Abstract
Ischemic preconditioning (IPC) represents an important adaptation of CNS to sub-lethal ischemia, which results in increased tolerance of CNS to the lethal ischemia. Ischemia-induced mitochondrial apoptosis is considered to be an important event leading to neuronal cell death after cerebral blood flow arrest. In presented study, we have determined the effect of IPC on ischemia/reperfusion-induced mitochondrial apoptosis. Global brain ischemia was induced by permanent occlusion of vertebral arteries and temporal occlusion of carotid arteries for 15 min. Rats were preconditioned by 5 min of sub-lethal ischemia and 2 days later 15 min of lethal ischemia was induced. With respect to mitochondrial apoptosis initiation, translocation of p53 to mitochondria was observed in hippocampus but not in cerebral cortex. However, level of both apoptotic bax and anti-apoptotic bcl-xl in both hippocampal and cortical mitochondria was unchanged after global brain ischemia. Detection of genomic DNA fragmentation as well as Fluoro-Jade C staining showed that ischemia induces apoptosis in vulnerable CA1 layer of rat hippocampus. IPC abolished completely ischemia-induced translocation of p53 to mitochondria and had significant protective effect on ischemia-induced DNA fragmentation. In addition, significant decrease of Fluoro-Jade C positive cells was observed as well. Our results indicate that IPC abolished almost completely both initiation and execution of mitochondrial apoptosis induced by global brain ischemia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Ischemic preconditioning is a well-known phenomenon in which brief ischemic insults confer neuroprotection against a subsequent severe ischemic challenge (Kirino et al. 1991; Kitagawa et al. 1991; Liu et al. 1992; Nishi et al. 1993; Simon et al. 1993). The molecular mechanisms underlying ischemic tolerance are not yet fully understood. Among the multiple paradigms for ischemic preconditioning (IPC), two windows have been identified. One window that represents very rapid and short-lasting protective preconditioning induced within minutes after a brief ischemic episode due to post-translational changes in ion channel permeability and protein phosphorylation (Perez-Pinzon and Born 1999). A second one develops slowly (over days) after initial insult and considerable delay from the preconditioning stimulus until onset of ischemic tolerance is consistent with a role for transcriptional changes in adaptation (Kirino 2002; Dirnagl et al. 2003; Gidday 2006; Obrenovitch 2008). In addition to transcriptional changes, alterations in signal transduction cascades are considered to be involved in mechanisms of IPC. Finally, an important difference between these two windows is that neuroprotection in the first window is transient while the neuroprotection in the second window is robust and long lasting.
Several observations suggest that apoptosis plays an important role in the central nervous system (CNS), where it regulates developmental and physiological cell death (for recent review see Buss et al. 2006). More recently, mitochondrial apoptosis has also been demonstrated to play significant role in pathological processes in the adult CNS (Polster and Fiskum 2004). Mitochondrial apoptosis induced by brain ischemia has been documented in several studies at the level of caspase 9 activation (Krajewski et al. 1999; Tanaka et al. 2004), cytochrome c release (Perez-Pinzon et al. 1999; Sugawara et al. 1999), bax-dependent initiation (Hetz et al. 2005), and translocation of p53 to mitochondria (Endo et al. 2006a; Racay et al. 2007). With respect to IPC, it has been shown that IPC acts downstream of caspase-3 activation and upstream of its target caspase-activated DNase to prevent the onset of apoptotic cell death (Tanaka et al. 2004). In addition, IPC prevented completely translocation of p53 to mitochondria (Racay et al. 2007).
In this study, we have determined the effect of IPC on ischemia/reperfusion-induced mitochondrial apoptosis. Besides ischemia-induced translocation of p53 to hippocampal mitochondria, effect of ischemia-reperfusion and IPC on mitochondrial level of both bax and bcl-xl was investigated. Finally, the impact of IPC on ischemia-induced genomic DNA fragmentation was assessed as well.
Materials and Methods
Ischemia-Reperfusion and Ischemic Preconditioning
Animal studies were performed under a protocol approved by the State Veterinary and Food Department of Slovak Republic. A total of 60 adult male Wistar rats from the breeding house of the Institute of Experimental Pharmacology of Slovak Academy of Science (Dobra voda, Slovak Republic) were used. All animals were maintained on a 12/12-h light/dark cycle. Food and water were available ad libitum until the beginning of the experiment. Transient global cerebral ischemia was produced using the four-vessel occlusion model. Briefly, on day 1, both vertebral arteries were irreversibly occluded by coagulation through the alar foramina after anesthesia with 2% halothane, 30% O2, and 68% N2O mixture. On day 2, both common carotid arteries were occluded for 15 min by small clips under anesthesia with 2% halothane, 30% O2, and 68% N2O mixture. Two minutes before carotid occlusion, the halothane was removed from the mixture. Body temperature was maintained using a homoeothermic blanket. Global ischemia was followed by 1, 3, 24, and 72 h of reperfusion. Ischemic preconditioning was induced by 5 min of sub-lethal ischemia followed by 2 days of reperfusion. The rats then underwent lethal ischemia for a duration of 15 min as above, followed by 1, 3, or 24 h of reperfusion. After ischemia and particular time of reperfusion, animals were sacrificed by decapitation and both the hippocampi were dissected and processed immediately. Control animals for both naïve ischemia group and preconditioned ischemia group underwent the same procedure except for carotid occlusion.
Isolation of Mitochondria
Mitochondria from both whole hippocampi were isolated as described previously (Racay et al. 2007). A protocol adapted from Polosa and Attardi (1991) with modifications was used to prepare metabolically active non-synaptic mitochondria from cerebral cortex. Dissected tissue was homogenized in ice-cold homogenization buffer (25 mM HEPES pH = 7.4, 250 mM sucrose, 4 mM MgCl2, 0.05 mM EGTA) using Potter Teflon-glass homogenizer. Homogenate was centrifuged at 400×g for 5 min and supernatant was collected. Sediment was rehomogenized and centrifuged at 400×g for 5 min. Pooled supernatants were then centrifuged at 12000×g for 10 min. Resulting sediment was resuspended in homogenization buffer (1 ml/g of original wet tissue). The suspension was then layered onto a one-step Percoll gradient (8 ml 16% Percoll, 0.25 M sucrose) and centrifuged at 15000×g for 20 min. Final sediment was resuspended in homogenization buffer supplemented with 0.25% BSA and stored on ice. Protein concentration was determined by protein Dc assay kit (Bio-Rad) using bovine serum albumin (BSA) as standard.
Isolation of Genomic DNA
Dissected and well minced hippocampus was incubated overnight in 0.6 ml of lysis buffer (0.5% SDS, 10 mM Tris–HCl, and 0.1 M EDTA) with 0.6 mg of proteinase K (AppliChem) at 55°C. DNA was precipitated by adding 120 μl of 5 M NaCl and 720 μl of isopropanol and precipitated overnight at −20°C. DNA was washed with 75% ethanol, air dried, and resuspended in DNase-free TE solution (10 mM Tris–HCl and 0.1 M EDTA). Concentration of isolated genomic DNA was determined spectrophotometrically.
DNA Fragmentation
Isolated genomic DNA (5 μg) was incubated with terminal deoxynucleotidyl transferase (TdT) (Roche) and biotin-16-uridine-5′-triphosphate (Roche) for 30 min at 37°C. Then the samples were mixed with loading buffer and subjected to electrophoresis on a 1.5% agarose gel. After electrophoresis, the gel was washed with 8 mM NaOH and 3 M NaCl. DNA was transferred to a positively charged nylon membrane (Roche). The membrane was first blocked by BSA blocking solution (Candor) for 60 min and then incubated with avidin–biotin–horseradish peroxidase (ABC kit; Vector Laboratories) for 30 min. After washing of membrane, the bands were visualized by the chemiluminescence method using SuperSignal West Pico Chemiluminescent Substrate (Pierce), and the membrane was exposed to Chemidoc XRS (Bio Rad).
In situ detection of cells with fragmented DNA was performed by the terminal deoxynucleotidyl transferase-mediated uridine 5′-triphosphate-biotin nick end labeling (TUNEL) method in the brains of ischemic and control rats. Brains fixed with 4% of freshly made paraformaldehyde solution were cut to 40 μm sections. After endogenous peroxidase was inactivated with 0.3% H2O2 for 30 min, the sections were immersed in terminal deoxynucleotidyl transferase (TdT) buffer (Roche) for 30 min and then incubated with TdT (Roche) and biotin-16-uridine-5′-triphosphate (Roche) for 60 min at 37°C. After incubation, the sections were washed twice with 0.5× sodium saline citrate solution (SSC). After blocking with BSA blocking solution (Candor), the sections were incubated with avidin–biotin–horseradish peroxidase (ABC kit; Vector Laboratories), washed, and visualized with 3 mM 3, 3′-diaminobenzidine tetrahydrochloride and 18 mM hydrogen peroxide in phosphate buffered saline (PBS). The air dried slides were then cleared in xylene for at least 1 min and then coverslipped with Fluoromont (Serva) nonfluorescent mounting media.
Semi-Quantitative Western Blot Analysis
Mitochondrial proteins were solubilized by addition of 20% sodium dodecyl sulphate (SDS) to final concentration of 10%. Proteins (50 μg) were separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) using 12.5% acrylamide–bis acrylamide gels, and then transferred on nitrocellulose membranes using a semi-dry transfer protocol. The membranes were controlled for even load and possible transfer artifacts by staining with Ponceau Red solution. After blocking with BSA blocking solution (Candor), membranes were first incubated for 90 min with primary antibodies against p53 (1:200, Santa Cruz), bax (1:200, Santa Cruz), bcl-xl (1:200, Santa Cruz), and cytochrome c oxidase subunit I (1:1000, Molecular Probes) dissolved in BSA blocking solution (Candor). Incubation of membranes with primary antibodies was followed by extensive washing using washing solution (Candor) and consequently by incubation of membranes with biotinylated anti-mouse antibodies (1:10 000, Vector Laboratories). After extensive washing, membranes were incubated with avidin–biotin conjugated peroxidase (Vector Laboratories) solution, washed again 4 times 15 min and then incubated in SuperSignal West Pico Chemiluminescent Substrate (Pierce) solution for 1 min. After exposition of membranes on Chemidoc XRS (BioRad), the corresponding bands were integrated using GeneTools software (SynGene).
Fluoro-Jade C Staining
Fixed brains were cut on a freezing sliding microtome at a thickness of 40 μm and mounted onto gelled slides. The sections were air dried for at least 30 min on a slide warmer at 50°C. Slides were first immersed in a basic alcohol solution consisting of 1% sodium hydroxide in 80% ethanol for 5 min. They were then rinsed for 2 min in 70% ethanol, for 2 min in distilled water, and then incubated in 0.06% potassium permanganate solution for 10 min. Following a 1–2 min water rinse, the slides were then transferred for 10 min to a 0.0001% solution of Fluoro-Jade C dissolved in 0.1% acetic acid. The slides were then rinsed through three changes of distilled water for 1 min per change. Excess water was drained onto a paper towel, and the slides were then air dried on a slide warmer at 50°C for at least 5 min. The air dried slides were then cleared in xylene for at least 1 min and then coverslipped with Fluoromont (Serva) nonfluorescent mounting media.
Statistical Analysis
All statistical analyses were done using GrafPhad InStat V2.04a (GrafPhad Software). For the comparison of ischemia-induced changes among all groups, a one-way ANOVA test was first carried out to test for differences among all experimental groups. Additionally, the unpaired Tukey’s test was used to determine differences between individual groups. Significance level was set at P < 0.05.
Results
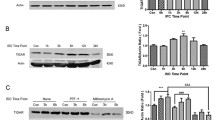
In order to asses impact of ischemia-reperfusion on the level of apoptotic and anti-apoptotic proteins in both cortical and hippocampal mitochondria, Western blot analysis of p53, bax, and bcl-xl was performed. Immunoreactivity of p53 in mitochondria isolated from rat hippocampus was evident as a band of approximately 50 kDa (Fig. 1a). We have observed that ischemia and consequent reperfusion led to increase of p53 level in hippocampal mitochondria, which was significant after 3 h (217.1 ± 42.2% of control, P < 0.01), 24 h (286.8 ± 65% of control, P < 0.001), and 72 h (232.9 ± 37.3% of control, P < 0.01) of reperfusion (Fig. 1b). Ischemia-induced increase of p53 level was not observed in mitochondria isolated from cerebral cortex (Fig. 1a). Level of both bax and bcl-xl in mitochondria isolated from either hippocampus or cerebral cortex was not significantly changed after ischemia and reperfusion (Fig. 1a).
Effect of naïve ischemia on mitochondrial level of p53, bax, and bcl-xl. a Western blot analysis of p53, bax, bcl-xl, and COXI in the mitochondria isolated from either rat hippocampus or cerebral cortex of control animals (CNTR), animals subjected to 15 min of global ischemia (ISCH), and animals subjected to 15 min of global ischemia followed by 1 (I1R), 3 (I3R), 24 (I24R), and 72 (I72R) h of reperfusion. Representative Western blots of five independent experiments are shown. b Semi-quantitative analysis of p53 level in mitochondria isolated from both hippocampi of animals subjected to naïve ischemia. Values are mean ± SD for five animals. Statistical significance was assessed by ANOVA, followed by Tukey’s test. ** P < 0.01 and *** P < 0.001
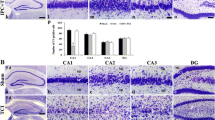
In addition to mitochondrial apoptosis initiation, we have examined apoptosis-related DNA fragmentation induced by global brain ischemia. Both biochemical and morphological approaches were performed. As documented by Southern blot analysis of TUNEL labeled genomic DNA, strong fragmentation of DNA was observed 24 and 72 h after 15 min of global brain ischemia (Fig. 2a). By performing morphological analysis, TUNEL positive nuclei were not observed in hippocampus of control animal (Fig. 2b). However, TUNEL positive nuclei were observed predominantly in CA1 layer of hippocampal formation 72 h after ischemia (Fig. 2c, d).
Effect of naïve ischemia on fragmentation of genomic DNA. a Southern blot analysis of genomic DNA fragmentation in rat hippocampus after naïve global ischemia. Genomic DNA was isolated from hippocampus of control animals (CNTR), animals subjected to 15 min of global ischemia (ISCH), and animals subjected to 15 min of global ischemia followed by 1 (I1R), 3 (I3R), 24 (I24R), and 72 (I72R) h of reperfusion and analyzed as described in section “Material and Methods”. In situ detection of genomic DNA fragmentation in rat hippocampus, b control animal, c animal subjected to 15 min of global ischemia followed by 3 days of reperfusion. d Higher magnification of TUNEL stained hippocampus of animal subjected to 15 min of global ischemia and 3 days of reperfusion. Coronal sections (40 μm) throughout the rat hippocampus were processed and stained by the terminal deoxynucleotidyl transferase-mediated uridine 5′-triphosphate-biotin nick end labeling (TUNEL) method as described in section “Material and Methods”. Space bars: −0.5 mm (b, c), −0.2 mm (d)
To examine molecular mechanisms underlying the neuroprotective effects of IPC, rats were subjected to sham operation; lethal ischemia for a duration of 15 min, sub-lethal ischemia for 5 min, and sub-lethal ischemia followed by lethal ischemia 2 days later (preconditioned ischemia). Neuronal death was assessed histologically using Fluoro-Jade C which stains all degenerating neurons, regardless of specific insult or mechanism of cell death (Schmued et al. 2005). In addition, effect of IPC on ischemia-induced translocation of p53 to mitochondria and genomic DNA fragmentation was investigated. Fluoro-Jade C positive cells were not observed in sham operated control animals (Fig. 3a). In hand with the concept of selective delayed neuronal ischemic death (Pulsinelli et al. 1982), Fluoro-Jade C positive bodies of degenerating neurons were observed in CA1 layer of hippocampal formation 6 days after naïve lethal ischemia (Fig. 3b). IPC did not induce neuronal damage (Fig. 3c), but afforded robust protection of CA1 neurons against subsequent lethal ischemic insult at 48 h (ischemic tolerance) as documented by Fluoro-Jade C staining of rat hippocampal formation 6 days after preconditioned ischemia (Fig. 3d). Ischemia-induced translocation of p53 to mitochondria (Fig. 4a) was completely abolished by IPC since no significant changes in mitochondrial p53 level were observed after preconditioned ischemia (Fig. 4b). Similar to naïve ischemia, mitochondrial levels of both bax and bcl-xl were not affected by IPC and subsequent lethal ischemia (Fig. 4a). Finally, ischemia-induced genomic DNA fragmentation was completely abolished 24 h after preconditioned ischemia while some residual fragmentation of genomic DNA was still observable 72 h after preconditioned ischemia (Fig. 5). However, morphological analysis performed 72 h after preconditioned ischemia did not reveal any TUNEL positive nuclei (not shown).
Detection of degenerating neurons after naïve ischemia and preconditioned ischemia. Coronal sections (40 μm) throughout rat hippocampus were processed and stained with the Fluoro-Jade C as described in section “Material and Methods”. a control animal, b animal subjected to 15 min of global ischemia followed by 6 days of reperfusion. c Animal subjected to 5 min of global ischemia followed by 2 days of reperfusion. d Animal subjected to 5 min of global ischemia followed 2 days later by 15 min of global ischemia and 6 days of reperfusion. Space bar −0.5 mm
Effect of ischemic preconditioning on mitochondrial level of p53, bax, and bcl-xl. a Western blot analysis of p53, bax, bcl-xl, and COXI in the rat hippocampus mitochondria after naïve ischemia and preconditioned ischemia. Mitochondria were isolated from both hippocampi of control animals (CNTR), animals subjected to 15 min of global ischemia (ISCH), and animals subjected to 15 min of global ischemia followed by 1 (I1R), 3 (I3R), and 24 (I24R) h of reperfusion and analyzed by Western blot as described in section “Material and Methods”. Representative Western blots of four independent experiments are shown. b Semi-quantitative analysis of p53 level in mitochondria isolated from both hippocampi of animals subjected to naïve ischemia and preconditioned ischemia. Values are mean ± SD for four animals. Statistical significance was assessed by ANOVA, followed by Tukey’s test. ** P < 0.01
Southern blot analysis of genomic DNA fragmentation in rat hippocampus after naïve ischemia and preconditioned ischemia. Genomic DNA was isolated from hippocampus of control animals (CNTR), animals subjected to 15 min of global ischemia (ISCH), and animals subjected to 15 min of global ischemia followed by 24 (I24R) and 72 (I72R) h of reperfusion as well as preconditioned control and preconditioned animals subjected to 15 min of global ischemia (ISCH) and to 15 min of global ischemia followed by 24 (I24R) and 72 (I72R) h of reperfusion. Genomic DNA fragmentation was analyzed as described in section “Material and Methods”
Discussion
Apoptosis is an evolutionarily conserved process that is crucial for development and homeostasis in CNS (for recent review see Buss et al. 2006) that, when deregulated, can elicit inappropriate cell death (Polster and Fiskum 2004). Global ischemia is a neuronal insult that induces intrinsic (mitochondrial) apoptotic pathway as it was documented in several previous studies (Krajewski et al. 1999; Perez-Pinzon et al. 1999; Sugawara et al. 1999; Tanaka et al. 2004; Hetz et al. 2005; Endo et al. 2006a; Racay et al. 2007). In this study, we have documented that global brain ischemia induces translocation of p53 to mitochondria which was observed in hippocampus but not in cerebral cortex. In addition, the mitochondrial level of two other key proteins of mitochondrial apoptosis, bax and bcl-xl, seems to be not affected by global brain ischemia and consequent reperfusion. Since bcl-xl can prevent mitochondrial membrane permeabilization by competing with bax (Billen et al. 2008), it seems that bax is already inserted in outer membrane of hippocampal mitochondria but the pore forming properties of bax are neutralized by high mitochondrial level of bcl-xl. Thus, our results are consistent with the recent view that p53 protein can directly induce permeabilization of the outer mitochondrial membrane by forming a complex with protective bcl-xl protein, resulting in oligomerization of bax, cytochrome c release, and initiation of neuronal apoptosis after cerebral ischemia (Endo et al. 2006a).
In hand with previous studies (Héron et al. 1993; MacManus et al. 1993; Jin et al. 1999), we have also documented that ischemia induces genomic DNA fragmentation which is observable after some days of reperfusion predominantly in cells of CA1 layer of rat hippocampal formation. DNA fragmentation is one of the most characteristic features of apoptotic cells and caspase-activated DNase is considered to be a major nuclease responsible for internucleosomal DNA fragmentation and chromatin condensation after cleavage by caspase-3 (Enari et al. 1998; Wolf et al. 1999; Slee et al. 2001). Caspase-3 can be activated by both extrinsic (receptor-mediated) and intrinsic (mitochondria-mediated) signaling pathways. The finding that global ischemia in naïve animals promotes Fas mRNA expression (Jin et al. 2001) and p75NTR protein expression (Tanaka et al. 2004) many hours after caspase-3 activation (Tanaka et al. 2004) argue against the extrinsic pathway of caspase-3 activation in early reperfusion after global brain ischemia.
Finally, we have also documented that ischemia-induced translocation of p53 to mitochondria was completely abolished while DNA fragmentation was significantly attenuated by IPC. IPC is a paradigm that affords robust protection of CA1 neurons against ischemic death for up to 14 days (Kirino 2002; Dirnagl et al. 2003; Gidday 2006; Obrenovitch 2008). The molecular mechanisms underlying a long-lasting ischemic tolerance induced by IPC are not yet fully understood. A considerable delay from the preconditioning stimulus until onset of ischemic tolerance is consistent with a role for transcriptional changes in adaptation (Kirino 2002; Dirnagl et al. 2003; Gidday 2006; Obrenovitch 2008). Tanaka and co-workers have shown that IPC acts downstream of caspase-3 activation and upstream of its target caspase-activated DNase to prevent the onset of apoptotic cell death (Tanaka et al. 2004). The IPC-induced inhibition of caspase-activated DNase was consistent with observations that IPC induces over expression of heat shock protein 70 kDa (Liu et al. 1993; Nishi et al. 1993; Tanaka et al. 2004), in which protective effect from cerebral ischemia via inhibition of caspase death cascade and mitochondrial apoptosis is well documented (Beere et al. 2000; Tsuchiya et al. 2003). Recently, it has also been shown that hsp70 inhibits apoptosis upstream of mitochondria by preventing bax translocation (Steel et al. 2004; Stankiewicz et al. 2005).
The molecular mechanisms driving translocation of p53 to mitochondria after brain ischemia are not yet known. Thus, we can only speculate about the possible mechanism involved in inhibition of mitochondrial p53 translocation observed after IPC. Since several different mechanisms, like IPC-induced over expression of heat shock protein 70 kDa (Liu et al. 1993; Nishi et al. 1993; Tanaka et al. 2004) or activation of Akt pathway (Brunet et al. 2001; Endo et al. 2006b; Miyawaki et al. 2008), might be considered, the exact mechanism of IPC-induced prevention of p53 translocation to mitochondria has to be clarified by further experiments.
In conclusion, our results showed that IPC acts at the level of both initiation and execution of ischemia-induced mitochondrial apoptosis. These results suggest that inhibition of the mitochondrial p53 pathway provides a potentially important mechanism of neuronal survival in the face of ischemic brain damage.
References
Beere HM, Wolf BB, Cain K, Mosser DD, Mahboubi A, Kuwana T, Tailor P, Morimoto RI, Cohen GM, Green DR (2000) Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat Cell Biol 2:469–475. doi:10.1038/35019501
Billen LP, Kokoski CL, Lovell JF, Leber B, Andrews DW (2008) Bcl-XL inhibits membrane permeabilization by competing with Bax. PLoS Biol 6:1268–1280. doi:10.1371/journal.pbio.0060147
Brunet A, Datta SR, Greenberg ME (2001) Transcription-dependent and -independent control of neuronal survival by the PI3 K-Akt signaling pathway. Curr Opin Neurobiol 11:297–305. doi:10.1016/S0959-4388(00)00211-7
Buss RR, Sun W, Oppenheim RW (2006) Adaptive roles of programmed cell death during nervous system development. Annu Rev Neurosci 29:1–35. doi:10.1146/annurev.neuro.29.051605.112800
Dirnagl U, Simon RP, Hallenbeck JM (2003) Ischemic tolerance and endogenous neuroprotection. Trends Neurosci 26:248–254. doi:10.1016/S0166-2236(03)00071-7
Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A, Nagata S (1998) A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature 391:43–50
Endo H, Kamada H, Nito C, Nishi T, Chan PH (2006a) Mitochondrial translocation of p53 mediates release of cytochrome c and hippocampal CA1 neuronal death after transient global cerebral ischemia in rats. J Neurosci 26:7974–7983. doi:10.1523/JNEUROSCI.0897-06.2006
Endo H, Nito C, Kamada H, Nishi T, Chan PH (2006b) Activation of the Akt/GSK3beta signaling pathway mediates survival of vulnerable hippocampal neurons after transient global cerebral ischemia in rats. J Cereb Blood Flow Metab 26:1479–1489. doi:10.1038/sj.jcbfm.9600303
Gidday JM (2006) Cerebral preconditioning and ischaemic tolerance. Nat Rev Neurosci 7:437–448. doi:10.1038/nrn1927
Héron A, Pollard H, Dessi F, Moreau J, Lasbennes F, Ben-Ari Y, Charriaut-Marlangue C (1993) Regional variability in DNA fragmentation after global ischemia evidenced by combined histological and gel electrophoresis observations in the rat brain. J Neurochem 61:1973–1976. doi:10.1111/j.1471-4159.1993.tb09843.x
Hetz C, Vitte PA, Bombrun A, Rostovtseva TK, Montessuit S, Hiver A, Schwarz MK, Church DJ, Korsmeyer SJ, Martinou JC, Antonsson B (2005) Bax channel inhibitors prevent mitochondrion-mediated apoptosis and protect neurons in a model of global brain ischemia. J Biol Chem 280:42960–42970. doi:10.1074/jbc.M505843200
Jin K, Chen J, Nagayama T, Chen M, Sinclair J, Graham SH, Simon RP (1999) In situ detection of neuronal DNA strand breaks using the Klenow fragment of DNA polymerase I reveals different mechanisms of neuron death after global cerebral ischemia. J Neurochem 72:1204–1214. doi:10.1046/j.1471-4159.1999.0721204.x
Jin K, Graham SH, Mao X, Nagayama T, Simon RP, Greenberg DA (2001) Fas (CD95) may mediate delayed cell death in hippocampal CA1 sector after global cerebral ischemia. J Cereb Blood Flow Metab 21:1411–1421. doi:10.1097/00004647-200112000-00005
Kirino T (2002) Ischemic tolerance. J Cereb Blood Flow Metab 22:1283–1296. doi:10.1097/00004647-200211000-00001
Kirino T, Tsujita Y, Tamura A (1991) Induced tolerance to ischemia in gerbil hippocampal neurons. J Cereb Blood Flow Metab 11:299–307
Kitagawa K, Matsumoto M, Kuwabara K, Tagaya M, Ohtsuki T, Hata R, Ueda H, Handa N, Kimura K, Kamada T (1991) “Ischemic tolerance” phenomenon detected in various brain regions. Brain Res 561:203–211. doi:10.1016/0006-8993(91)91596-S
Krajewski S, Krajewska M, Ellerby LM, Welsh K, Xie Y, Deveraux OL, Salvesen GS, Bredesen DE, Rosenthal RE, Fiskum G, Reed JC (1999) Release of caspase-9 from mitochondria during neuronal apoptosis and cerebral ischemia. Proc Natl Acad Sci USA 96:5752–5757. doi:10.1073/pnas.96.10.5752
Liu Y, Kato H, Nakata N, Kogure K (1992) Protection of rat hippocampus against ischemic neuronal damage by pretreatment with sub-lethal ischemia. Brain Res 586:121–124. doi:10.1016/0006-8993(92)91380-W
Liu Y, Kato H, Nakata N, Kogure K (1993) Temporal profile of heat shock protein 70 synthesis in ischemic tolerance induced by preconditioning ischemia in rat hippocampus. Neuroscience 56:921–927. doi:10.1016/0306-4522(93)90138-6
MacManus JP, Buchan AM, Hill IE, Rasquinha I, Preston E (1993) Global ischemia can cause DNA fragmentation indicative of apoptosis in rat brain. Neurosci Lett 164:89–92. doi:10.1016/0304-3940(93)90864-H
Miyawaki T, Mashiko T, Ofengeim D, Flannery RJ, Noh KM, Fujisawa S, Bonanni L, Bennett MV, Zukin RS, Jonas EA (2008) Ischemic preconditioning blocks BAD translocation, Bcl-xL cleavage, and large channel activity in mitochondria of postischemic hippocampal neurons. Proc Natl Acad Sci USA 105:4892–4897. doi:10.1073/pnas.0800628105
Nishi S, Taki W, Uemura Y, Higashi T, Kikuchi H, Kudoh H, Satoh M, Nagata K (1993) Ischemic tolerance due to the induction of HSP70 in a rat ischemic recirculation model. Brain Res 615:281–288. doi:10.1016/0006-8993(93)90039-P
Obrenovitch TP (2008) Molecular physiology of preconditioning-induced brain tolerance to ischemia. Physiol Rev 88:211–247. doi:10.1152/physrev.00039.2006
Perez-Pinzon MA, Born JG (1999) Rapid preconditioning neuroprotection following anoxia in hippocampal slices: role of the K+ ATP channel and protein kinase C. Neuroscience 89:453–459. doi:10.1016/S0306-4522(98)00560-0
Perez-Pinzon MA, Xu GP, Born J, Lorenyo J, Busto R, Rosenthal M, Sick TJ (1999) Cytochrome C is released from mitochondria into the cytosol after cerebral anoxia or ischemia. J Cereb Blood Flow Metab 19:39–43. doi:10.1097/00004647-199901000-00004
Polosa PL, Attardi G (1991) Distinctive pattern and translational control of mitochondrial protein synthesis in rat brain synaptic endings. J Biol Chem 266:10011–10017
Polster BM, Fiskum G (2004) Mitochondrial mechanisms of neural cell apoptosis. J Neurochem 90:1281–1289. doi:10.1111/j.1471-4159.2004.02572.x
Pulsinelli WA, Brierley JB, Plum F (1982) Temporal profile of neuronal damage in a model of transient forebrain ischemia. Ann Neurol 11:491–498. doi:10.1002/ana.410110509
Racay P, Tatarkova Z, Drgova A, Kaplan P, Dobrota D (2007) Effect of ischemic preconditioning on mitochondrial dysfunction and mitochondrial p53 translocation after transient global cerebral ischemia in rats. Neurochem Res 32:1823–1832. doi:10.1007/s11064-007-9437-3
Schmued LC, Stowers CC, Scallet AC, Xu L (2005) Fluoro-Jade C results in ultra high resolution and contrast labeling of degenerating neurons. Brain Res 1035:24–31. doi:10.1016/j.brainres.2004.11.054
Simon RP, Niiro M, Gwinn R (1993) Prior ischemic stress protects against experimental stroke. Neurosci Lett 163:135–137. doi:10.1016/0304-3940(93)90364-Q
Slee EA, Adrain C, Martin SJ (2001) Executioner caspase-3, -6, and -7 perform distinct, non-redundant roles during the demolition phase of apoptosis. J Biol Chem 276:7320–7326. doi:10.1074/jbc.M008363200
Stankiewicz AR, Lachapelle G, Foo CP, Radicioni SM, Mosser DD (2005) Hsp70 inhibits heat-induced apoptosis upstream of mitochondria by preventing Bax translocation. J Biol Chem 280:38729–38739. doi:10.1074/jbc.M509497200
Steel R, Doherty JP, Buzzard K, Clemons N, Hawkins CJ, Anderson RL (2004) Hsp72 inhibits apoptosis upstream of the mitochondria and not through interactions with Apaf-1. J Biol Chem 279:51490–51499. doi:10.1074/jbc.M401314200
Sugawara T, Fujimura M, Morita-Fujimura Y, Kawase M, Chan PH (1999) Mitochondrial release of cytochrome c corresponds to the selective vulnerability of hippocampal CA1 neurons in rats after transient global cerebral ischemia. J Neurosci 19:1–6
Tanaka H, Yokota H, Jover T, Cappuccio I, Calderone A, Simionescu M, Bennett MV, Zukin RS (2004) Ischemic preconditioning: neuronal survival in the face of caspase-3 activation. J Neurosci 24:2750–2759. doi:10.1523/JNEUROSCI.5475-03.2004
Tsuchiya D, Hong S, Matsumori Y, Shiina H, Kayama T, Swanson RA, Dillman WH, Liu J, Panter SS, Weinstein PR (2003) Overexpression of rat heat shock protein 70 is associated with reduction of early mitochondrial cytochrome c release and subsequent DNA fragmentation after permanent focal ischemia. J Cereb Blood Flow Metab 23:718–727. doi:10.1097/01.WCB.0000054756.97390.F7
Wolf BB, Schuler M, Echeverri F, Green DR (1999) Caspase-3 is the primary activator of apoptotic DNA fragmentation via DNA fragmentation factor-45/inhibitor of caspase-activated DNase inactivation. J Biol Chem 274:30651–30656. doi:10.1074/jbc.274.43.30651
Acknowledgements
This work was supported by the Ministry of Education of Slovak Republic (grant VEGA 1/4255/07 to P.R.). Authors are grateful to Zdenka Cetlova for her excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Racay, P., Chomova, M., Tatarkova, Z. et al. Ischemia-Induced Mitochondrial Apoptosis is Significantly Attenuated by Ischemic Preconditioning. Cell Mol Neurobiol 29, 901–908 (2009). https://doi.org/10.1007/s10571-009-9373-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-009-9373-7