Abstract
Silver-functionalized textiles, as the most common protective medical materials, have attracted considerable attention. However, poor antibacterial durability and relatively tedious preparation process limit their applications. In this study, a kind of multifunctional cotton fabric was prepared through simultaneous dip-coating of silver-silica hybrid nanoparticles (Ag-MSNs) and fluorinated MSNs (F-MSNs), providing excellent antifouling ability due to superhydrophobicity and long-term antibacterial properties from the sustained release of Ag ions (Ag+). Detailed studies were performed to evaluate their structure and protective performance, especially the long-term antibacterial properties of the obtained fabrics were tested by the inhibition zone experiment for 25 d. The as-prepared fabrics showed good non-wetting properties and sustained antibacterial activity against Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus). Further washing and abrasion experimentations demonstrated that the as-prepared fabrics had stable hydrophobicity and antibacterial properties, much more reliable than commercial antibacterial gauze. Considering the inexpensive and simple preparation process as well as long-term and efficient effect for sterilization, the strategy paves a new and facile way for the fabrication of multifunctional fabrics with robust hydrophobic and antibacterial activity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cotton fabrics have a wide range of application scenarios in civil and military fields for their good air permeability, wearing comfort, and special protective properties (Xu et al. 2017; Wu et al. 2016). Although cotton fabrics provide a lot of unique protective properties, the porous structure and hydrophilic properties of the fabrics make them easily absorb numbers of bacteria or fungi on the surface, further resulting in adverse effects on the properties of the fabrics (Elshaarawy et al. 2019). Especially, when cotton fabrics infected by microorganisms are in close contact with the wound, the microorganisms easily grow and multiply in large numbers, and eventually leading to serious wound infection (Zhang et al. 2015; Li et al. 2011). Recently, much effort has been dedicated to the development of functionalized fabrics with antibacterial properties (Xu et al. 2017; Liu et al. 2012; Holt et al. 2018). Surface modification of cotton fabrics with significantly antibacterial substances is one of the most acceptable methods. A variety of metal ions (Rizzello and Pompa 2014), polymers (Lin et al. 2018; Fan et al. 2018), and nanomaterials (Fu et al. 2017) with antibacterial properties have been widely used to design antibacterial cotton fabrics. Among them, silver ions are the optimum because of their broad-spectrum bactericidal properties on both Gram-negative and Gram-positive bacteria (Jin et al. 2018). As the most common antibacterial fabrics, silver-functionalized fabrics are widely used in medical care and special military equipment due to their excellent sterilization and applicability (Ouadil et al. 2019; Syafiuddin et al. 2019; Krishnan et al. 2020).
Currently, there are two main methods for preparing silver-functionalized fabrics. The first one is to add silver nanoparticles (Ag NPs) during the spinning process to form antibacterial fibers and then Ag NP-doped fibers are woven by the fibril method (Yu et al. 2021), by which Ag NPs can be introduced well. However, this method is relatively complicated, and the prepared nanofibers have lower mechanical and tensile strength compared with ordinary fibers. The flexibility and comfort of the functionalized fabrics would also deteriorate (Zhang et al. 2016). The second method is to form Ag NPs on the surface of the fabrics in situ or to coat the prepared Ag NPs on the fabrics by finishing methods such as coating, sputtering, and printing (Krishnan et al. 2020; Xu et al. 2019; Chauhan et al. 2019; Ou et al. 2019). Compared with the fibril method, the finishing method is simpler in operation, but the Ag NPs easily fall off or suffer from uncontrolled release (Qiu et al. 2020). As a result, the silver-functionalized fabrics prepared by this method have poor durability and low antibacterial stability. In addition, it is worth pointing out that the aggregation of Ag NPs is often unavoidable during the preparation of silver-functionalized fabrics, which makes it hard for large-scale industrial production (Yu et al. 2021). Consequently, it is highly worthy to explore a facile and efficient approach to introduce suitable Ag NPs onto the fabrics to produce antibacterial fibers with the durable coalition and controlled distribution of Ag NPs.
For current silver-functionalized fabrics, their applications are still limited by low antibacterial durability. As the concentration of Ag+ reduces through daily use, the antibacterial properties of the silver-functionalized fabrics will gradually decrease and eventually disappear, which render it unable to defend microorganisms (Kim et al. 2018). Therefore, it is particularly important to achieve the long-term antibacterial properties of silver-functionalized fabrics. To achieve the long-term high-efficiency antibacterial effect, it is necessary to load more Ag NPs or to slow the release rate of Ag+ without reducing the antibacterial effect. Unfortunately, the amount of Ag NPs coated on the fabrics will be limited by the aggregation of Ag NPs. In addition, a large amount of Ag NPs coated on the surface of the fabrics will deteriorate the fabrics’ flexibility, breathability, and sense of comfort (Li et al. 2017a, b). Slowing down the release of Ag+ can be realized by coated certain silver-loaded functionalized nanoparticles on the fabrics, while the antibacterial ability of the fabrics will be greatly reduced (Jin et al. 2018). There is a big challenge to resolve the tradeoff between effective antibacterial efficacy by sufficient release of Ag+ and long-term antibacterial properties by slow consumption of Ag+. Recently, the superhydrophobic modifications by introducing low surface energy substances and surface micro-nano structures have attracted great attention as the main antifouling strategies for the fabrication of antibacterial surfaces. Superhydrophobic surfaces are defined as contact angles (CAs) larger than 150º, and sliding angles (SAs) less than 10º (Wang et al. 2020), which possess low adhesion to bacteria (Li et al.2017a, b). In this case, the amounts of bacteria attached per unit area of the superhydrophobic fabrics can be significantly reduced. As a result, the small amounts of adhering bacteria can be easily killed by a low concentration of Ag+. In another word, even if the release of Ag+ from silver-functionalized superhydrophobic fabrics is slow, the outstanding bactericidal effect can be achieved.
Additionally, superhydrophobic surfaces can be easily realized based on low-surface-energy materials and micro/nanoscale surface roughness, minimizing contact between water and the solid surface (Wang et al. 2020). In our previous study, F-MSNs were necessary nanoparticles which possessed both low surface energy and nanostructures to endow the functionalized fabrics superhydrophobicity (Ye et al. 2021). While, in the present study, we investigated the release-killing antibacterial and antifouling properties of superhydrophobic functionalized coating composed of Ag-MSNs and F-MSNs (Scheme 1) through simultaneous dip-coating, aiming to efficiently fabricate a long-term antibacterial functionalized cotton fabrics that could inhibit the growth of drug-resistant bacteria and avoid wound infection.
Experimental section
Materials
Silver nitrate (AgNO3, 0.0242 mol/L), Tetraethyl orthosilicate (TEOS, ≥ 99%), trimethoxy (octadecafluorodecyl) silane (FAS, 97%), Poly(vinylpyrrolidone) (PVP, Mw≈30 k, powder), PDMS (prepolymer named as PDMS-A and the thermal curing agent named as PDMS-B) was purchased from Dow Corning Corp. Diethanolamine (DEA, ≥ 99.5%), methanol, ethanol, tetrahydrofuran (THF, ≥ 99.5%), hexadecyltrimethylammonium chloride (CTAC, 25 wt.%), and hydrochloric acid (37%, analytical grade), were purchased from Sigma-Aldrich. Gram-positive bacteria Staphylococcus aureus (S. aureus, ATCC 6538) and Gram-negative bacteria Escherichia coli (E. coli, ATCC 25,922) were incubated at 37ºC for 24 h before use. The cotton fabrics (200 g/m2, 21 × 16/107 × 59) were purchased from a local store. All these above-mentioned reagents were used as received.
Preparation of two nanoparticles (MSNs and F-MSNs)
Mesoporous silicon nanoparticles (MSNs) were synthesized based on the Stöber method according to the previous paper (Wu et al. 2019). Firstly, the solution of ethanol (9 mL) and water (64 mL) with 0.10 g of CTAC was magnetically stirred for 3 min. After that, the DEA (0.02 g) was added to control the pH of the solution. And then, 7 mL of TEOS was added dropwise while the above solution was still stirred. The reaction mixture gradually became milky white due to the formation of silica particles at 60ºC. After 3 h, the silica particles were obtained by centrifugation at 8500 rpm for 15 min. To remove the unreacted materials, the above silica particles were washed three times with ethanol and water respectively. In addition, F-MSNs were synthesized by the same process except that 1.0 g of FAS was slowly added to the solution. To remove the CTAC which was the template in the above silica particles, both particles were all dispersed in 500 mL of the methanol solution with hydrochloric acid (30 mL), and the solution was refluxed for 24 h at 40ºC, respectively. To ensure that there was no residual hydrochloric acid, the particles after centrifuged were washed with distilled water and ethanol three times, respectively. Finally, two kinds of mesoporous nanoparticles were obtained after drying in a vacuum oven for 24 h.
Preparation of Ag-MSNs
Ag-MSNs were synthesized according to a previous report with appropriate modifications (Nie et al. 2018). Firstly, 1.0 g of MSNs was ultrasonically dispersed in 100 mL of ethanol, and then the mixture was stirred for 7 h at 45ºC. Whereafter, 87.5 mg of AgNO3 was slowly added into the above mixture under vigorous stirring and refluxed in the dark. And then, 2 mL ethanol mixed with 0.02 g NaBH4 was added dropwise and stirred for 3 h. Finally, Ag-MSNs were separated by using a centrifuge and washed with ethanol and water to remove the superfluous Ag+ if any, and the precipitates were dried at 25ºC.
Preparation of functionalized fabrics
To obtain functionalized fabrics, the 0.15 g of Ag-MSNs and 0.12 g F-MSNs were immersed into 30 mL THF containing 0.15 g of PDMS -A and 0.015 g PDMS-B by ultrasonic for 45 min. After that, clear cotton fabrics (4 cm × 10 cm) were put into the above solution under vortex shocking for 15 min. Finally, the fabrics were placed into the beaker and cured at 70ºC for 3 h.
Characterization of F-MSNs, Ag-MSNs and functionalized fabrics
Surface microstructures and morphology of F-MSNs, Ag-MSNs, and functionalized fabrics were obtained by TEM and SEM, and element mapping images were obtained from an energy-dispersive spectroscope which was attached to the SEM. The chemical functional groups of the nanoparticles were analyzed by a Fourier transform infrared spectroscopy (FTIR, Thermo Fisher Scientific Inc.) which covers a 250–4000 cm−1 infrared range with a resolution of 2 cm−1. A Kruss Easy Drop goniometer (Kruss Germany) was used to measure the water contact angles (CAs) and sliding angles (SAs) of each sample. Distilled water droplets with a volume of 8 μL were deposited on the samples at 25ºC. The functionalized fabrics were glued to the glass plate by double-sided tape before measured to ensure the accuracy of CAs and SAs, and average CAs and SAs had been measured on 5 different positions.
Wetting evaluations of functionalized fabrics
The non-wetting properties of the functionalized fabrics were evaluated by the droplets’ static contact and rolling on the fabrics according to the previous report (Su et al. 2017; Pan et al. 2018). Besides, the floating experiment of glass glued with functionalized fabrics on the water was also obtained.
Antibacterial activity of Ag-MSNs and functionalized fabrics
The antibacterial activity of Ag-MSNs was determined by the colony count method, and the detailed steps were described in our previous report (Ye et al. 2021). E. coli and S. aureus were cultivated at 37ºC for 2 h. After cultivation, the bacterial solution (E. coli and S. aureus, respectively) were diluted by PSB buffer to 106 CFU mL−1. And then, 1 mL of the diluted solution was mixed with 1 mL of PBS buffer containing Ag-MSNs with a concentration of 40 μg mL−1, the newly mixed solution was incubated at a constant temperature and shaking table (37ºC, 150 rpm min−1) for 24 h. Finally, 100 μL resulting bacterial solution was quickly dispersed onto the LB agar plate by bio-clean SS-Spreader. Bacterial colonies on the cultural plates were counted after incubation at 37ºC for 24 h.
To evaluate the antibacterial activity of functionalized fabrics, the fabrics were cut into 1 cm × 2 cm, and three samples were tested in parallel. The functionalized fabrics and the pristine fabrics were all immersed in 5 mL of bacterial suspension (S. aureus or E. coli, 105 CFU mL−1) and subjected to Vortex oscillation for 3 min to make the bacteria solution contacted with the fabrics well. Then, the samples were taken out and held vertically to allow the droplets remaining on the surface to slide away. Additionally, the samples were transferred into tubes at 37ºC for 24 h. After the incubation, the fabrics were put into test tubes containing 5 mL of PBS and experienced a water bath ultrasonication for 10 min, respectively. Finally, 100 μL of above-detached bacteria in PBS was spread onto the LB agar plates to incubate at 37ºC for 24 h. The antibacterial activity of functionalized fabrics was calculated by counting the number of colonies on plates.
To further test the antibacterial activity, inhibition zone test of functionalized fabrics against S. aureus, which is regarded as a quantitative technology against S. aureus, was conducted according to the AATCC100-2004 standard. As for the inhibition zone test, 100 μL of bacterial solution (S. aureus, 106 CFU mL−1) was spread onto LB agar plate, and then the sample (1.5 cm × 1.5 cm circles of functionalized fabrics) was put on it to incubate at 37ºC for 24 h.
Mechanical robustness and chemical durability of functionalized fabrics
The stability of the antibacterial properties of the fabrics was also tested by ultrasonic washing. And the fabrics (5 cm × 5 cm) were submerged in 50 mL distilled water for 4 h under ultrasonication (40 kHz, 180 W). After that, the samples were collected at certain intervals and dried at 105ºC for 2 h, followed by the measurements of CAs and antibacterial properties, respectively.
Abrasion resistance of fabrics is particularly critical in daily use. Herein, the wear resistance of functionalized fabrics was tested by sandpaper abrasion. A 200 g weight was put on the fabric while it was placed facedown to the sandpaper (standard sandpaper, grit no. 360). Then, the fabric was moved back and forth at about 2 cm/s. Each iteration was counted as a cycle, and CAs and antibacterial properties of the fabric were tested after every 100 abrasion cycles.
As for chemical stability, the functionalized fabrics were cut into 3 cm × 5 cm, and then were immersed in the prepared hydrochloric acid (HCl, pH = 2), sodium hydroxide (NaOH, pH = 12) solution and liquid nitrogen for 12 h, after which the fabrics were taken out and dried in an oven for 2 h. The contact angles were determined by measuring five random positions of the dried fabrics.
Air permeability of functionalized fabrics
Air permeability was one of the important characteristics of functionalized fabrics and measured by Air Permeability Tester with a pressure applied of 128 Pa, according to standard test method (Fabrics1 D737-04). Additionally, the water vapor permeability was also tested. The beaker containing water was sealed with a functionalized fabrics (9 cm × 9 cm, circle), and then the bottom was heated with an alcohol lamp until the water in the beaker began to boil, and the generation of water mist above the fabric was recorded with a camera.
Results and discussion
Characterization of Ag-MSNs and F-MSNs
The morphologies of the as-prepared Ag-MSNs and F-MSNs were shown in Fig. 1. As shown in Fig. 1a, the black spots were silver particles, which were bonded with MSNs, and the synthetic Ag-MSNs presented a ball-and-stick structure (Fig. 1c). The sphericity of F-MSNs was irregular, with some roughness (Fig. 1b), and the particle size of F-MSNs was about 50–60 nm (Fig. 1d). The stretching modes of the Si–O-Si groups belonged to MSNs and F-MSNs were shown at 804 cm−1 and 463 cm−1, while the hydroxyl stretching of Si–OH groups was shown at 3372 cm−1. As for F-MSNs, the absorption peaks at 1147 cm−1 and 1207 cm−1 could be ascribed to antisymmetric and symmetric C-F stretching modes (Fig. 1e). Additionally, the EDS spectrum mapping of Ag, Si and O elements indicated the successful synthesis of Ag-MSNs (Fig. S1a). Besides, the clearly visible lattice fringes with spacing of about 0.236 nm of Ag NPs were directly detected in the HRTEM image (Fig. S1b). The survey XPS spectra (Fig. S1c) of the Ag-MSNs showed the featured bands corresponding to Ag, Si and O. The signature of Ag 3d doublet presented in high-resolution XPS spectra (Fig. S1d) at 368.8 and 374.8 eV was ascribed to the binding energies of Ag 3d5/2 and Ag 3d3/2 electronic states, respectively. These results indicated the Ag-MSNs and F-MSNs were successfully synthesized.
Characterizations of functionalized fabrics
The optical photo of THF solution mixed with two functionalized nanoparticles after 15 min sonication was shown in Fig. 2a. The solution was black because of the presence of Ag-MSNs. As shown in Fig. 2b, the surface of functionalized fabrics was covered with nanoparticles, and four elements: Si, O, Ag, and F were well shown in the element mapping. Due to the nanostructure of the two kinds of nanoparticles and the microstructure of the fabrics surface, combined with the hydrophobic properties of F-MSNs and PDMS, the functionalized fabrics had excellent superhydrophobicity (CA = 151º, SA = 6º) shown in Fig. 2c. To further evaluate the superhydrophobicity of functionalized fabrics, Fig. 2d, e showed the photos of a water droplet touching and leaving the pristine fabrics and functionalized fabrics, respectively. The droplet was forced to contact the fabrics surface sufficiently with an obvious change in sphericity, and when it was lifted lightly, the water droplet showed almost no deformation in Fig. 2e, while the water droplet was quickly immersed in the pristine fabrics in Fig. 2d, which confirmed the extremely low water adhesion for functionalized fabrics (Gu et al. 2019).
Non-wetting properties of the functionalized fabrics
The superhydrophobicity of the functionalized fabrics can reduce the adhesion of bacteria in the aqueous solution (Zhu et al. 2020). To intuitively reflect the superhydrophobicity of functionalized fabrics, related experiments were designed as follows. Through the static and sliding experiments of droplets, we further verified that the fabrics had excellent superhydrophobicity. As shown in Fig. 3a left and Video. S1, five kinds of common water-based droplets (from left to right: milk, cola, coffee, juice, water) were dropped on the functionalized fabrics (bottom) and the pristine fabric (top), respectively. Compared with the pristine fabrics in which the droplets permeated rapidly, the functionalized fabrics possessed well non-wetting properties that the droplets remained spherical. In addition, the droplets would be easily absorbed away by the paper and there were no droplets left on the surface of the functionalized fabrics (Fig. 3a right and Video. S2). After that, we glued the functionalized fabrics and the pristine fabrics to the glass sheet with double-sided adhesive, respectively. As shown in Fig. 3b and Video. S3, when they were placed in the dyed water at the same time, we found that the functionalized fabrics with the glass plate could float on the water surface, while the pristine fabrics with the glass plate would sink in the water, which further proved that the functionalized fabrics had excellent hydrophobicity. As shown in Fig. 3c and Video. S4, the droplets could slide quickly from the top to the bottom of the functionalized fabrics. The experiment of droplets’ roll-off further confirmed the non-stick performance of the functionalized fabrics. The above experiments further showed that low surface energy and nanostructures of F-MSNs coated to the surface of the fabrics by PDMS, resulted in superhydrophobic and antifouling properties of functionalized fabrics.
Non-wetting properties of functionalized fabrics. a The still images of different droplets on functionalized fabrics (bottom) and pristine fabrics (top). b the glass which was glued with functionalized fabrics (left) and pristine fabrics (right). c The sliding images of milk droplets on functionalized fabrics
Antibacterial properties of Ag-MSNs and the functionalized fabrics
The antibacterial ability of Ag-MSNs is a key factor in determining the success of functionalized fabrics, and we quantitatively evaluated the antibacterial properties of Ag-MSNs. As shown in Fig. S2, when the concentration of Ag-MSNs reached 20 μg mL−1, the antibacterial rates for E. coli and S. aureus were both as high as 99.9%. As we all known, long-term antibacterial properties of the functionalized fabrics play an important role in daily use, and the stability of the antibacterial properties can provide reliable protection (Lu et al. 2015). As shown in Fig. 4a, functionalized fabrics named TF had excellent antibacterial properties compared with the pristine fabrics named F. In addition, the long-term antibacterial performance of functionalized fabrics was verified through the colony counting method. As time goes by, the antibacterial rates of the functionalized fabrics decreased gradually due to the continuous release of Ag+ (Fig. 4b). Thanks to the synergistic effect of superhydrophobic antifouling and Ag-MSNs antibacterial properties, the antibacterial rates of the functionalized fabrics could still reach 92.3% (E. coli) and 90.1% (S. aureus) even after 30 d, which was sufficient to ensure that the functionalized fabrics had stable protective effects during daily use.
Antibacterial properties of the functionalized fabrics were directly reflected in Fig. 5. As-prepared fabrics could keep an excellent zone of inhibition for 25 d because of the continuous release of Ag+ from the Ag-MSNs coated on the surface of the fabrics (Nie et al. 2018). The diameters of the inhibition zones were well observed, which intuitively revealed that the as-prepared fabrics possessed prominent antibacterial properties. In addition, we further explored the influence of the concentration of PDMS in the dip-coating solution on the zone of inhibition and found that when the concentration of PDMS reached 0.02 g mL−1, the zone of inhibition did not appear (Fig. S3a), which meant that the thicker PDMS coating would completely prevent the release of Ag+.
The long-term antibacterial mechanism could be explained from three points: Firstly, the superhydrophobic antifouling surface can greatly reduce the infiltration of water droplets on its surface, thereby reducing the adhesion of bacteria, fungi and other microorganisms which are multiplied in the droplets. Thus, few microorganisms attached can be killed by a small amount of Ag+; secondly, mesoporous silicon nanoparticles (MSNs) can avoid a large amount of agglomeration of silver nanoparticles, and the large specific area of MSNs can make the nanoparticles bond more firmly on the fabrics, which ensures that there are abundant antibacterial materials on the functional fabrics; thirdly, PDMS is not only used as a binder, but also as a layer of coating material to form a barrier on the surface of Ag-MSNs, thereby slowing the release rate and extending the release time of Ag+. Owing to the superhydrophobic antibacterial properties of the surface, the few bacteria attached on the fabrics could be killed by a small number of Ag+. Therefore, the high loading of Ag-MSNs on the functional fabrics and the slower release rate of Ag+ realize the long-term antibacterial properties of the fabric.
Mechanical robustness and chemical durability of the functionalized fabrics
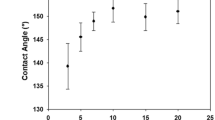
Excellent mechanical robustness and chemical durability are prerequisites for the reliable protection of functionalized fabrics. Ultrasonic washing and sandpaper abrasion tests were used to evaluate the robustness of the fabrics, and shown in Fig. 6. The difference of morphology was compared by SEM in Fig. 6a and c. After ultrasonic washing for 150 min, there were still many functionalized nanoparticles on the surface of the fabrics (Fig. 6a). Besides, the CAs and antibacterial rates (S. aureus) of the functionalized fabrics could still reach 144º and 94.3%, which meant the fabrics were extremely stable to keep their hydrophobicity and antibacterial properties. During the daily use of cotton fabrics, wear occurs over time. The wear resistance of functionalized fabrics was investigated through a mechanical wear test by using sandpaper abrasion. As shown in Fig. 6c, the texture structure of the surface of fabrics was greatly destroyed after 500 times abrasion. Interestingly, the CAs and the antibacterial rate (S. aureus) only decreased to 140º and 90.2%, which proved that the fabrics still had excellent antifouling and antibacterial properties. The reason why the worn fabric still had good antibacterial properties was that the functionalized nanoparticles were firmly bonded to the surface of the fabric fibers by PDMS. Even if the surface fibers were damaged, the silver nanoparticles on the remaining fibers could continuously release Ag+ to achieve the sterilization effect. Acid/alkali and liquid nitrogen resistance of functional fabrics was tested according to previous studies (Ye et al. 2021). Briefly, the functional fabrics were immersed in HCl (pH = 2), NaOH (pH = 12) solution and liquid nitrogen, respectively for 12 h and then the changes of CAs were evaluated. As shown in the Fig. S4, after treated by acid/alkali solution or liquid nitrogen, the CAs of the fabrics were only reduced to 139 ± 3º, 142 ± 2º and 148 ± 2º, respectively. Owing to the stability of PDMS and the superhydrophobicity of the fabrics surface which reduced the contact between the coating and the solution, functionalized fabrics had excellent chemical durability.
Air permeability of the functionalized fabrics
Air permeability is one of the important characteristics of fabrics. As shown in Fig. S5a, water vapor could directly penetrate functionalized fabrics and condensed to visible mist above the fabrics, which directly indicated that the functionalized fabrics had good water vapor permeability. In addition, the air permeability of functionalized fabrics (289 ± 25 mm/s) had no significant decrease compared with the pristine fabrics (300 ± 20 mm/s) owing to the thin coating would not affect the pristine cotton fabric gap too much (Fig. S5b), which proved that the functionalized fabrics we prepared still had good air permeability compared to the pristine fabrics.
Conclusion
In this study, two kinds of functionalized nanoparticles, Ag-MSNs and F-MSNs, were well synthesized. Functionalized nanoparticles, together with PDMS, were added into the THF solution to prepare a functionalized coating solution, which was coated onto the cotton fabrics through the dip-coating method. Furthermore, the functionalized fabrics with both long-term antibacterial effect and superhydrophobic antifouling function were successfully tested. Experimental studies showed that the prepared fabrics had effective long-term inhibition effects on S. aureus for up to 25 d. Besides, the superhydrophobic properties resulted from the existence of F-MSNs, which makes the functionalized fabrics inhibit the antiadhesion of bacteria. It is because of the synergy of antibacterial properties and antiadhesion of bacteria, the antibacterial rates of functionalized fabrics up to 92.3% (E. coli) and 90.1% (S. aureus) even after 30 d. More significantly, considering the simple preparation process, low cost, antifouling, and long-term antibacterial properties of as-prepared fabrics, these functionalized fabrics will have great application prospects in medical materials and special textiles in the future.
References
Chauhan P, Kumar A, Bhushan B (2019) Self-cleaning, stain-resistant and anti-bacterial superhydrophobic cotton fabric prepared by simple immersion technique. J Colloid Interface Sci 535:66–74. https://doi.org/10.1016/j.jcis.2018.09.087
Elshaarawy RFM, Seif GA, El-Naggar ME, Mostafa TB, El-Sawi EA (2019) In-situ and ex-situ synthesis of poly-(imidazolium vanillyl)-grafted chitosan/silver nanobiocomposites for safe antibacterial finishing of cotton fabrics. Eur Polym J 116:210–221. https://doi.org/10.1016/j.eurpolymj.2019.04.013
Fan XL, Hu M, Qin ZH, Wang J, Chen XC, Lei WX, Ye WY, Jin Q, Ren KF, Ji J (2018) Bactericidal and hemocompatible coating via the mixed-charged copolymer. ACS Appl Mater Interfaces 10:10428–10436. https://doi.org/10.1021/acsami.7b18889
Fu Y, Jiang J, Zhang Q, Zhan X, Chen F (2017) Robust liquid-repellent coatings based on polymer nanoparticles with excellent self-cleaning and antibacterial performances. J Mater Chem A 5:275–284. https://doi.org/10.1039/c6ta06481g
Gu Z, Kothary P, Sun CH, Gari A, Zhang Y, Taylor C, Jiang P (2019) Evaporation-induced hierarchical assembly of rigid silicon nanopillars fabricated by a scalable two-level colloidal lithography approach. ACS Appl Mater Interfaces 11:40461–40469. https://doi.org/10.1021/acsami.9b12388
Holt BA, Gregory SA, Sulchek T, Yee S, Losego MD (2018) Aqueous Zinc Compounds as Residual Antimicrobial Agents for Textiles. ACS Appl Mater Interfaces 10:7709–7716. https://doi.org/10.1021/acsami.7b15871
Jin C, Liu X, Tan L, Cui Z, Yang X, Zheng Y, Yeung KWK, Chu PK, Wu S (2018) Ag/AgBr-loaded mesoporous silica for rapid sterilization and promotion of wound healing. Biomater Sci 6:1735–1744. https://doi.org/10.1039/c8bm00353j
Kim J-H, Mirzaei A, Kim HW, Kim SS (2018) Novel superamphiphobic surfaces based on micro-nano hierarchical fluorinated Ag/SiO2 structures. Appl Surf Sci 445:262–271. https://doi.org/10.1016/j.apsusc.2018.03.148
Krishnan PD, Banas D, Durai RD, Kabanov D, Hosnedlova B, Kepinska M, Fernandez C, Ruttkay-Nedecky B, Nguyen HV, Farid A, Sochor J, Narayanan VHB, Kizek R (2020) Silver nanomaterials for wound dressing applications. Pharmaceutics 12:12090821. https://doi.org/10.3390/pharmaceutics12090821
Li P, Poon YF, Li W, Zhu HY, Yeap SH, Cao Y, Qi X, Zhou C, Lamrani M, Beuerman RW, Kang ET, Mu Y, Li CM, Chang MW, Leong SS, Chan-Park MB (2011) A polycationic antimicrobial and biocompatible hydrogel with microbe membrane suctioning ability. Nat Mater 10:149–156. https://doi.org/10.1038/nmat2915
Li S, Zhu T, Huang J, Guo Q, Chen G, Lai Y (2017a) Durable antibacterial and UV-protective Ag/TiO2@ fabrics for sustainable biomedical application. Int J Nanomed 12:2593–2606. https://doi.org/10.2147/IJN.S132035
Li S, Huang J, Chen Z, Chen G, Lai Y (2017b) A review on special wettability textiles: theoretical models, fabrication technologies and multifunctional applications. J Mater Chem A 5:31–55. https://doi.org/10.1039/c6ta07984a
Lin J, Chen X, Chen C, Hu J, Zhou C, Cai X, Wang W, Zheng C, Zhang P, Cheng J, Guo Z, Liu H (2018) Durably antibacterial and bacterially antiadhesive cotton fabrics coated by cationic fluorinated polymers. ACS Appl Mater Interfaces 10:6124–6136. https://doi.org/10.1021/acsami.7b16235
Liu T, Yin B, He T, Guo N, Dong L, Yin Y (2012) Complementary effects of nanosilver and superhydrophobic coatings on the prevention of marine bacterial adhesion. ACS Appl Mater Interfaces 4:4683–4690. https://doi.org/10.1021/am301049v
Lu Z, Xiao J, Wang Y, Meng M (2015) In situ synthesis of silver nanoparticles uniformly distributed on polydopamine-coated silk fibers for antibacterial application. J Colloid Interface Sci 452:8–14. https://doi.org/10.1016/j.jcis.2015.04.015
Nie W, Dai X, Li D, McCoul D, Gillispie GJ, Zhang Y, Yu B, He C (2018) One-pot synthesis of silver nanoparticle incorporated mesoporous silica granules for hemorrhage control and antibacterial treatment. ACS Biomater Sci Eng 4:3588–3599. https://doi.org/10.1021/acsbiomaterials.8b00527
Ou J, Wu B, Xue M, Wang F (2019) Silver ions anchored to fabric via coordination: Evaluation on washing durability and antibacterial activity. Mater Lett 237:134–136. https://doi.org/10.1016/j.matlet.2018.11.090
Ouadil B, Amadine O, Essamlali Y, Cherkaoui O, Zahouily M (2019) A new route for the preparation of hydrophobic and antibacterial textiles fabrics using Ag-loaded graphene nanocomposite. Colloids Surf Physicochem Eng Aspects 579:123713. https://doi.org/10.1016/j.colsurfa.2019.123713
Pan S, Guo R, Bjornmalm M, Richardson JJ, Li L, Peng C, Bertleff-Zieschang N, Xu W, Jiang J, Caruso F (2018) Coatings super-repellent to ultralow surface tension liquids. Nat Mater 17:1040–1047. https://doi.org/10.1038/s41563-018-0178-2
Qiu Q, Chen S, Li Y, Yang Y, Zhang H, Quan Z, Qin X, Wang R, Yu J (2020) Functional nanofibers embedded into textiles for durable antibacterial properties. Chem Eng J 384:123241. https://doi.org/10.1016/j.cej.2019.123241
Rizzello L, Pompa PP (2014) Nanosilver-based antibacterial drugs and devices: mechanisms, methodological drawbacks, and guidelines. Chem Soc Rev 43:1501–1518. https://doi.org/10.1039/c3cs60218d
Su X, Li H, Lai X, Zhang L, Wang J, Liao X, Zeng X (2017) Vapor-liquid sol-gel approach to fabricating highly durable and robust superhydrophobic polydimethylsiloxane@silica surface on polyester textile for oil-water separation. ACS Appl Mater Interfaces 9:28089–28099. https://doi.org/10.1021/acsami.7b08920
Syafiuddin A (2019) Toward a comprehensive understanding of textiles functionalized with silver nanoparticles. J Chin Chem Soc 66:793–814. https://doi.org/10.1002/jccs.201800474
Wang D, Sun Q, Hokkanen MJ, Zhang C, Lin FY, Liu Q, Zhu SP, Zhou T, Chang Q, He B, Zhou Q, Chen L, Wang Z, Ras RHA, Deng X (2020) Design of robust superhydrophobic surfaces. Nature 582:55–59. https://doi.org/10.1038/s41586-020-2331-8
Wu M, Ma B, Pan T, Chen S, Sun J (2016) Silver-Nanoparticle-Colored Cotton Fabrics with Tunable Colors and Durable Antibacterial and Self-Healing Superhydrophobic Properties. Adv Funct Mater 26:569–576. https://doi.org/10.1002/adfm.201504197
Wu D, Shi X, Zhao F, Chilengue STF, Deng L, Dong A, Kong D, Wang W, Zhang J (2019) An injectable and tumor-specific responsive hydrogel with tissue-adhesive and nanomedicine-releasing abilities for precise locoregional chemotherapy. Acta Biomater 96:123–136. https://doi.org/10.1016/j.actbio.2019.06.033
Xu Q, Xie L, Diao H, Li F, Zhang Y, Fu F, Liu X (2017) Antibacterial cotton fabric with enhanced durability prepared using silver nanoparticles and carboxymethyl chitosan. Carbohydr Polym 177:187–193. https://doi.org/10.1016/j.carbpol.2017.08.129
Xu Q, Li R, Shen L, Xu W, Wang J, Jiang Q, Zhang L, Fu F, Fu Y, Liu X (2019) Enhancing the surface affinity with silver nano-particles for antibacterial cotton fabric by coating carboxymethyl chitosan and l-cysteine. Appl Surf Sci 497:143673. https://doi.org/10.1016/j.apsusc.2019.143673
Ye Z, Li S, Zhao S, Deng L, Zhang J, Dong A (2021) Textile coatings configured by double-nanoparticles to optimally couple superhydrophobic and antibacterial properties. Chem Eng J 420:127680. https://doi.org/10.1016/j.cej.2020.127680
Yu W, Li X, He J, Chen Y, Qi L, Yuan P, Ou K, Liu F, Zhou Y, Qin X (2021) Graphene oxide-silver nanocomposites embedded nanofiber core-spun yarns for durable antibacterial textiles. J Colloid Interface Sci 584:164–173. https://doi.org/10.1016/j.jcis.2020.09.092
Zhang LJ, Guerrero-Juarez CF, Hata T, Bapat SP, Ramos R, Plikus MV, Gallo RL (2015) Innate immunity. Dermal adipocytes protect against invasive Staphylococcus aureus skin infection. Science 347:67–71. https://doi.org/10.1126/science.1260972
Zhang S, Tang Y, Vlahovic B (2016) A review on preparation and applications of silver-containing nanofibers. Nanoscale Res Lett 11:80. https://doi.org/10.1186/s11671-016-1286-z
Zhu R, Liu M, Hou Y, Zhang L, Li M, Wang D, Fu S (2020) One-pot preparation of fluorine-free magnetic superhydrophobic particles for controllable liquid marbles and robust multifunctional coatings. ACS Appl Mater Interfaces 12:17004–17017. https://doi.org/10.1021/acsami.9b22268
Acknowledgments
We gratefully acknowledge the financial support provided by National Natural Science Foundation of China (32171380, 31971306).
Funding
This study was funded by [National Natural Science Foundation of China (32171380, 31971306)].
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by [YZ], [RH], and [LS]. The first draft of the manuscript was written by [YZ] and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
All authors certify that they have no affiliation with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Availability of data and material
All authors make sure that all data and materials support the published claims and comply with field standards.
Code availability
All authors make sure that software applications or custom codes support the published claims and comply with field standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file2 (MP4 13102 kb)
Supplementary file3 (MP4 6985 kb)
Supplementary file4 (MP4 5738 kb)
Supplementary file5 (MP4 5669 kb)
Rights and permissions
About this article
Cite this article
Ye, Z., Rong, H., Li, S. et al. A facile strategy to fabricate silver-functionalized superhydrophobic cotton fabrics with long-term antibacterial properties. Cellulose 29, 1163–1174 (2022). https://doi.org/10.1007/s10570-021-04289-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-021-04289-5