Abstract
Glutamate neurotoxicity has been postulated to play a prominent role in glaucoma. In this study the possible roles of two subunits of glutamate receptors during the early phase of retinal ganglion cell (RGC) loss in a rat chronic ocular hypertension (COH) model were investigated. COH was induced by applying argon laser to the episcleral and limbal veins of the right eye of rats, the observation times were at 4, 14 and 28 days after the first laser. RGCs were retrogradely labeled by putting Fluoro-Gold (FG) on the surface of both side superior colliculus. Immunohistochemical staining using specific antibodies against N-methyl-d-aspartate receptor 1 (NR1) or glutamate receptor 2/3 (GluR2/3) was performed on the retinal sections of normal and COH eyes. Fluorescent images were captured using confocal laser scanning microscope and the number of NR1 and GluR2/3 labeled cells were counted and cell size was measured using Stereo Investigator. During the observation period, the numbers of NR1 and GluR2/3 positive RGCs in the RGC layer were reduced parallel to the loss of RGC. The dramatic loss of GluR2/3 immunoreactive neurons occurred starting immediately after the first laser to 4 days while the dramatic loss of NR1 immunoreactive neurons occurred from 14 to 28 days after the first laser. Size difference was detected in NR1 immunoreactive RGCs, large ones were more sensitive to the high ocular pressure. These results suggest that both NR1 and GluR2/3 are involved in the mediation of RGC death in the early stage of COH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glaucoma is an aging-related neurodegenerative disease associated with irreversible loss of retinal ganglion cells (RGCs) and their axons. It is the second leading cause of blindness worldwide (Resnikoff et al. 2004). Clinically, glaucoma patients have characteristic visual field loss, suggesting that a subpopulation of the RGCs is more vulnerable to injury. Morphologically, large RGCs which are related to the magnocellular pathway are selectively damaged in human and experimental glaucoma in monkey (Glovinsky et al. 1991, 1993; Quigley et al. 1989). However, the mechanism of differential loss is not known yet and glutamate neurotoxicity has been postulated to play a prominent role (Luo et al. 2001; Zhou et al. 2008). The elevated level of glutamate in the vitreous body of glaucomatous eyes was detected in the animal models using rats, monkeys and dogs (Dreyer et al. 1996; Brooks et al. 1997). Both in vivo and in vitro exposure to glutamate or NMDA can cause damage in RGCs (Lam et al. 1999; Zhou et al. 2008), and their toxic effects can be blocked by antagonist of NMDA, MK-801(Chaudhary et al. 1998).
Glutamate exerts its toxic effect on RGCs through the glutamate receptor subtypes. Glutamate receptors are divided into ionotropic and metabotropic (G protein-coupled) receptor subtypes. According to their preferred agonists, ionotropic glutamate receptors are subdivided into N-methyl-d-aspartate (NMDA) and non-NMDA receptors. The latter includes α-amino-3-hydroxyl-5-methyl-isoxazol-4-propionic acid (AMPA) and kainate subtypes. In addition, many subunits of the subtypes have been identified. They are NMDA receptor 1 (NR1), NR2A-D and NR3A-B of NMDA receptors; glutamate receptor 1-4 (GluR1-4) of AMPA receptors; GluR5-7 of kainate receptors; and metabotropic glutamate receptor 1-8 (mGluR1-8) (Hollmann and Heinemann 1994; Nakanishi et al. 1998; Chatterton et al. 2002). All subunits of NMDA and AMPA receptors are distributed in the RGCs and amacrine neurons of the rat retina visualized with immunohistochemistry (Grunder et al. 2000a, b). We hypothesize that the distribution and function of the glutamate receptor subunits (NR1 and GluR2/3) might be involved in the RGC vulnerability at early stage of ocular hypertensive insult.
Using the laser photocoagulation induced rat chronic ocular hypertension (COH) model (Chan et al. 2007; Ji et al. 2004; Li et al. 2006), retrograde labeling and immunohistochemical analysis were used together to detect the temporal change of NR1 or GluR2/3 labeled RGC at early stage of this COH model. Result showed that the dramatic loss of GluR2/3 immunoreactive neurons occurred before that of the NR1 immunoreactive neurons. Loss of NR1 immunoreactive RGCs were positively related with the cell size under COH.
Materials and Methods
Experimental Glaucoma Model
Twenty normal adult female Sprague-Dawley (SD) rats (250–280 g) were obtained from the Laboratory Animal Unit of the LKS Faculty of Medicine, The University of Hong Kong, and were maintained in a temperature-controlled room with a 12 h light/dark cycle. Handling of animals adhered to the ARVO statement for the use of animals in ophthalmic and vision research and was approved by the University of Hong Kong Committee for the Use of Live Animals in Teaching and Research (CULATR). Prior to IOP or any other operation, the rats were anesthetized with an intra-peritoneal injection of a ketamine/xylazine mixture (ketamine 80 mg/kg and xylazine 8 mg/kg; Alfasan, Woerden, Holland). One drop of proparacaine hydrochloride (0.5% alcaine, Alcon-Couvreur, Belgium) was applied to the eyes as a topical anesthetic.
Experimental glaucoma was induced in the right eye of each animal using laser photocoagulation according to our previous publications (Chiu et al. 2007; Ji et al. 2004; Li et al. 2006; Fu et al. 2008). Left eye of each rat did not receive laser treatment and served as contralateral control for IOP. Briefly, the limbal vein and the three radical episcleral aqueous humor drainage veins (superior nasal, superior temporal and inferior temporal) were photocoagulated (power 1,000 mV; spot size 50–100 μm; duration, 0.1 s) using an Argon laser (Ultima 2000SE Argon Laser, Coherent, USA). About 60 laser spots around the limbal vein (except the nasal area) and 15–20 laser spots on each episcleral aqueous humor drainage vein were applied. To maintain high IOP, a second laser treatment at the same setting was applied 7 days later to block any reconnected vascular flow. After each laser treatment, ophthalmic Tobrex ointment (3% tobramyxin, Alcon-Couvreur, Belgium) was applied topically to prevent infection.
Five rats were used as normal control and experimental glaucoma was induced in the right eye of other fifteen rats. The animals survived for 4, 14 and 28 days (n = 5) after the first laser photocoagulation. IOP was measured with a Tonopen XL tonometer (Mentor®, Norwell, USA) before and on day 2, 4, 8, 14, 21, 28 after the first laser treatment. To avoid diurnal variation and the effect of anesthesia, all IOP measurements were taken at 10 a.m. and within 15–30 min after anesthesia using a ketamine and xylazine mixture (i.p.). Every time an average of ten records was used to determine the IOP of each eye.
About 7 days prior to euthanization, a small piece of gel foam (Upjohn, Kalamazoo, MI, USA) soaked with 6% Fluoro-Gold (FG; Fluorochrome, Denver, CO, USA) was placed over the entire surface of superior colliculus (SC) for retrograde labeling of the surviving RGCs (Chan et al. 2007; Chiu et al. 2008; Ji et al. 2004). Analgesic rimadyl (0.025 mg/ml) was applied in drinking water for 5 days after the surgery. Rats were killed with an over-dose of a mixture of ketamine/xylazine and transcardiac infused with 0.9% normal saline to flush out the blood. Both eyes were enucleated and then post-fixed with 4% paraformaldehyde in 0.1 M PB (pH 7.4) at 4°C overnight and then immersed in 30% sucrose. Ten-micron-thick retinal sections containing whole-length retinas and optic nerve heads were cut using a cryostat (CM1900, Leica) and mounted. Three consecutive sections containing optic nerve head were collected from each retina.
Immunofluorescence Staining of Retina
Immunofluorescence histochemistry was carried out following our previous method (Ng and Yung 2001). Briefly, the radial sections of retina were washed with 0.01 M PBS for 5 min and incubated in a mixture of 4% normal goat serum (NGS) and 0.01 M PBS containing 0.1% Triton X–100 for 20 min at room temperature. The sections were incubated in the primary antibodies of NR1 (1:200; Chemicon) or GluR2/3 (1:200; Chemicon) overnight at 4°C. The specificity of antibody was tested by omission of the primary antibody. After washing, the sections were incubated in the secondary antibodies of Alexa 568 (conjugated with anti-rabbit IgG, 1:1,000; Molecular Probe, Invitrogen, Calsbad, USA) for 2 h at room temperature. After thorough washes, the retinal sections were mounted using fluorescent mounting medium (Dako, Glostrup, Denmark) and retinal sections were scanned by a LSM-510 laser scan confocal microscope (Carl Zeiss). Confocal images from four areas in each retinal section from all groups were captured. The four areas are selected from both sides of the optic nerve head at 1,000 μm (paracentral) and 3,000 μm (peripheral) from the central of the optic nerve head. The length of the retina measured in each visual field was 225 μm. The cell size in each image was measured using Stereo Investigator® (MicroBrightField, Colchester, USA).
Statistical Analysis
The cell counts for each marker of all the groups were statistically analyzed with one-way ANOVA and Turkey test. Only cells labeled with FG were counted and therefore, amacrine cells were excluded from the analysis. The percentage of NR1 or GluR2/3 positive RGCs was calculated by obtaining the ratio of number of NR1 or GluR2/3 positive RGCs/total number of FG labeled RGCs. To analyze the correlation of the cell size and cell loss, the data were divided into two groups: normal and glaucoma. SigmaStat® was used to perform the linear regression analysis between cell number and cell size of RGCs in the control and glaucomatous retinas.
Results
Intraocular Pressure
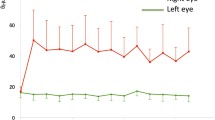
In consistence with our previous study using the same animal model (Chan et al. 2007; Fu et al. 2008; Ji et al. 2004; Li et al. 2006), the average IOP of normal eyes was around 15 mmHg. After photocoagulation using argon laser, the IOP of the right experimental eyes was increased on the second day after operation and reached the highest level (~27 mmHg) after the second laser treatment. The IOP was maintained at a high level (~22 mmHg) for 1 month after the first laser. At the same time, the IOP of the contralateral left control eyes remained at a much lower level (~15.5 mmHg; Fig. 1a). The IOP of laser treated eyes was significantly elevated compared with those of control eyes (* P < 0.001).
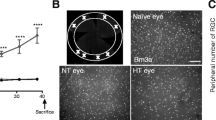
The changes of IOP and RGCs after the first laser photocoagulation. a IOP of the glaucomatous right eyes increased at second day after the first laser and the elevated IOP can be maintained for 1 month after two consecutive laser treatments on the limbal and episcleral drainage veins. IOP of the left eyes remained at normal level. At the same time point, the means of IOP from right eyes compared with that of contralateral left eyes were statistically different (* P < 0.01). b Density of RGC (per mm2) significantly decreased after the first laser photocoagulation. Compared with normal retina, there was significant percentage of RGC loss starting from 2 weeks after the first laser photocoagulation (* P = 0.011). Error bars represent the SEM
Loss of RGC in the Ocular Hypertension Rat Eyes
It is well known that about 98% of RGCs project to the contralateral SC in rat (Forrester and Peters 1967). Applying FG on both side of SC retrogradely labels the RGCs and provides an useful tool for investigating cell survival in the retina. On the flat mounted retina, the density of RGCs in the normal control eyes was 2,241 ± 27 cells/mm2 (Fig. 1b) and this was comparable with our previous reports (Li et al. 2006; Yip et al. 2006). Compared with normal retina, there was significant loss of RGC in the experimental eyes starting at 2 weeks after the first laser treatment (* P = 0.011) and there was about 1,964 RGCs/mm2 left at 1 month after the first laser (#P = 0.002, Fig. 1b). The loss of RGC was about 17% of the normal retina.
Same trend of RGC loss in the experimental glaucomatous retina was also observed in the radial retinal sections, there was progressive loss of FG labeled RGC in the retinal ganglion cell layer (RGCL) starting from 4 days and statistically significant from 14 days after the first laser photocoagulation (* P < 0.05, Fig. 2a).
Effects of ocular hypertension on the survival RGCs labeled with FG, NR1 and GluR2/3. a The number of FG labeled RGCs reduced from 4 to 14 (P < 0.05) and 28 days (P < 0.01). Compared with the normal control, NR1 positive RGCs was significantly decreased in the 14 and 28 days glaucoma (P < 0.01); GluR2/3 immunoreactive RGCs was statistically reduced in 14 and 28 days glaucoma (P < 0.01). b Percentage of NR1 positive RGCs was relatively increased at 4 days after the first laser and then dramaticaly decreased at 14 and 28 days after the first laser. Percentage of Glu2/3 positive RGCs decreased right after the first laser and stabilized since 14 days after the first laser (spotted line)
Differential Loss of NR1 or GluR2/3 Labeled RGC in the Ocular Hypertension Eyes
In normal retinal section, about 80% of the FG labeled RGCs expressed NR1 (Figs. 2b, 3c) while about 85% of FG labeled RGCs expressed GluR2/3 (Figs. 2b, 4c). A few of NR1 or GluR2/3 positive neurons in the RGCL that were small in size did not contain FG particles in the cytoplasm, which might be displaced amacrine cells (Figs. 3, 4).
Confocal micrographs of FG and NR1 positive RGCs in normal and glaucomatous retinas. FG labeled RGCs (a, d, g, j), immunohistochemical staining of NR1 (b, e, h, k, arrowheads) and merged (c, f, i, l, arrows) in normal control (a–c) and glaucomatous retinas (d–l). Some of the large RGCs were double labeled by FG and NR1 (yellow in c, f,) while reduced in 14 and 28 days glaucoma (i, l). Scale bar 20 μm. INL, inner nuclear layer; IPL, inner plexiform layer; RGCL, retinal ganglion cell layer. (Color figure online)
Confocal micrographs of FG and GluR2/3 positive RGCs in normal and glaucomatous retinas. FG labeled RGCs (a, d, g, j), immunohistochemical staining of GluR2/3 (b, e, h, k, arrowheads) and merged (c, f, i, l, arrows) in normal control (a–c) and glaucomatous retinas (d–l). Most of the double labeled RGCs by FG and GluR2/3 are medium in size (yellow in c, f, i, l). Scale bar 20 μm. INL, inner nuclear layer; IPL, inner plexiform layer; RGCL, retinal ganglion cell layer. (Color figure online)
NR1 positive neurons were detected in both the RGCL and the inner nuclear layer (INL) (Fig. 3b, e, h, k, arrowheads). At 4 days after the first laser, the number of NR1 positive neurons in the RGCL was similar to normal retina (Figs. 2a, 3c, f, arrows). NR1 positive RGC was significantly reduced starting from 14 days (#P < 0.05, Figs. 2a, 3i, l). When calculated the percentage of NR1 positive FG labeled RGC loss, there was an upregulation at 4 days in the surviving RGCs after the first laser. The dramatic loss of NR1 positive RGCs mainly occurred from 14 to 28 days after the first laser (Fig. 2b).
The similar localization of GluR2/3 immunoreactive neurons could be found in the RGCL and the INL of retina (Fig. 4b, e, h, k, arrowheads). FG and GluR2/3 double labeled RGCs were mainly localized in the small to medium sized neurons in the RGCL (Fig. 4c, f, i, l, arrows). The loss of GluR2/3 positive RGCs started immediately after the first laser (Fig. 2b). In COH retina, the largest reduce in the number of GluR2/3 positive RGCs was from immediate after the first laser to day 4 (Fig. 2) and there was significant loss at 14 days after the first laser (* P < 0.05, Figs. 2a, 4i, l). Quantitatively, at 28 days after the first laser, there was an overall 5.8% loss of GluR2/3 immunoreactive RGC and 26.7% loss of NR1 immunoreactive RGC in the COH eye compared with the normal control eye.
Loss of NR1 Immunoreactive RGCs was Size Dependent
In the radial retinal sections the size of FG retrograde labeled RGCs was different, ranging from 50 to 350 μm2. Most of lost RGCs were larger than 150 μm2 while the RGCs smaller than 150 μm2 was relatively increased in the glaucomatous retinas (Fig. 5). NR1 positive large ganglion cells are more sensitive to the elevated IOP. The loss of NR1 positive RGCs was limited to cells larger than 100 μm2 (Fig. 6). And regressive analysis of NR1 positive RGCs in the glaucomatous retinas showed that correlating factor was 0.76, indicating that there was a positive correlation (#P < 0.05) between the loss of cell and cell size. The size of GluR2/3 positive neurons was relatively small with largest at 250 μm2 and the majority at around 100 μm2. And the loss of GluR2/3 positive RGCs was not significantly related with cell size (R = 0.56, P > 0.36, Fig. 7).
Sectional area of FG labeled RGCs showing the number of RGCs was decreased in the glaucomatous retinas. It shows that the number of the small RGCs relatively increased while the large RGCs decreased severely but the correlation between the number of cell loss and cell size in the glaucomatous retinas was not significant (R = 0.13, P > 0.5)
Sectional areas of GluR2/3 positive RGCs showing that most of GluR2/3 positive RGCs were medium to small sized. It shows that the number of small to large GluR2/3 positive RGCs was slightly reduced in the glaucomatous retinas. There was no significant positive correlation between cell size and the number of cell loss (R = 0.52, P > 0.05)
Discussion
Glutamate and its agonists are involved in the RGC loss in the pathology of glaucoma. The neurotoxic effect of glutamate is mediated by various subunits of glutamate receptors. The mRNA of the glutamate receptor subunits NR1, NR2A-C, KA2 and GluR6–7 are shown to be expressed in the RGCL of the rat retina using in situ hybridization (Brandstatter et al. 1994). Our study using retrograde labeling and immunohistochemical methods identify simultaneously FG and NR1 or GluR2/3 in the same neuron has enabled us to study the distribution of NR1 or GluR2/3 in the surviving RGC in the normal and glaucomatous retinas.
In normal rat retina, about 80.4% RGC expressed NR1 and 85.4% RGC expressed GluR2/3 (Fig. 2a). Most of the NR1 positive RGCs were medium to large sized (Figs. 3, 6). While, the size of the RGCs labeled with GluR2/3 were of small to medium size (Figs. 4, 7). These findings suggest that there are selective expressions of glutamate receptor subunits in the RGCs and for a certain portion of RGCs, there were both NR1 and GluR2/3 expression.
NR1 is the major NMDA receptor subunit that is known to form the NMDA ion channel (Kutsuwada et al. 1992; Meguro et al. 1992; Monyer et al. 1992; Moriyoshi et al. 1991). A functional NMDA channel is suggested to be one of the key players to mediate glutamate neurotoxicity (Choi 1994). During the 28 days period of COH, the loss of NR1 positive RGC was about 26.7%. Compared with the 5.8% loss of GluR2/3 positive RGC and the overall loss of RGC at about 17%, the present results indicate that the subgroups of RGCs that are NR1-immunoreactive are more vulnerable to degeneration in the early phase of COH. However, the loss of NR1-positive RGCs did not start until around 4 days after the first laser and the expression of NR1 in RGCs was increased at the very first stage of onset of glaucoma (Fig. 2b). The upregulation of NR1 expression in the RGCs at early onset of ocular hypertension was consistent with the finding that the mRNA of NR1 was upregulated in the 1 week glaucomatous rat retina in the cauterization model (Kim et al. 2007). The excessive expression of NR1 in the RGCs may be involved in the course of cellular degeneration of the RGCs in the early stage of glaucoma and leading to progressive RGC loss thereafter. This was proved in the monkeys chronic experimental glaucoma model with 21 to 416 weeks survival time, not only NR1 but also GluR2 positive neurons with neurofilament labeling were dramatically reduced over long observation period (Hof et al. 1998).
Compared with the upregulation of NR1 in RGCs during the first 4 days after the first laser, there was loss of GluR2/3 expression in RGCs. After that, the loss of GluR2/3 positive RGCs was stabilized at about 5.8% till the 28th day after the first laser. This may be related to the fact that GluR2 is considered as a “molecular switch” of the AMPA channel related to Ca2+ permeability in neurons (Bennett et al. 1996; Vandenberghe et al. 2000). With the expression of GluR2 subunit, the AMPA channels are less permeable to Ca2+. The neurons with a low Ca2+ permeability, i.e., those displayed GluR2/3 immunoreactivity in this case, are likely to be less susceptible to glutamate-induced neurotoxicity. Internalization and/or downregulation of GluR2 were found in the early stage of ischemic or degenerative disease, that may related to excitotoxic neuronal death (Liu et al. 2006; Zhao et al. 2008). Similarly, in our study those RGCs showing down regulated expression of GluR2/3 at 4 days after the first laser might link to the switching on the NR1 overexpression in the same RGC and lead to dramatic loss of RGCs in the following time points.
The selective cell death, where by large RGCs were selectively lost in human and experimental glaucoma has been demonstrated previously by Glovinsky et al. (1991). Furthermore, glutamate or NMDA has been found to be more toxic to large RGCs both in tissue culture and in the intact rat eye while cells smaller than 10 μm diameter were relatively unaffected by glutamate or NMDA (Dreyer et al. 1994). Vickers et al.(1995) reported that large ganglion cells labeled with neurofilament specific antibodies were severely damaged in the glaucomatous eyes. Our study further confirmed these findings and demonstrated that compared with GluR2/3 positive RGCs, the NR1 positive RGCs were more vulnerable to increased intraocular pressure and the loss of NR1 positive RGCs were size dependent.
In summary, our data showed that NR1 positive large RGCs were the most vulnerable type under the stress of ocular hypertension. Early down regulation of GluR2/3 in the RGCs may trigger up regulation of the NR1 in the same RGC and involved in the early phase of ocular hypertension. However, the RGC death in glaucoma is a very complicated process involving the interaction of two or more receptors. Further works are needed to elucidate the mechanism of RGC death in glaucoma.
References
Bennett MVL, Pellegrini-Giampietro DE, Gorter JA, Aronica E, Connor JA, Zukin RS (1996) The GluR2 hypothesis: Ca++-permeable AMPA receptors in delayed neurodegeneration. Cold Spring Harb Symp Quant Biol 61:373–384
Brandstatter JH, Hartveit E, Sassoepognetto M, Wassle H (1994) Expression of NMDA and high-affinity kainite receptor subunit messenger-RNAs in the adult-rat retina. Eur J NeuroSci 6:1100–1112. doi:10.1111/j.1460-9568.1994.tb00607.x
Brooks DE, Garcia GA, Dreyer EB, Zurakowski D, Franco-Bourland RE (1997) Vitreous body glutamate concentration in dogs with glaucoma. Am J Vet Res 58:864–867
Chan HC, Chang RCC, Ip AKC et al (2007) Neuroprotective effects of Lycium barbarum Lynn on protecting retinal ganglion cells in an ocular hypertension model of glaucoma. Exp Neurol 203:269–273. doi:10.1016/j.expneurol.2006.05.031
Chatterton JE, Awobuluyi M, Premkumar LS et al (2002) Excitatory glycine receptors containing the NR3 family of NMDA receptor subunits. Nature 415:793–798
Chaudhary P, Ahmed F, Sharma SC (1998) MK801—a neuroprotectant in rat hypertensive eyes. Brain Res 792:154–158. doi:10.1016/S0006-8993(98)00212-1
Chiu K, Chang RCC, So KF (2007) Laser induced rat chronic ocular hypertension model. Journal of Visualized Experiments 10: http://www.jove.com/index/Details.stps?ID=549
Chiu K, Lau WM, Yeung SC, Chang RCC, So KF (2008) Retrograde labeling of retinal ganglion cells by application of Fluoro-Gold on the surface of superior colliculus. Journal of Visualized Experiments 16: http://www.jove.com/index/Details.stp?ID=819
Choi DW (1994) Glutamate receptors and the induction of excitotoxic neuronal death. Prog Brain Res 100:47–51
Dreyer EB, Pan ZH, Storm S, Lipton SA (1994) Greater sensitivity of larger retinal ganglion-cells to NMDA-mediated cell death. Neuroreport 5:629–631. doi:10.1097/00001756-199401000-00024
Dreyer EB, Zurakowski D, Schumer RA, Podos SM, Lipton SA (1996) Elevated glutamate levels in the vitreous body of humans and monkeys with glaucoma. Arch Ophthalmol 114:299–305
Forrester J, Peters A (1967) Nerve fibres in optic nerve of rat. Nature 214:245–247. doi:10.1038/214245a0
Fu QL, Wu W, Wang H, Li X, Lee VWH, So KF (2008) Up-regulated endogenous erythropoietin/erythropoietin receptor system and exogenous erythropoietin rescue retinal ganglion cells after chronic ocular hypertension. Cell Mol Neurobiol 28:317–329. doi:10.1007/s10571-007-9155-z
Glovinsky Y, Quigley HA, Dunkelberger GR (1991) Retinal ganglion-cell loss is size dependent in experimental glaucoma. Invest Ophthalmol Vis Sci 32:484–491
Glovinsky Y, Quigley HA, Pease ME (1993) Foveal ganglion-cell loss is size dependent in experimental glaucoma. Invest Ophthalmol Vis Sci 34:395–400
Grunder T, Kohler K, Guenther E (2000a) Distribution and developmental regulation of AMPA receptor subunit proteins in rat retina. Invest Ophthalmol Vis Sci 41:3600–3606
Grunder T, Kohler K, Kaletta A, Guenther B (2000b) The distribution and developmental regulation of NMDA receptor subunit proteins in the outer and inner retina of the rat. J Neurobiol 44:333–342. doi:10.1002/1097-4695(20000905)44:3<333::AID-NEU4>3.0.CO;2-S
Hof PR, Lee PY, Yeung G, Wang RF, Podos SM, Morrison JH (1998) Glutamate receptor subunit GluR2 and NMDAR1 immunoreactivity in the retina of macaque monkeys with experimental glaucoma does not identify vulnerable neurons. Exp Neurol 153:234–241. doi:10.1006/exnr.1998.6881
Hollmann M, Heinemann S (1994) Cloned glutamate receptors. Annu Rev Neurosci 17:31–108. doi:10.1146/annurev.ne.17.030194.000335
Ji JZ, Elyaman W, Yip HK et al (2004) CNTF promotes survival of retinal ganglion cells after induction of ocular hypertension in rats: the possible involvement of STAT3 pathway. Eur J NeuroSci 19:265–272. doi:10.1111/j.0953-816X.2003.03107.x
Kim JH, Lee NY, Jung SW, Park CK (2007) Expression of N-methyl-d-aspartate receptor 1 in rats with chronic ocular hypertension. Neuroscience 149:908–916. doi:10.1016/j.neuroscience.2007.07.056
Kutsuwada T, Kashiwabuchi N, Mori H et al (1992) Molecular diversity of the NMDA receptor channel. Nature 358:36–41. doi:10.1038/358036a0
Lam TT, Abler AS, Kwong JMK, Tso MOM (1999) N-methyl-d-aspartate (NMDA)-induced apoptosis in rat retina. Invest Ophthalmol Vis Sci 40:2391–2397
Li RS, Chen BY, Tay DK, Chan HHL, Pu ML, So KF (2006) Melanopsin-expressing retinal ganglion cells are more injury-resistant in a chronic ocular hypertension model. Invest Ophthalmol Vis Sci 47:2951–2958. doi:10.1167/iovs.05-1295
Liu BS, Liao MX, Mielke JG et al (2006) Ischemic insults direct glutamate receptor subunit 2-lacking AMPA receptors to synaptic sites. J Neurosci 26:5309–5319. doi:10.1523/JNEUROSCI.0567-06.2006
Luo XM, Heidinger V, Picaud S et al (2001) Selective excitotoxic degeneration of adult pig retinal ganglion cells in vitro. Invest Ophthalmol Vis Sci 42:1096–1106
Meguro H, Mori H, Araki K et al (1992) Functional-characterization of a heteromeric NMDA receptor channel expressed from cloned cDNAs. Nature 357:70–74. doi:10.1038/357070a0
Monyer H, Sprengel R, Schoepfer R et al (1992) Heteromeric NMDA receptors—molecular and functional distinction of subtypes. Science 256:1217–1221. doi:10.1126/science.256.5060.1217
Moriyoshi K, Masu M, Ishii T, Shigemoto R, Mizuno N, Nakanishi S (1991) Molecular-cloning and characterization of the rat NMDA receptor. Nature 354:31–37. doi:10.1038/354031a0
Nakanishi S, Nakajima Y, Masu M et al (1998) Glutamate receptors: brain function and signal transduction. Brain Res Brain Res Rev 26:230–235. doi:10.1016/S0165-0173(97)00033-7
Ng TKY, Yung KKL (2001) Differential expression of GABA(B)R1 and GABA(B)R2 receptor immunoreactivity in neurochemically identified neurons of the rat neostriatum. J Comp Neurol 433:458–470. doi:10.1002/cne.1153
Quigley HA, Dunkelberger GR, Green WR (1989) Retinal ganglion-cell atrophy correlated with automated perimetry in human eyes with glaucoma. Am J Ophthalmol 107:453–464
Resnikoff S, Pascolini D, Etya’ale D et al (2004) Global data on visual impairment in the year 2002. Bull World Health Organ 82:844–851
Vandenberghe W, Robberecht W, Brorson JR (2000) AMPA receptor calcium permeability, GluR2 expression, and selective motoneuron vulnerability. J Neurosci 20:123–132
Vickers JC, Schumer RA, Podos SM, Wang RF, Riederer BM, Morrison JH (1995) Differential vulnerability of neurochemically identified subpopulations of retinal neurons in a monkey model of glaucoma. Brain Res 680:23–35. doi:10.1016/0006-8993(95)00211-8
Yip HKF, Lai TYY, So KF, Kwok AKH (2006) Retinal ganglion cells toxicity caused by photosensitising effects of intravitreal indocyanine green with illumination in rat eyes. Br J Ophthalmol 90:99–102. doi:10.1136/bjo.2005.076042
Zhao PW, Ignacio S, Beattie EC, Abood ME (2008) Altered presymptomatic AMPA and cannabinoid receptor trafficking in motor neurons of ALS model mice: implications for excitotoxicity. Eur J NeuroSci 27:572–579. doi:10.1111/j.1460-9568.2008.06041.x
Zhou W, Zhu X, Zhu L et al (2008) Neuroprotection of muscarinic receptor agonist pilocarpine against glutamate-induced apoptosis in retinal neurons. Cell Mol Neurobiol 28:263–275. doi:10.1007/s10571-007-9251-0
Acknowledgments
This study was supported by funding from the Jessie Ho Professorship in Neuroscience (The University of Hong Kong Foundation for Educational Development and Research Limited, and donation from Mr. George Ho), and donations from Madame Tung Shai Yun, and Madame Annie Tsao Wen Wei. This study was also supported by the National Natural Science Foundation of China (No 30170303 and 30671100). We would like to thank Mrs. CH So for her comments in the writing and Ms. Wen for her technical assistance in the use of the confocal microscope.
Author information
Authors and Affiliations
Corresponding authors
Additional information
X.-G. Luo and K. Chiu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Luo, XG., Chiu, K., Lau, F.H.S. et al. The Selective Vulnerability of Retinal Ganglion Cells in Rat Chronic Ocular Hypertension Model at Early Phase. Cell Mol Neurobiol 29, 1143–1151 (2009). https://doi.org/10.1007/s10571-009-9407-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-009-9407-1