Abstract
Alzheimer’s disease (AD) is the leading cause of dementia, a condition that gradually destroys brain cells and leads to progressive decline in mental functions. The disease is characterized by accumulation of misfolded neuronal proteins, amyloid and tau, into insoluble aggregates known as extracellular senile plaques and intracellular neurofibrillary tangles, respectively. However, only tau pathology appears to correlate with the progression of the disease and it is believed to play a central role in the progression of neurodegeneration. In AD, tau protein undergoes various types of posttranslational modifications, most notably hyperphosphorylation and truncation. Using four proteomics approaches we aimed to uncover the key steps leading to neurofibrillary degeneration and thus to identify therapeutic targets for AD. Functional neuroproteomics was employed to generate the first transgenic rat model of AD by expressing a truncated misordered form of tau, “Alzheimer’s tau”. The rat model showed that Alzheimer’s tau toxic gain of function is responsible for the induction of abnormal tau cascade and is the driving force in the development of neurofibrillary degeneration. Structural neuroproteomics allowed us to determine partial 3D structure of the Alzheimer’s filament core at a resolution of 1.6 Å. Signaling neuroproteomics data lead to the identification and characterization of relevant phosphosites (the tau phosphosignalome) contributing to neurodegeneration. Interaction neuroproteomics revealed links to a new group of proteins interacting with Alzheimer’s tau (tau interactome) under normal and pathological conditions, which would provide novel drug targets and novel biomarkers for treatment of AD and other tauopathies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s disease (AD) is the leading cause of dementia, a condition that gradually destroys brain cells and leads to progressive decline in mental function. As the society is graying, the number of elderly people at risk of developing dementia is growing rapidly as a consequence of increasing life span. Recently, the prevalence of AD and its demographic development in the near future has been analyzed by a panel of 12 international experts (Ferri et al. 2005). They estimated that 24.3 million people have dementia today, with 4.6 million new cases of dementia every year (one new case every 7 s).
Histopathologically, AD is characterized by accumulation of misordered proteins in the form of two types of insoluble fibrous material: extracellular senile plaques and intracellular neurofibrillary deposits, originally reported by Alois Alzheimer (Alzheimer 1907). Advances in the proteomics in 1980s allowed the identification of the constituents of these structures at the molecular level. Both appeared to result from aberrant folding of proteins, senile plaques being composed of amyloid β (Aβ) (Glenner and Wong 1984) and neurofibrillary deposits (or tangles, NFT) being composed of the tau protein (Grundke-Iqbal et al. 1986; Wischik et al. 1988a, 1988b).
Tau and Aβ became the objects of intense research. Currently, it is assumed that both of these proteins are responsible for the development of AD pathology. The role of Aβ in the AD pathology was proposed in the Aβ cascade hypothesis (for detailed review see Seabrook et al. 2007; and Walsh and Selkoe 2004), which also became the basis for the development of therapeutic approaches and drug discovery for AD.
In our work we focused on tau protein, which is essential for the propagation of neurofibrillary degeneration in AD and is also able to induce neurofibrillary pathology in other tauopathies (Baker et al. 1997; Goedert et al. 1998; Hutton et al. 1998; Rapoport et al. 2002; Roberson et al. 2007).
Tau Proteome
Tau belongs to the family of microtubule-associated proteins (MAPs) and is involved in establishing neuronal polarity and axonal outgrowth during development and for maintaining axonal morphology and axonal transport in mature neurons (Caceres and Kosik 1990; Dawson et al. 2001; Esmaeli-Azad et al. 1994; Hanemaaijer and Ginzburg 1991; Harada et al. 1994).
At the proteomics level, tau belongs to the family of the intrinsically disordered proteins (IDPs) that are characterized by the absence of a rigid three-dimensional structure (Skrabana et al. 2006; Uversky 2002). However, IDPs attain defined structure upon binding to their partners. Thus, not surprisingly, the IDPs are mostly found between signaling and regulatory proteins that have multiple interaction partners.
The intrinsically disordered character endows tau with high structural flexibility allowing interaction with many proteins. This interaction flexibility is further increased by the presence of six isoforms in the brain (Goedert et al. 1989). Further multiplication of tau forms is achieved through many posttranslational modifications. Eighty-five phosphorylatable Ser/Thr/Tyr residues are present on tau, out of which 45 were already found to be phosphorylated in AD (Hanger et al. 2007). Tau isolated from AD is ubiquitinated and oxidized (Horiguchi et al. 2003; Morishima-Kawashima et al. 1993). Finally, AD tau is also characterized by truncation (Gamblin et al. 2003; Novak et al. 1991, 1989).
Thus, in AD, the combination of all of these modifications leads to a population of hundreds of multiply modified tau proteins, better characterized by the term “AD tau proteome” (Fig. 1). Consequently, giving this multitude of tau species, the following questions arise:
The AD tau proteome. Tau is expressed as six isoforms in adult human brain. This complexity is multiplied by phosphorylation at least 45 known sites in AD, truncation, ubiquitination, and other known modifications taking place in AD. This multitude of tau proteins makes it difficult to identify the key molecular steps of the transition of the disordered normal tau into misordered aggregated protein
-
(a)
Which of these individual tau proteins are responsible for the initiation of neurodegeneration in AD and related tauopathies?
-
(b)
Which represent protective attempts of a neuron against the environmental insults?
-
(c)
What are the pathological and protective pathways leading to AD?
In order to address this complex task, decoding the AD tau proteome, we have to undertake a multipart proteomic approach. This approach should take place at four main platforms: functional, structural, signaling and, interaction neuroproteomics.
Functional Neuroproteomics
The appearance of truncated tau is a characteristic feature of AD and other tauopathies (Binder et al. 2005; Novak 1994). We have identified in sporadic AD a truncation event in tau that converts the intrinsically disordered normal tau into a mis-disordered Alzheimer’s tau protein with toxic gain of function (Novak et al. 1989; Zilka et al. 2006).
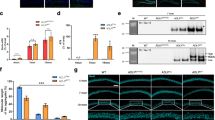
In order to determine the toxic potential of Alzheimer’s tau, we expressed this protein as a transgene in the rat brain (Zilka et al. 2006). Expression of the mis-disordered tau protein led to the induction of a complete tau cascade of neurofibrillary degeneration (Table 1), consisting of tau hyperphosphorylation, formation of argyrophilic tangles and sarcosyl-insoluble tau complexes with a typical A68 triplet (Fig. 2). These pathological changes resulted in the neuronal dysfunction consequently leading to the progressive neurobehavioral impairment and premature death (Hrnkova et al. 2007; Koson et al. 2008). Furthermore, the rat model of AD also revealed that expression of the Alzheimer’s tau protein led to the accumulation of reactive oxygen species and sensitized rat cortical neurons to cell death induced by oxidative stress (Cente et al. 2006). This indicates that truncation of tau may precede oxidative stress in the pathogenesis of neurodegenerative diseases such as AD and other tauopathies.
Expression of Alzheimer’s tau in the rat brain induces complete tau cascade of neuropathology including the sarcosyl-insoluble tau with the A68 triplet characteristic of human AD neurofibrillary degeneration. The western blot represents sarcosyl insoluble brain extracts from wild type (WT), transgenic rat expressing Alzheimer’s tau (TG) and human AD brain. The blot was probed with a pan tau antibody (DC25) or with PHF1 antibody specific for phosphorylated Ser396/Ser404. Numbers on the left indicate positions of molecular mass markers (kDa)
Thus, we have discovered the inducer and driving force of pathogenesis leading to full development of neurofibrillary degeneration of Alzheimer’s type, including histopathological hallmarks—NFTs, oxidative stress, neuronal dysfunction, and death.
The expression of Alzheimer’s tau in the rat brain also induced its hyperphosphorylation, which seems to be an integral part of the tau cascade. The hyperphosphorylation occurred immediately after its expression started, while sarcosyl-insoluble tau appeared only late in the life of the transgenic animals (Zilka et al. 2006). Thus, there are additional steps except truncation and hyperphosphorylation that ultimately lead to the formation of the insoluble misordered tau.
In order to identify the events leading neurofibrillary pathology, we employ molecular mapping of the tau modifications and structures using state-of-the-art proteomics approaches.
Structural Neuroproteomics
Inherent physical properties of the intrinsically disordered protein tau have precluded so far its detailed structural analysis by X-ray crystallography or nuclear magnetic resonance. The tau protein undergoes disorder-to-misorder transition in AD, whereby a highly soluble protein forms insoluble aggregates, neurofibrillary tangles, which contain polymeric structures. Determination of the structure of the polymerized tau—paired helical filaments (PHFs) will provide vital information for therapeutic intervention to halt the pathological process. Many laboratories struggle to determine the structure of the core PHF using different techniques, such as circular dichroism, nuclear magnetic resonance, X-ray diffraction, and some others. Several models of the arrangement of tau molecules within PHF or in solution have been suggested (Berriman et al. 2003; Inouye et al. 2006; Mukrasch et al. 2007; Ruben et al. 1991). In spite of that effort, the structure of misordered tau long escaped its determination.
In order to solve the structure of misordered tau assembled into PHF, we have used a specific monoclonal antibody MN423 raised against the PHF core, the protease resistant product of proteolytic cleavage of Alzheimer’s PHF, which retains its characteristic morphological features and consists mainly of tau protein (Novak et al. 1989). The MN423 has unequivocal specificity for tau ordered into the PHF structure (Khuebachova et al. 2002; Skrabana et al. 2004; Wischik et al. 1988a, 1988b) and represents an in vivo imprint of the core PHF. The antibody, serving as a mold, induced disorder-to-misorder transition of tau upon interaction, i.e., forcing the tau fragment to adopt the PHF core conformation. Crystallization of the complex of monoclonal antibody MN423 with the C-terminal hexapeptide of the core PHF tau led to the identification of its structure at a resolution of 1.65 Å (Sevcik et al. 2007).
This structural analysis suggests important role of the core PHF C-terminus for PHF assembly. This approach shows new possibilities in crystallographic analysis of intrinsically disordered proteins involved in pathogenesis of neurodegenerative diseases. Using several monoclonal antibodies recognizing different parts of the PHF core could lead to the solution of the 3D structure of the whole PHF core.
It is reasonable to expect that this approach will help to reveal the structural principles underlying the tau protein assembly into PHF and possibly will facilitate rationale drug design for inhibition of Alzheimer neurofibrillary changes.
Signaling Neuroproteomics
Hyperphosphorylation of tau is an important “cascade-like” molecular event implicated in tau pathology and a prerequisite for generation of insoluble forms—misordered tau assembled in paired helical filaments in AD and other tauopathies (Iqbal et al. 2005; Pevalova et al. 2006). Alonso et al. (1994, 1996) have shown that hyperphosphorylated tau isolated from human brains exhibits severely reduced ability to bind microtubules (loss of function), and on the other hand gains ability to sequester normal tau and prevents it from executing its functions (gain of toxic function).
However, the role of (hyper)phosphorylation in the transition of tau into its misordered pathological form is still not fully elucidated, since phosphorylation of the individual Ser/Thr residues has different effects on tau function (microtubule binding) and self-assembly into filaments. Some reports showed reduced self-assembly of tau protein phosphorylated in MT domain or Pro-rich domain (Eidenmuller et al. 2001; Schneider et al. 1999), while others reported a weakly promoting effect (Liu et al. 2007). It has recently been found that although hyperphosphorylation of tau is essential for tau induced toxicity, no single phosphorylation event plays a dominant role in controlling tau toxicity (Steinhilb et al. 2007a, 2007b). Finally, many protein kinases are able to phosphorylate tau in vitro and in vivo (for recent reviews see Iqbal and Grundke-Iqbal 2008; Pevalova et al. 2006).
These findings showed that tau is an acceptor of many modification/regulatory events originating in multiple signaling pathways. Thus, as it is typical for IDPs, the high number of regulatory sites (more than 45 phosphosites already identified on tau) allows tau to efficiently participate in both one-to-many and many-to-one signaling pathways (Uversky et al. 2008). They also suggest that the final outcome of tau phosphorylation depends on the combination of various phosphorylated sites on one tau molecule.
In order to understand these regulatory signals, it is necessary to decode the combination of signaling phosphosites on tau—the “tau phosphosignalome”. Towards this goal, we employed state-of-the-art proteomic approaches in combination with the rat model of AD (Zilka et al. 2006). In this model, the transgene Alzheimer’s tau undergoes further modifications (phosphorylation) and becomes misordered, aggregated in AD-like PHF. First, rapid, efficient, and simple procedure for purification of tau proteins from AD brain tissue had to be developed, which would faithfully preserve all phosphorylated residues on tau and thus render tau amenable to further analysis (Ivanovova et al. 2008). Next, efficient separation techniques have to be designed in order to separate and purify the individual populations of tau proteins, based on their phosphorylation pattern and truncation. For their classification, both bottom-up and top-down proteomics approaches have to be employed. The bottom-up approach usually starts with a gel-based protein separation followed by excision of the protein band of interest, in-gel digested with trypsin and the resulting pool of peptides analyzed by mass spectrometry for protein identification and the presence of modifications (Domon and Aebersold 2006). This method can provide information on all existing modifications, e.g., phosphorylation, in the pool of tau proteins in the sample. However, it cannot provide information on the combination of the individual phosphosites (or other modifications) that exists simultaneously on one tau molecule, the key information that distinguishes normal tau from misordered tau.
Therefore, it is necessary to analyze intact tau proteins by top-down mass spectrometry. This technology will provide the essential information on the abundance of the individual tau protein forms, information on the combination of phosphosites existing on tau in diseased brains as well as identity of additional modifications like truncation (Siuti and Kelleher 2007). This approach needs to be supported with high-end instrumentation like Fourier-transform tandem MS (FTMS/MS), Orbitrap, and latest MALDI-TOF MS/MS, which provide both high resolution and high mass accuracy (for a review see Aebersold and Mann 2003).
These approaches would allow us to understand the pathological metamorphosis of normal tau into misordered tau during the disease progression by decoding AD tau signalome. The pathways will be exploited as potential drug targets. The tau molecular species will also be utilized as biomarkers for development of preclinical AD diagnostic assays.
Interaction Neuroproteomics
The intrinsically disordered proteins are characterized by a large number of interacting partners. Tau is no exception to this rule, since many proteins have already been found to interact with tau in vitro and some also in vivo. These include structural proteins like tubulin and actin, peptidyl-prolyl isomerase, motor protein complexes, protein chaperones, cytoplasmic and membrane-bound kinases, phosphatase PP2A, proteases, S100 calcium binding protein B, α-synuclein, and other potential targets (Baudier and Cole 1988; Binder et al. 2005; Dickey et al. 2007; Iqbal et al. 2005; Jensen et al. 1999; Lee 2005; Lim and Lu 2005; Magnani et al. 2007; Mesco and Timiras 1991). Thus, given the high number of phosphorylation sites on tau and diversity of interaction partners, tau most likely sits in a crossroad of many signaling pathways, a typical many-to-one and one-to-many signaling protein (Uversky et al. 2008).
For that reason, uncovering the molecular underpinnings of tau neurodegeneration requires not only mapping the “AD tau signalome” (see above), but it is necessary to map the crosstalk between tau, normal and pathologically altered, and the proteome of a neuronal and glial cell. We set up a proteomics platform for purification and identification of tau interaction partners, the “tau interactome”. The approach is based on immunopurification of tau complexes, their resolution on 2-dimensional electrophoresis and mass spectrometry. We took advantage of the rat model of AD expressing Alzheimer’s tau. This approach already yielded more than 40 interacting partners of tau, many of which are differentially expressed between wild type and transgenic animals (Hanes et al., manuscript in preparation). The differentially interacting proteins will provide essential information on both protective and pathological pathways that are active at different stages of the disease process. Furthermore, these pathways might become targets for therapeutical intervention to halt the progression of the disease or even reverse it.
The second part of the interaction proteomics involves kinetic measurements of tau-partner interactions using optical biosensors (Visser and Heck 2008). The variability in the interaction should provide information on the structure of the tau proteins and the level of “misorder” caused by various modifications, a kind of staging of the transition process from disordered to misordered tau.
Conclusion
The prerequisite for a successful accomplishment of the aforementioned proteomics strategy for decoding tau proteome is that all four platforms work in concert, i.e., there is sufficient flow of information between them. In addition, analysis of tau proteome has to be done in both AD model systems and AD patients. The model systems, from cellular to animal, have advantage of precise staging of the pathology, known pathological starter (transgene, environmental insult), unlimited supply, sample homogeneity and existence of a wild type counterpart of the same genetic background. The human samples have to be used, however, for validation of all observations made in the model systems.
Modern era of proteomics brings about rapid development and implementation of novel technologies in protein analysis. Decoding AD tau proteome will uncover novel targets and detailed knowledge on molecular nature of neurodegeneration induced by pathologically misordered tau protein.
References
Aebersold R, Mann M (2003) Mass spectrometry-based proteomics. Nature 422(6928):198–207. doi:10.1038/nature01511
Alonso AC, Zaidi T, Grundke-Iqbal I, Iqbal K (1994) Role of abnormally phosphorylated tau in the breakdown of microtubules in Alzheimer disease. Proc Natl Acad Sci USA 91(12):5562–5566. doi:10.1073/pnas.91.12.5562
Alonso AC, Grundke-Iqbal I, Iqbal K (1996) Alzheimer’s disease hyperphosphorylated tau sequesters normal tau into tangles of filaments and disassembles microtubules. Nat Med 2(7):783–787. doi:10.1038/nm0796-783
Alzheimer A (1907) Über eine eigenartigeErkrankung der Hirnrinde. Allg Z Psychiatry Psych-Gerichtl Med 64:146–148
Baker M, Kwok JB, Kucera S, Crook R, Farrer M, Houlden H, Isaacs A, Lincoln S, Onstead L, Hardy J, Wittenberg L, Dodd P, Webb S, Hayward N, Tannenberg T, Andreadis A, Hallupp M, Schofield P, Dark F, Hutton M (1997) Localization of frontotemporal dementia with parkinsonism in an Australian kindred to chromosome 17q21–22. Ann Neurol 42(5):794–798. doi:10.1002/ana.410420516
Baudier J, Cole RD (1988) Interactions between the microtubule-associated tau proteins and S100b regulate tau phosphorylation by the Ca2+/calmodulin-dependent protein kinase II. J Biol Chem 263(12):5876–5883
Berriman J, Serpell LC, Oberg KA, Fink AL, Goedert M, Crowther RA (2003) Tau filaments from human brain and from in vitro assembly of recombinant protein show cross-beta structure. Proc Natl Acad Sci USA 100(15):9034–9038. doi:10.1073/pnas.1530287100
Binder LI, Guillozet-Bongaarts AL, Garcia-Sierra F, Berry RW (2005) Tau, tangles, and Alzheimer’s disease. Biochim Biophys Acta 1739(2–3):216–223
Caceres A, Kosik KS (1990) Inhibition of neurite polarity by tau antisense oligonucleotides in primary cerebellar neurons. Nature 343(6257):461–463. doi:10.1038/343461a0
Cente M, Filipcik P, Pevalova M, Novak M (2006) Expression of a truncated tau protein induces oxidative stress in a rodent model of tauopathy. Eur J NeuroSci 24(4):1085–1090. doi:10.1111/j.1460-9568.2006.04986.x
Dawson HN, Ferreira A, Eyster MV, Ghoshal N, Binder LI, Vitek MP (2001) Inhibition of neuronal maturation in primary hippocampal neurons from tau deficient mice. J Cell Sci 114(Pt 6):1179–1187
Dickey CA, Kamal A, Lundgren K, Klosak N, Bailey RM, Dunmore J, Ash P, Shoraka S, Zlatkovic J, Eckman CB, Patterson C, Dickson DW, Nahman NS Jr, Hutton M, Burrows F, Petrucelli L (2007) The high-affinity HSP90-CHIP complex recognizes and selectively degrades phosphorylated tau client proteins. J Clin Invest 117(3):648–658. doi:10.1172/JCI29715
Domon B, Aebersold R (2006) Mass spectrometry and protein analysis. Science 312(5771):212–217. doi:10.1126/science.1124619
Eidenmuller J, Fath T, Maas T, Pool M, Sontag E, Brandt R (2001) Phosphorylation-mimicking glutamate clusters in the proline-rich region are sufficient to simulate the functional deficiencies of hyperphosphorylated tau protein. Biochem J 357(Pt 3):759–767. doi:10.1042/0264-6021:3570759
Esmaeli-Azad B, McCarty JH, Feinstein SC (1994) Sense and antisense transfection analysis of tau function: tau influences net microtubule assembly, neurite outgrowth and neuritic stability. J Cell Sci 107(Pt 4):869–879
Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, Hall K, Hasegawa K, Hendrie H, Huang Y, Jorm A, Mathers C, Menezes PR, Rimmer E, Scazufca M (2005) Global prevalence of dementia: a Delphi consensus study. Lancet 366(9503):2112–2117. doi:10.1016/S0140-6736(05)67889-0
Gamblin TC, Chen F, Zambrano A, Abraha A, Lagalwar S, Guillozet AL, Lu M, Fu Y, Garcia-Sierra F, LaPointe N, Miller R, Berry RW, Binder LI, Cryns VL (2003) Caspase cleavage of tau: linking amyloid and neurofibrillary tangles in Alzheimer’s disease. Proc Natl Acad Sci USA 100(17):10032–10037. doi:10.1073/pnas.1630428100
Glenner GG, Wong CW (1984) Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun 120(3):885–890. doi:10.1016/S0006-291X(84)80190-4
Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA (1989) Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer’s disease. Neuron 3(4):519–526. doi:10.1016/0896-6273(89)90210-9
Goedert M, Crowther RA, Spillantini MG (1998) Tau mutations cause frontotemporal dementias. Neuron 21(5):955–958. doi:10.1016/S0896-6273(00)80615-7
Grundke-Iqbal I, Iqbal K, Quinlan M, Tung YC, Zaidi MS, Wisniewski HM (1986) Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem 261(13):6084–6089
Hanemaaijer R, Ginzburg I (1991) Involvement of mature tau isoforms in the stabilization of neurites in PC12 cells. J Neurosci Res 30(1):163–171. doi:10.1002/jnr.490300117
Hanger DP, Byers HL, Wray S, Leung KY, Saxton MJ, Seereeram A, Reynolds CH, Ward MA, Anderton BH (2007) Novel phosphorylation sites in tau from Alzheimer brain support a role for casein kinase 1 in disease pathogenesis. J Biol Chem 282(32):23645–23654. doi:10.1074/jbc.M703269200
Harada A, Oguchi K, Okabe S, Kuno J, Terada S, Ohshima T, Sato-Yoshitake R, Takei Y, Noda T, Hirokawa N (1994) Altered microtubule organization in small-calibre axons of mice lacking tau protein. Nature 369(6480):488–491. doi:10.1038/369488a0
Horiguchi T, Uryu K, Giasson BI, Ischiropoulos H, LightFoot R, Bellmann C, Richter-Landsberg C, Lee VM, Trojanowski JQ (2003) Nitration of tau protein is linked to neurodegeneration in tauopathies. Am J Pathol 163(3):1021–1031
Hrnkova M, Zilka N, Minichova Z, Koson P, Novak M (2007) Neurodegeneration caused by expression of human truncated tau leads to progressive neurobehavioural impairment in transgenic rats. Brain Res 1130(1):206–213. doi:10.1016/j.brainres.2006.10.085
Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, Pickering-Brown S, Chakraverty S, Isaacs A, Grover A, Hackett J, Adamson J, Lincoln S, Dickson D, Davies P, Petersen RC, Stevens M, de Graaff E, Wauters E, van Baren J, Hillebrand M, Joosse M, Kwon JM, Nowotny P, Che LK, Norton J, Morris JC, Reed LA, Trojanowski J, Basun H, Lannfelt L, Neystat M, Fahn S, Dark F, Tannenberg T, Dodd PR, Hayward N, Kwok JB, Schofield PR, Andreadis A, Snowden J, Craufurd D, Neary D, Owen F, Oostra BA, Hardy J, Goate A, van Swieten J, Mann D, Lynch T, Heutink P (1998) Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP–17. Nature 393(6686):702–705. doi:10.1038/31508
Inouye H, Sharma D, Goux WJ, Kirschner DA (2006) Structure of core domain of fibril-forming PHF/Tau fragments. Biophys J 90(5):1774–1789. doi:10.1529/biophysj.105.070136
Iqbal K, Grundke-Iqbal I (2008) Alzheimer neurofibrillary degeneration: significance, etiopathogenesis, therapeutics and prevention. J Cell Mol Med 12(1):38–55. doi:10.1111/j.1582-4934.2008.00225.x
Iqbal K, Alonso Adel C, Chen S, Chohan MO, El-Akkad E, Gong CX, Khatoon S, Li B, Liu F, Rahman A, Tanimukai H, Grundke-Iqbal I (2005) Tau pathology in Alzheimer disease and other tauopathies. Biochim Biophys Acta 1739(2–3):198–210
Ivanovova N, Handzusova M, Hanes J, Kontsekova E, Novak M (2008) High-yield purification of fetal tau preserving its structure and phosphorylation pattern. J Immunol Methods 339(1):17–22. doi:10.1016/j.jim.2008.07.014
Jensen PH, Hager H, Nielsen MS, Hojrup P, Gliemann J, Jakes R (1999) Alpha-synuclein binds to Tau and stimulates the protein kinase A-catalyzed tau phosphorylation of serine residues 262 and 356. J Biol Chem 274(36):25481–25489. doi:10.1074/jbc.274.36.25481
Khuebachova M, Verzillo V, Skrabana R, Ovecka M, Vaccaro P, Panni S, Bradbury A, Novak M (2002) Mapping the C terminal epitope of the Alzheimer’s disease specific antibody MN423. J Immunol Methods 262(1–2):205–215. doi:10.1016/S0022-1759(02)00006-6
Koson P, Zilka N, Kovac A, Kovacech B, Korenova M, Filipcik P, Novak M (2008) Truncated tau expression levels determine life span of a rat model of tauopathy without causing neuronal loss or correlating with terminal neurofibrillary tangle load. Eur J NeuroSci 28(2):239–246. doi:10.1111/j.1460-9568.2008.06329.x
Lee G (2005) Tau and src family tyrosine kinases. Biochim Biophys Acta 1739(2–3):323–330
Lim J, Lu KP (2005) Pinning down phosphorylated tau and tauopathies. Biochim Biophys Acta 1739(2–3):311–322
Liu F, Li B, Tung EJ, Grundke-Iqbal I, Iqbal K, Gong CX (2007) Site-specific effects of tau phosphorylation on its microtubule assembly activity and self-aggregation. Eur J NeuroSci 26(12):3429–3436
Magnani E, Fan J, Gasparini L, Golding M, Williams M, Schiavo G, Goedert M, Amos LA, Spillantini MG (2007) Interaction of tau protein with the dynactin complex. EMBO J 26(21):4546–4554. doi:10.1038/sj.emboj.7601878
Mesco ER, Timiras PS (1991) Tau-ubiquitin protein conjugates in a human cell line. Mech Ageing Dev 61(1):1–9. doi:10.1016/0047-6374(91)90002-H
Morishima-Kawashima M, Hasegawa M, Takio K, Suzuki M, Titani K, Ihara Y (1993) Ubiquitin is conjugated with amino-terminally processed tau in paired helical filaments. Neuron 10(6):1151–1160. doi:10.1016/0896-6273(93)90063-W
Mukrasch MD, von Bergen M, Biernat J, Fischer D, Griesinger C, Mandelkow E, Zweckstetter M (2007) The “jaws” of the tau-microtubule interaction. J Biol Chem 282(16):12230–12239. doi:10.1074/jbc.M607159200
Novak M (1994) Truncated tau protein as a new marker for Alzheimer’s disease. Acta Virol 38(3):173–189
Novak M, Wischik CM, Edwards P, Pannell R, Milstein C (1989) Characterisation of the first monoclonal antibody against the pronase resistant core of the Alzheimer PHF. Prog Clin Biol Res 317:755–761
Novak M, Jakes R, Edwards PC, Milstein C, Wischik CM (1991) Difference between the tau protein of Alzheimer paired helical filament core and normal tau revealed by epitope analysis of monoclonal antibodies 423 and 7.51. Proc Natl Acad Sci USA 88(13):5837–5841. doi:10.1073/pnas.88.13.5837
Pevalova M, Filipcik P, Novak M, Avila J, Iqbal K (2006) Post-translational modifications of tau protein. Bratisl Lek Listy (Tlacene Vyd) 107(9–10):346–353
Rapoport M, Dawson HN, Binder LI, Vitek MP, Ferreira A (2002) Tau is essential to beta -amyloid-induced neurotoxicity. Proc Natl Acad Sci USA 99(9):6364–6369. doi:10.1073/pnas.092136199
Roberson ED, Scearce-Levie K, Palop JJ, Yan F, Cheng IH, Wu T, Gerstein H, Yu GQ, Mucke L (2007) Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer’s disease mouse model. Science 316(5825):750–754. doi:10.1126/science.1141736
Ruben GC, Iqbal K, Grundke-Iqbal I, Wisniewski HM, Ciardelli TL, Johnson JE Jr (1991) The microtubule-associated protein tau forms a triple-stranded left-hand helical polymer. J Biol Chem 266(32):22019–22027
Schneider A, Biernat J, von Bergen M, Mandelkow E, Mandelkow EM (1999) Phosphorylation that detaches tau protein from microtubules (Ser262, Ser214) also protects it against aggregation into Alzheimer paired helical filaments. Biochemistry 38(12):3549–3558. doi:10.1021/bi981874p
Seabrook GR, Ray WJ, Shearman M, Hutton M (2007) Beyond amyloid: the next generation of Alzheimer’s disease therapeutics. Mol Interv 7(5):261–270. doi:10.1124/mi.7.5.8
Sevcik J, Skrabana R, Dvorsky R, Csokova N, Iqbal K, Novak M (2007) X-ray structure of the PHF core C-terminus: insight into the folding of the intrinsically disordered protein tau in Alzheimer’s disease. FEBS Lett 581(30):5872–5878. doi:10.1016/j.febslet.2007.11.067
Siuti N, Kelleher NL (2007) Decoding protein modifications using top-down mass spectrometry. Nat Methods 4(10):817–821. doi:10.1038/nmeth1097
Skrabana R, Kontsek P, Mederlyova A, Iqbal K, Novak M (2004) Folding of Alzheimer’s core PHF subunit revealed by monoclonal antibody 423. FEBS Lett 568(1–3):178–182. doi:10.1016/j.febslet.2004.04.098
Skrabana R, Sevcik J, Novak M (2006) Intrinsically disordered proteins in the neurodegenerative processes: formation of tau protein paired helical filaments and their analysis. Cell Mol Neurobiol 26(7–8):1085–1097. doi:10.1007/s10571-006-9083-3
Steinhilb ML, Dias-Santagata D, Fulga TA, Felch DL, Feany MB (2007a) Tau phosphorylation sites work in concert to promote neurotoxicity in vivo. Mol Biol Cell 18(12):5060–5068. doi:10.1091/mbc.E07-04-0327
Steinhilb ML, Dias-Santagata D, Mulkearns EE, Shulman JM, Biernat J, Mandelkow EM, Feany MB (2007b) S/P and T/P phosphorylation is critical for tau neurotoxicity in Drosophila. J Neurosci Res 85(6):1271–1278. doi:10.1002/jnr.21232
Uversky VN (2002) Natively unfolded proteins: a point where biology waits for physics. Protein Sci 11(4):739–756. doi:10.1110/ps.4210102
Uversky VN, Oldfield CJ, Dunker AK (2008) Intrinsically disordered proteins in human diseases: introducing the D2 concept. Annu Rev Biophys 37:215–246. doi:10.1146/annurev.biophys.37.032807.125924
Visser NF, Heck AJ (2008) Surface plasmon resonance mass spectrometry in proteomics. Expert Rev Proteomics 5(3):425–433. doi:10.1586/14789450.5.3.425
Walsh DM, Selkoe DJ (2004) Deciphering the molecular basis of memory failure in Alzheimer’s disease. Neuron 44(1):181–193. doi:10.1016/j.neuron.2004.09.010
Wischik CM, Novak M, Edwards PC, Klug A, Tichelaar W, Crowther RA (1988a) Structural characterization of the core of the paired helical filament of Alzheimer disease. Proc Natl Acad Sci USA 85(13):4884–4888. doi:10.1073/pnas.85.13.4884
Wischik CM, Novak M, Thogersen HC, Edwards PC, Runswick MJ, Jakes R, Walker JE, Milstein C, Roth M, Klug A (1988b) Isolation of a fragment of tau derived from the core of the paired helical filament of Alzheimer disease. Proc Natl Acad Sci USA 85(12):4506–4510. doi:10.1073/pnas.85.12.4506
Zilka N, Filipcik P, Koson P, Fialova L, Skrabana R, Zilkova M, Rolkova G, Kontsekova E, Novak M (2006) Truncated tau from sporadic Alzheimer’s disease suffices to drive neurofibrillary degeneration in vivo. FEBS Lett 580(15):3582–3588. doi:10.1016/j.febslet.2006.05.029
Acknowledgments
This work was supported by research grants VEGA 2/6183/26 and 2/5101/27, APVV 0631-07, APVV 0603-06, LPP-0326-06, LPP-0353-06, LPP-0354-06 and LPP-0363-06.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kovacech, B., Zilka, N. & Novak, M. New Age of Neuroproteomics in Alzheimer’s Disease Research. Cell Mol Neurobiol 29, 799–805 (2009). https://doi.org/10.1007/s10571-009-9358-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-009-9358-6