to NMDA or H2O2. The results were analyzed using WinMDI 2.7 computing program.
Hydrolysis of Carnosine Derivatives by Serum Carnosinase
Enzymatic hydrolysis of carnosine and its derivatives was measured using method described previously (Lenny et al. 1982). The method is based on measuring the disappearance of l-carnosine (or its derivatives) and subsequent histidine accumulation after incubation of potential substrates for carnosinase with human blood plasma. Accordingly to modified protocol (Pegova et al. 2000), donor’s blood was freshly collected in sterile-heparinized syringe, centrifuged at 500g for 10 min, and the supernatant was used in the experiments. Samples of 200 μl serum plasma were preincubated for 30 min at 37°C in a medium containing 800 μl of 125 mM Tris–HCl (pH 8.5), 300 μl of 5 mM CdCl2 (in 30 mM sodium citrate) and 300 μl of distilled water. The stock solutions of carnosine, STC and RTC (50 mM solution in deionized water) and trolox (250 mM solution in DMSO) were adjusted to pH 8.5—the value at which hydrolytic reaction is measured as described earlier (Lenny et al. 1982; Pegova et al. 2000). Then, 400 μl of the substrate solution was added to a final concentration of 10 mM. Aliquots of 100 μl were collected after appropriate incubation time (0–180 min), and the reaction was stopped by addition of 300 μl cold ethanol. Denatured proteins were removed by centrifugation (15 min at 3500g), and supernatant was used for HPLC analysis after 20-fold dilution.
Statistical Analysis
Results are presented as mean ± S.E.M. Statistical evaluation was performed using the Student’s t test; differences with a value of P < 0.05 were considered significant.
Results
Direct Oxidation of DPPH Radical
The data obtained from interaction of the compounds tested with DPPH radical are presented in Table 1 where the rate of decrease in optical density was used as an indicator of the ability to neutralize DPPH radical, reflecting direct antioxidant capacity of the compounds. Trolox and both carnosine derivatives, RTC and STC, demonstrated pronounced ability to quench DPPH radical, and at lower concentrations (0.025–0.100 mM), STC and RTC were more effective compared to trolox. At higher concentration of the compounds (0.3 mM), trolox had as high activity as STC while RTC still was more effective. Carnosine (0.025–0.150 mM) did not demonstrate an ability to interact with DPPH and interacted with the reagent very slowly at higher concentrations (0.3–0.6 mM). Thus, the compounds tested showed more efficient antioxidant capacity compared to carnosine or trolox.
Oxidative Hemolysis of Human Red Blood Cells
All the compounds tested did not induce RBC hemolysis themselves but slowed down the rate of oxidative hemolysis of human RBC. Figure 3 shows that the compounds tested taken at 0.05 mM concentration decreased the rate of hemolysis in the following rank of efficiency: STC < RTC ≪ carnosine. In Table 2, experimental results are presented for all four compounds tested, being used in concentration range between 0.025 and 1.2 mM. As shown in Table 2, efficiency of STC and RTC in protection against RBC hemolysis comparable to that of carnosine achieved at 2–3 times higher concentrations of the derivatives. Trolox demonstrated the smallest antihemolytic effect among all the compounds tested.
Fe2+-Induced Lipoprotein Oxidation
Before experiment, the compounds were tested on the ability to generate ChL flash (h) in the presence of Fe2+ ions with no lipoproteins added in order to check their auto-oxidation. All the compounds in concentrations up to 1 mM demonstrated negligible signal (2–5 mV vs. 129.0 ± 3.1, when ChL burst was induced by addition of Fe2+ to lipoproteins).
The results of comparative analysis of the ability to suppress Fe2+-induced lipoprotein oxidation are presented in Table 3. It is seen in Table 3 that carnosine sufficiently decreases the stationary level of hydroperoxides (h), and 50% inhibition of the hydroperoxide accumulation is achieved at about 0.5 mM concentration. Simultaneously, carnosine increased the lag period of oxidation, τ (which evidences the increase in resistance against oxidation), and decreased the rate of oxidation proportionally to its concentration. Trolox affected these parameters in a similar manner, being more effective than carnosine in terms of lag period of oxidation and oxidative rate (Table 2). It is important to note that the effect of trolox, RTC and STC appeared at lower concentrations (0.025–0.05 mM). At 0.5 mM, RTC and STC were more effective than carnosine in terms of suppression of hydroperoxide levels (h) and increase in resistance to oxidation (see changes in τ and v values in Table 3). Antioxidant capacity of trolox was lower than that of RTC and STC. Comparing all ChL parameters, one can conclude that RTC and STC are more effective than either carnosine or trolox (see Table 3).
Protection of Neuronal Cells From Oxidative Stress by l-Carnosine and its Derivatives
In order to estimate an ability of the compounds tested to protect neuronal cells from oxidative stress, we have applied flow cytometry, which is often used for direct measurement of intracellular ROS levels and viability of neuronal cells (Boldyrev et al. 2000, 2004). Oxidative stress was induced in our experiments by 30 min exposure of cerebellum granule cells with 0.25–1.0 mM NMDA that corresponds to excitotoxic conditions inducing oxidative stress in neurons (Boldyrev et al. 2000).
Figure 4 shows that after exposure of cells to NMDA, percentage of PI-labeled (necrotic) neurons (located in the upper part of dot plots, A and B) is slightly increased (from 15 ± 3% to 24 ± 5%, not statistically significant), and the viable cells (lower part of these dot plots) are moved to the right along the ROS axis. In viable cells (Fig. 4, lower part of dot plots in A and B and histograms in C and D), NMDA induces redistribution of neurons from low ROS area (M1) to high ROS area (M2) in such a manner that mean fluorescence of the cells was increased proportionally to time of incubation or NMDA concentration. In further experiments, mean fluorescence was used to characterize ROS levels in neuronal suspension. Effect of NMDA on this parameter in the range of 0.25–1 mM was proportional to its concentration and at the higher concentration, induced 25–30% increase in ROS levels, which was characteristic of oxidative stress in cells (Boldyrev et al. 2000).
The distribution of neuronal cells between viable and dead sub-populations (a and b) and intracellular level of reactive oxygen species, ROS (c and d) for the intact neurons (a and c) and after 30 min incubation with 0.5 mM NMDA (b and d) (typical experiment is present, FL1 and FL2 correspond to fluorescence of PI (ordinate, FL2) and DCF (abscissa, FL1)
Preincubation of cell suspension with carnosine, trolox, STC or RTC (0.25 mM) resulted in decrease in stationary ROS levels within the neurons, and the compounds tested demonstrated the following rank of efficiency: carnosine < trolox < STC < RTC (Fig. 5a, light gray bars). In the same manner, these compounds affected NMDA-induced increase in the intracellular ROS levels (Fig. 5a, dark gray bars).
Effect of carnosine, trolox, STC and RTC (0.25 mM each) on mean fluorescence of DCF (corresponding to intracellular ROS level) measured in the neuronal cells exposed during 30 min to NMDA (1 mM, A) or H2O2 (20 mM, B). Light gray bars represent ROS levels in the cells before, and dark gray bars those after exposure to the earlier mentioned inducers of oxidation stress. “*” and “&” correspond to statistically significant difference (P < 0.05) from the intact cells incubated with or without oxidative stress inducers
When the cell suspension was exposed to 20 mM H2O2 (a non-specific inducer of oxidative stress), accumulation of ROS in cell suspension was more pronounced, and similar protection was found in the presence of all four compounds tested (Fig. 5b).
Hydrolysis of l-Carnosine and its Derivatives by Human Serum Carnosinase
Comparative kinetics of hydrolysis of carnosine, STC and RTC in vitro are presented in Fig. 6. Carnosine is easily hydrolyzed under conditions used in agreement with the previous data (Pegova et al. 2000). Decrease in carnosine levels has been accompanied by histidine accumulation. STC and RTC were totally resistant to human serum carnosinase: even incubation during 3 h does not result in a measurable decrease in their amount in the medium; no histidine was also found.
Hydrolysis of carnosine (white columns), STC (light gray columns) and RTC (dark gray columns) during their incubation in the presence of human serum carnosinase; control sample contained no carnosinase (black columns). Initial concentration of each compound was 10 mkmoles/ml. “*” correspond to statistically significant difference (P < 0.05) from the control sample
Discussion
STC and RTC are novel compounds being not described before. They have been shown to possess better ability than carnosine or trolox to interact in vitro with hydrophobic DPPH radical (Table 1) thus demonstrating higher antioxidant capacity in the hydrophobic environment.
In addition, trolox, STC and RTC are able to protect human red blood cells against oxidative hemolysis; however, their efficiency is much less than that of carnosine (Table 2). It was found recently (Formazyuk et al. 1992; Hipkiss et al. 1998) that carnosine is able to interact with hypochlorous anion and to preserve cell structures against oxidative damage. β-Amino group of carnosine has been found to take part in this protection thus it is not surprising that trolox, STC and RTC are not able to prevent RBC from hypochlorite-induced hemolysis as effectively as carnosine.
At the same time, it is necessary to note that neither STC nor RTC induces themselves hemolytic damage of human RBC. This observation may be important for further pharmacological studies of these compounds as possible in vivo protectors of the organism against oxidative stress.
In addition, RTC and SRC were able to protect plasma lipoproteins against Fe2+-induced oxidation to a higher extent than carnosine (Table 3). Compared to carnosine, they suppressed the rate of lipoperoxides’ accumulation (ν), prolongated lag period (τ) and decreased the stationary level of hydroperoxides. This last parameter was affected by STC better than carnosine at all concentrations used and by RTC—at only high concentrations (Table 3). Thus, STC and RTC are able to interact directly with lipid hydroperoxides and to increase resistance of lipid moiety toward oxidative damage.
Antioxidant capacity of RTC and STC and their ability to protect neurons from oxidative stress induced by NMDA or hydrogen peroxide showed good correlation with each other. As shown in Fig. 5a, both RTC and STC restricted accumulation of ROS to the lower extent compared to carnosine and trolox. Figure 5b shows that the same is true for the oxidative stress induced by hydrogen peroxide. In other words, both specific (NMDA receptors-dependent) and unspecific (H2O2 induced) rise in ROS levels is efficiently prevented by both RTC and STC evidencing their strong antioxidant capacity showed in DPPH test (see Table 1). Carnosine and trolox decreased both stationary levels of ROS in neuronal cells and stress-induced ROS accumulation to much lower extent.
Trolox is able to prevent the oxidative damage induced by hydrogen peroxide and superoxide radical (Zeng et al. 1991; Forrest et al. 1994) or β-amyloid (Quintanilla et al. 2005) in vitro. It also demonstrates the efficiency in protection of laboratory animals at ischemia/reperfusion of liver (Zeng et al. 1991), methanol intoxication (Farbiszewski et al. 2000) or global brain ischemia (Gupta and Sharma 2006) at doses till 30 mg/kg body weight (use of higher doses is limited by its solubility). Effective concentrations of carnosine in the in vivo experiments are also high enough (50–100 mg/kg) (Boldyrev 2007). Recently, carnosine was successfully used to treat human beings under Parkinsonian disease (Boldyrev et al. 2008) or discirculatory encephalopathy (Fedorova et al. 2009), and the dose of carnosine used (1.5–2.0 g per day) was rather high. Thus, it is important to have drugs demonstrating such protecting effects at lower concentrations.
With this respect, it is important to stress that novel carnosine derivatives not only effectively prevent the cells against oxidative stress, but demonstrate high stability against serum carnosinase. As Fig 6 showed, they are totally resistant to enzymatic hydrolysis contrary with carnosine which content progressively decreased with time of incubation. This property of these carnosine derivatives may be useful to decrease the effective doses and to prolong their action in the organism. Thus, further studies are necessary to analyze the pharmacokinetics of these compounds in the body.
The data presented show that novel derivatives of carnosine described here, RTC and STC, may be interesting candidates to substitute carnosine in treatment of brain under oxidative stress in vivo. They may have an effect under both hydrophobic (DPPH test, Table 1) and hydrophilic (Fe2+-induced lipoprotein oxidation, Table 3) conditions. In addition, they may protect biological structures against oxidative injury more effectively (Fig. 5) and at lower concentrations (Table 3) than carnosine and exist in body fluids longer time than carnosine does. Thus, from our results, we can conclude that the novel carnosine derivatives can be considered as promising compounds for the further assay of their pharmaceutical activity.
References
Babizhayev MA (2006) Biological activities of the natural imidazole-containing peptidomimetics N-acetylcarnosine, carcinine and L-carnosine in ophthalmic and skin care products. Life Sci 78:2343–2357
Boldyrev AA (2007) Carnosine and oxidative stress in cells and tissues. Nova Science Publishers, Inc, New York
Boldyrev A, Johnson P (2002) Carnosine and related compounds: antioxidant dipeptides. In: Boldyrev AA, Johnson P (eds) Oxidative stress at molecular, cellular and organ levels. Research Signpost, Trivandrum, India, pp 101–113
Boldyrev AA, Severin SE (1990) The histidine-containing dipeptides, carnosine and anserine: distribution, properties and biological significance. Adv Enz Regul 30:175–194
Boldyrev A, Song R, Dyatlov V, Lawrence D, Carpenter D (2000) Neuronal cell death and reactive oxygen species. Cel Molec Neurobiol 20:433–450
Boldyrev A, Bulygina E, Leinsoo T, Petrushanko I, Tsubone S, Abe H (2004) Protection of neuronal cells against reaction oxygen species by carnosine and related compounds. Comp Biochem Physiol B Biochem Mol Biol 137:81–88
Boldyrev A, Fedorova T, Stepanova M, Dobrotvorskaya I, Kozlova E, Boldanova N, Bagyeva G, Ivanova-Smolenskaya I, Illarioshkin S (2008) Carnosine increases efficiency of DOPA therapy of Parkinson’s disease: a pilot study. Rejuv Res 11:821–827
Dobrota D, Fedorova T, Stvolinsky S, Babushikova E, Likavcanova K, Drgova A, Strapkova A, Boldyrev A (2005) Carnosine protects the brain of rats and mongolian gerbils against ischemic injury: after-stroke-effect. Neurochem Res 30:1283–1288
Farbiszewski R, Witek A, Skrzydlewska E (2000) N-acetylcysteine or Trolox derivative mitigate the toxic effects of methanol on the antioxidant system of rat brain. Toxicology 156:47–55
Fedorova TN, Boldyrev AA, Gannushkina IV (1999) Lipid peroxidation in experimental ischemia of the brain. Biochemistry (Moscow) 64:75–79
Fedorova TN, Belyaev MS, Trunova OA, Gnezditsky VV, Maximova MYu, Boldyrev AA (2009) Neuropeptide carnosine increases stability of lipoproteins and red blood cells as well as efficiency of immune competent system of patients with discirculatory encephalopathy. Biochemistry (Moscow) Suppl. A: Membrane and Cell Biol 3:62–65
Formazyuk VE, Gorshkova TYu, Boldyrev AA, Sergienko VI (1992) Characterization of chloramine complexes of carnosine with hypochlorite anion. Biokhimiia 57:1324–1329 Russian
Forrest VJ, Kang YH, McClain DE, Robinson DH, Ramakrishnan N (1994) Oxidative stress-induced apoptosis prevented by Trolox. Free Radic Biol Med 16:675–684
Friaa O, Brault D (2006) Kinetics of the reaction between the antioxidant Trolox® and the free radical DPPH in semi-aqueous solution. Org Biomol Chem 4:2417–2423
Grasborn-Frodl EM, Nakao N, Brundin P (1996) The lazaroid U-83836E improves the survival of rat embryonic mesencephalic tissue stored at 4°C and subsequently used for cultures or intracerebral transplantation. Brain Res Bull 39:341–347
Gupta S, Sharma SS (2006) Neuroprotective effects of trolox in global cerebral ischemia in gerbils. Biol Pharm Bull 29:957–961
Hipkiss AR, Worthington VC, Himsworth DT, Herwig W (1998) Protective effects of carnosine against protein modification mediated by malondialdehyde and hypochlorite. Biochim Biophys Acta 1380:46–54
Leinsoo T, Abe H, Boldyrev A (2006) Carnosine and related compounds protect double-helices DNA against oxidative injury. J Evol Biochem Physiol 42:453–456
Lenny JF, George RP, Weiss AM, Kucera CM, Chan PWH, Rinzler GS (1982) Human serum carnosinase: characterization, distinction from cellular carnosinase, and activation by cadmium. Clin Chim Acta 123:221–231
Mahmood A, Fitzgerald AJ, Marchbank T, Nyatsaki E, Murray D, Ghosh S, Playford RJ (2007) Zinc-carnosine, a health food supplement that stabilizes small bowel integrity and stimulates gut repair processes. Gut 56:168–175
Matsukura T, Tanaka H (2000) Applicability of zinc complex of L-carnosine for medical use. Biochemistry (Moscow) 65:817–823
O’Dowd JJ, Cairns MT, Trainor M, Robins DJ, Miller DJ (1990) Analysis of carnosine, homocarnosine, and other histidyl derivatives in rat brain. J Neurochem 55:446–452
Oyama Y, Carpenter DO, Chikahisa L, Okasaki E (1996) Flow-cytometric estimation on glutamate- and kainate-induced increases in intracellular Ca2+ of brain neurons: a technical aspects. Brain Res 728:121–124
Pegova A, Abe H, Boldyrev A (2000) Hydrolysis of carnosine and related compounds. Comp Biochem Physiol B Biochem Mol Biol 127:443–446
Prokopieva VD, Bohan NA, Johnson P, Abe H, Boldyrev AA (2000) Effects of carnosine and related compounds on the stability and morphology of erythrocytes from alcoholics. Alcohol Alcohol 35:44–48
Quintanilla RA, Muñoz FJ, Metcalfe MJ, Hitschfeld M, Olivares G, Godoy JA, Inestrosa NC (2005) Trolox and 17β-estradiol protect against amyloid β -peptide neurotoxicity by a mechanism that involves modulation of the Wnt signaling pathway. J Biol Chem 280:11615–11625
Rona C, Vailati F, Berardesca E (2004) The cosmetic treatment of winkles. J Cosmet Dermatol 3:26–34
Schlesier K, Harwat M, Bőhm V, Bitsch R (2002) Assessment of antioxidant activity by using different in vitro methods. Free Rad Res 36:177–187
Sharma SS, Kaundal RK (2007) Neuroprotective effects of 6-hydroxy-2, 5, 7, 8-tetramethylchroman-2-carboxylic acid (trolox), an antioxidant in middle cerebral artery occlusion induced focal cerebral ischemia in rats. Neurol Res 29:304–309
Sureda FX, Camins A, Pallas M, Trullas R, Escubedo E, Camarasa J (1998) Reactive oxygen production by glutamate agonists in dissociated cerebellar cells: a flow cytometric study. Gen Pharmacol 30:507–511
Zeng LH, Wu J, Carey D, Wu TW (1991) Trolox and ascorbate: are they synergistic in protecting liver cells in vitro and in vivo? Biochem Cell Biol 69:198–201
Acknowledgments
This work was supported by Hamari Chemicals Ltd and Russian Foundation for Basic Research (grants no. 07-04-00557 and 09-04-00507).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stvolinsky, S.L., Bulygina, E.R., Fedorova, T.N. et al. Biological Activity of Novel Synthetic Derivatives of Carnosine. Cell Mol Neurobiol 30, 395–404 (2010). https://doi.org/10.1007/s10571-009-9462-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-009-9462-7