Abstract
It is significant to treat cotton fabrics with flame retardant, which can effectively protect them from damage in the fire. A novel reactive flame retardant coating, ammonium salt of N,N-dimethylene-piperazine-(methylphosphonic acid) (ASNDP), was prepared and employed to enhance the anti-burning effect of pure cotton fabrics. Fourier transform infrared spectroscopy and nuclear magnetic resonance spectrum were used to analyze the chemical composition of the synthesized ASNDP. The combustion behavior and anti-burning performance of coated cotton fabrics were fully investigated with using cone calorimetry test, limited oxygen index (LOI) test and vertical flammability test. The total heat release value of the sample coated with ASNDP (450 g/L) decreased to 3.7 ± 0.1 MJ/m2 and the LOI value of the sample with a weight gain of 17.4 ± 0.3% raised to 29.5 ± 0.1%. The thermal and thermo-oxidative stability of coated cotton fabrics were researched with thermogravimetric analysis. The integral procedural decomposition temperature (IPDT) value of coated cotton fabrics in nitrogen atmosphere increased from 610.1 to 1402.8 °C, which proved that ASNDP inhibited the thermal degradation of fiber unit by promoting the growth of the char layer. Besides, the morphological structure of uncoated and coated samples before and after combustion was characterized by scanning electron microscopy. It can be observed that the fiber skeleton of the coated cotton fabrics was evenly distributed and remained intact after combustion. The conclusion was that ASNDP endowed cotton fabrics with good flame retardancy, and effectively performed the flame-retardant effect in both gas phase and the condensed phase.
Graphic abstract
A novel phosphorus–nitrogen synergistic flame retardant containing multiple reactive groups was successfully synthesized and applied to improve the flame retardancy and thermal stability of pure cotton fabrics.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Natural cellulose polymer materials, especially of cotton fabrics, are one of the most widely used textile substrates in daily life and are favored by building and interior decoration industry for their excellent hygroscopicity, comfort, softness and biodegradation properties (Laufer et al. 2012; Zhu et al. 2020). However, the materials made from cotton fabrics have the disadvantage of high flammability, which severely limits their application in certain special fields (Liu et al. 2020; Zhang et al. 2020). Consequently, the cotton fabrics with flame resistance are studied to reduce fire accidents and contributed to the protection of various materials made from cotton fabrics.
At present, there are some flame retardant methods for cotton fabrics, such as compound coating, surface chemical grafting, layer-by-layer assembly and so on (Carosio et al. 2015; Jia et al. 2017; Jiang et al. 2019; Zhao et al. 2017). Among them, the technology of chemically grafting the reactive flame retardant to cotton fabrics by the impregnation method is most widely used because of its simple finishing process and excellent durability (Jiang et al. 2019). Based on these advantages, some scholars have conducted research on flame retardants suitable for impregnation method to treat cotton fabrics. The common flame retardants synthesized earlier are a series of compounds containing N-methylol structure (Zhao et al. 2017). Among this type of flame retardant, the most prominent representative is N-methylol of Pyrovatex CP (Huang et al. 2019). The cotton fabrics coated with it have highly efficient flame retardancy and extraordinary thermal stability, which made it one of the most frequently applied products in the industry for a period of time. The only drawback is that cotton fabrics coated with Pyrovatex CP easily release free formaldehyde during use (Alongi and Malucelli 2015). With the increasing health and environmental protection concept of consumers, this flame retardant is gradually being replaced. The other common flame retardant is that it contains active chemical components that can be grafted onto pure cotton fabrics, which can chemically react with the –OH groups of cellulose units to generate covalent bonds that are resolutely bound (Zhao et al. 2014). In this category of flame retardants, organic compounds containing triazine group have been studied most (Dong et al. 2017). During the impregnation process, the C–Cl bond existing in their chemical structure can react with the cotton fabrics to form a C–O–C bond without the release of any harmful substances (He et al. 2018). However, no matter what kind of flame retardant, its chemical components must embody elements that play a flame retardant role to achieve top-quality results (Wang et al. 2014). The effect of flame retardants prepared by halogen elements is extraordinary. The biggest disadvantage of these compounds is that they emit abundant smoke and corrosive substances during burning process, which causes their application to be extremely restricted (Ling and Guo 2020). The flame resistance of phosphorus and nitrogen is also outstanding, and no toxic by-products are generated after they are burned (Thota et al. 2020; Wan et al. 2020; Wei et al. 2019). According to previous literature, it can be concluded that phosphorus-containing flame retardants can be decomposed into phosphoric acid and polyphosphoric acid compounds during thermal degradation process (Cheng et al. 2020; van der Veen and de Boer 2012), and these compounds undergo esterification and cross-linking reactions with cotton cellulose to promote formation of char layer (Barbalini et al. 2020; Lee and Jang 2020); nitrogen-containing flame retardants can generate low-density incombustible gas during combustion, which plays a role in blocking oxygen from entering the fiber (Fang et al. 2016; Li et al. 2019b). Therefore, many researchers are committed to develop more stable flame retardants containing phosphorus and nitrogen in structure and apply them to cotton fabrics in recent years.
In this study, a novel organic coating (ASNDP) was successfully prepared, which can greatly ameliorate the flame retardancy of cotton fabrics. Its chemical structure has multiple –P=O(O−NH4+) groups (as reaction component), which are combined with control cotton fabrics to constitute strong P–O–C chemical bonds under the action of a catalyst. The chemical composition of ASNDP was characterized by FTIR and NMR, and the differences in surface morphology and elemental composition between uncoated and coated samples were determined by SEM and energy dispersive spectrometer (EDS). The thermal degradation stability and flame retardancy of uncoated and coated samples were measured by TG analysis, LOI test and vertical flammability test. The combustion behavior of cotton fabrics before and after coating was evaluated by cone calorimetry test. Moreover, the chemical structures of the char layers formed by the coated cotton fabrics after burning and at different heating temperatures were researched by FTIR, and the crystal structure of cotton fabrics coated with ASNDP had been investigated by X-ray diffraction (XRD).
Experimental
Materials
The control cotton fabrics (20.1 × 23.4 tex2, 136 g/m2) was obtained from Qingdao Phoenix Printing and Dyeing Co., Ltd. (Qingdao, China). N-Aminoethylpiperazine was purchased by Chengdu Aikeda Chemical Reagent Co., Ltd. (Chengdu, China). Phosphorous acid and formaldehyde solution (40%) were provided by TianJin Fuyu Fine Chemical Co., Ltd. (Tianjin China). Urea, dicyandiamide and ethanol were supplied by Tianjin Zhiyuan Chemical Reagent Co., Ltd. (Tianjin China).
Preparation of ASNDP
In a 50 ml beaker, phosphorous acid (9.840 g, 0.12 mol) and formaldehyde (9.009 g, 0.12 mol) were dissolved in deionized water (20 ml). After sufficient stirring, the mixture was added to a 250 ml three-necked flask outfitted with a magnetic stirrer, a condenser tube and a thermometer. The temperature was raised to 50 °C, and a solution of N-Aminoethylpiperazine (5.168 g, 0.04 mol) dissolved in deionized water (20 ml) in advance was added to the reaction device. Then, the temperature was increased to 90 °C and kept for 4 h. After the reaction was completed, urea (9.609 g, 0.16 mol) was added to the three-necked flask. The synthesis system was continuously heated to 100 °C, and the reaction was carried out for 2 h while stirring. Subsequently, the obtained products were purified with ethanol to obtain a yellow sticky solid (ASNDP, yield 89.27%). The preparation process of ASNDP was presented in Scheme 1.
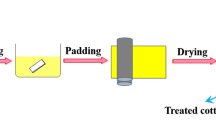
Preparation of flame retardant coating on cotton fabrics
The ASNDP was added to deionized water to produce different concentrations of flame-retardant solutions (150 g/L, 250 g/L, 350 g/L, and 450 g/L) containing 5% dicyandiamide (as catalyst), which were fully stirred at 50 °C for 20 min. The control cotton fabrics were immersed in the mixture with a bath ratio of 1:20 for 1 h. After completion of soaking, the coated samples were padded with using a laboratory padder (Shanghai Changcai Textile Equipment Co., Ltd., EL-400) to achieve a wet pick-up rate of about 100%. The process of dips and nips was repeated twice, and the obtained samples were dried in a blast dryer at 110 °C for 2 h. Then, the coated cotton fabrics were washed once with deionized water and dried to constant weight at 80 °C. The reaction process between ASNDP and cellulose unit was described in Scheme 2.
The weight gain (WG) of the samples coated with ASNDP was calculated by the following formula:
where W0 indicates the weight of the control cotton fabrics and W1 indicates the weight of ASNDP-coated cotton fabrics.
Characterizations
Fourier transform infrared (FTIR) spectroscopy of ASNDP and coated cotton fabrics (after burning and at different heating temperatures) was obtained with using a Nicolet iS 50 FTIR spectrometer (Thermo Fisher Scientific, USA) with a resolution of 4 cm−1 in the range of 4000–400 cm−1.
A Bruker AVANCE III HD 400 MHz spectrometer (Bruker, Germany) was used to measure the 1H nuclear magnetic resonance (1H-NMR), 13C nuclear magnetic resonance (13C-NMR) and 31P nuclear magnetic resonance (31P-NMR) of ASNDP dissolved in D2O solvent.
The crystal structures of uncoated and coated cotton fabrics were analyzed using a X-ray diffraction (XRD) apparatus (Dandong Haoyuan Instrument Co. Ltd., China) at a current of 20 mA (k = 0.154 nm) and an accelerating voltage of 36 kV.
The surface morphologies of the control cotton fabrics, coated cotton fabrics before and after combustion were observed with using scanning electron microscopy (SEM) (TESCAN, Czechoslovakia) at an acceleration voltage of 15 kV. All samples were sputter-coated with a gold layer before observation.
The contents of carbon (C), oxygen (O), nitrogen (N) and phosphorus (P) for the cotton fabrics coated with ASNDP before and after burning were determined by energy dispersive spectrometer (EDS) (JEOL-6300F).
The limiting oxygen index (LOI) and vertical flammability tests are important means to determine the flame retardancy of materials. LOI values of samples were assessed twice according to GB/T 5454-1997 by a LFY-606B digital limiting oxygen index apparatus (Shandong Institute of Textile Science, China). The vertical flammability of control and coated cotton fabrics was determined twice by using a LFY-601A vertical combustion apparatus (Shandong Textile Science Research Institute, China) according to GB/T 5455-2014.
In the temperature range from 40 to 750 °C, the thermogravimetric analyzer STA6000 (PerkinElmer, USA) was used to conduct thermogravimetric (TG) analysis on multiple samples under nitrogen and air atmosphere at a heating rate of 10 °C min−1.
According to ISO 5660 standard, the combustion behaviors of control and coated cotton fabrics were evaluated twice by a FTT0007 cone calorimeter (Fire Testing Technology, UK) under an external heat flux of 35 kW/m2.
The washing durability of the coated cotton fabrics was measured by a shaking bath device (SHA-BA, Qingdao Lantern Science and Education Equipment Co., Ltd, China). The coated cotton fabrics were immersed in a 250 ml conical beaker containing 0.15 wt% detergent solution. Then, the beaker was immobilized in a shaking water bath at 49 °C. The samples were washed at 80 rpm for 45 min, which was defined as five washing cycles. After 5, 10, 15, 20 washing cycles, the samples were repeatedly rinsed with tap water to remove the detergent solution. Finally, the samples were dried in an oven at 60 °C for 1 h, and their flame retardancy was measured by LOI test.
The whiteness of control and coated cotton fabrics was determined by Intelligent whiteness tester (WSB-V, China). The tensile strength of control and coated cotton fabrics was evaluated by multifunctional electronic fabric strength meter (HD026PC, Hongda Experimental Instrument Co., Ltd., Nantong, China) according to GB/T 3923.1-2013. In addition, the bending rigidity of control and coated cotton fabrics was measured by electronic stiffness tester (YM-01c, Laizhou Yuanmao Instrument Co. Ltd, China) according to GB/T 18318.1-2009.
Results and discussion
Synthesis and characterization of ASNDP
The ASNDP was prepared by Mannich reaction, and its chemical composition was analyzed by FTIR, 1H-NMR, 13C-NMR and 31P-NMR spectra as shown in Fig. 1. Figure 1a is the FTIR spectrum of ASNDP. The characteristic peaks of P=O and C–N are observed at 1240 cm−1 and 1036 cm−1, respectively (He et al. 2018). The characteristic peak at 757 cm−1 is attributed to P–C absorption (Jin et al. 2017), which indicates that a Mannich reaction has occurred between phosphorous acid, formaldehyde and N-Aminoethylpiperazine. Moreover, the characteristic peak at 2385 cm−1 is assigned to –POOH absorption (Xu et al. 2019), the characteristic peaks at 3029 cm−1 and 3201 cm−1 are corresponded to NH4+ units (Ran et al. 2019). The results prove that the addition of urea cause some phosphorus hydroxyl groups to be converted into ammonium phosphonate active groups.
The chemical structure of ASNDP was further determined with using NMR spectrum, and the data obtained were plotted in Fig. 1b–d. As presented in Fig. 1b for 1H-NMR, the peak at 4.70 ppm belongs to hydrogen atoms in D2O. The multiplets at around 3.11–3.17 ppm (Ha), 2.74 ppm (Hb), and 2.67–2.72 ppm (Hc) are correspond to the –CH2– groups (P–CH2–N, linear N–CH2–CH2–N, ring N–CH2–CH2–N). For the 13C-NMR spectrum of ASNDP (Fig. 1c), the peak at 53.01 ppm (Ca) is attributed to carbon atoms in P–CH2–N–CH2–P, the peaks at 50.44 ppm (Cb) and 49.19 ppm (Cc) are assigned to carbon atoms in linear N–CH2–CH2–N, and the double peaks at around 42.45–42.94 ppm (Cd) are attributed to carbon atoms in ring N–CH2–CH2–N. In addition, the peak at 53.60 ppm (Ce) belongs to carbon atom in P–CH2–N. In the 31P-NMR spectrum displayed in Fig. 1d, the peaks at 0.55 ppm (Pa) and 2.63 ppm (Pb) are assigned to the phosphorus of ASNDP. All the discussion above confirms that the target product ASNDP has been successfully synthesized.
Crystal structure analysis of ASNDP-coated cotton fabrics
The crystal structure of uncoated and coated cotton fabrics was compared by XRD spectra. As depicted in Fig. 2, control and coated cotton fabrics display similar diffraction peaks at approximately the same position. The diffraction peak at 15.34° is ascribed to the (1–10) plane, the diffraction peak at 16.59° is attributed to (110) plane, and the diffraction peaks at 22.85° and 34.68° are assigned to (200) plane and (004) plane (Zheng et al. 2018), respectively, which proves that the representative cellulose crystal structure of cotton fabrics coated with ASNDP has not changed significantly. However, all the diffraction peak intensity of the coated cotton fabrics is slightly stronger than that of the control cotton fabrics. It can be inferred that ammonium phosphonate active groups on ASNDP react with cotton fiber, reducing the –OH content in cellulose, which changes the distribution of the amorphous and crystalline regions of original cotton fabrics (Huang et al. 2019). In short, this change is extremely small, which does not have any serious impact on the crystal morphology of coated cotton fabrics.
Flame retardancy analysis of ASNDP-coated cotton fabrics
The anti-burning properties of uncoated and coated sample were researched by LOI test and vertical flammability test. The photos of cotton fabrics coated with different concentrations of ASNDP after vertical flammability test and the test data of all samples were shown in Fig. 3 and Table 1. It can be seen that the control cotton fabrics are entirely burned during the vertical flammability test with 17.8 ± 1.9 s of after-flame time and 18.4 ± 2.1 s of after-glow time, indicating that they did not exhibit any flame retardancy. In contrast, cotton fabrics coated with ASNDP can effectively prevent flame growth (Fig. 3), and the after-glow time of all coated samples disappears. With the increase of ASNDP amount (Table 1: increase from 150 to 350 g/L), the char length after combustion of coated cotton fabrics gradually decreases (Table 1: decrease from 30 ± 0.0 to 7.9 ± 0.2 cm). When the flame retardant concentration is increased to 450 g/L, the char length of Cotton-ASNDP-4 sample is shortened to 5.6 ± 0.2 cm. The LOI refers to the lowest oxygen volume fraction concentration that sustains polymer burning in a mixed atmosphere of oxygen and nitrogen (Li et al. 2019a). Compared with control cotton fabrics, Cotton-ASNDP-3 and Cotton-ASNDP-4 samples show excellent LOI values, increasing from 18.6 ± 0.2 to 28.2 ± 0.2% and 29.5 ± 0.1%, respectively. In addition, the WG of Cotton-ASNDP-3 and Cotton-ASNDP-4 samples are 14.8 ± 0.4% and 17.4 ± 0.3%, respectively. The reason for the speculation is that a small finishing bath ratio (1:20) leads to increased utilization of ASNDP, which makes the reaction between flame retardant and cotton fabrics more complete. Therefore, the conclusion is that ASNDP is a flame retardant with excellent fire resistance and active reactivity.
Thermal degradation process of ASNDP-coated cotton fabrics
The thermo-oxidative and thermal stability of the control and 450 g/L ASNDP-coated cotton fabrics were assessed with TG test under air and N2 atmospheres. Figure 4 depicts the thermal degradation curves and Table 2 lists the data obtained by the test. In air (Fig. 4a, b), the thermo-oxidative of cotton fabrics under intense heat is divided into two steps. First of all, the control cotton fabrics begin to decompose at 256 ± 3 °C (Tonset), and the maximum thermal mass loss occur at 344 ± 1 °C (Tmax). The cause of this phenomenon is the pyrolysis of fiber units and the generation of unstable char residue (Castellano et al. 2019). As the temperature increases, the char residue is converted into aromatic products and accompanied by the generation of CO2 and CO, which triggers the second thermal degradation of the control cotton (Rosace et al. 2018). In contrast, coated cotton fabrics show the earlier onset degradation temperature (Tonset: 157 ± 5 °C) and reach the maximum mass loss temperature at 276 ± 2 °C. This phenomenon is credited to premature pyrolysis of the chemical constituents of phosphorus-containing in flame retardant system, which catalyzes the decomposition of cotton fabrics to form a char layer (Castellano et al. 2019). In addition, the char residue of coated cotton fabrics is significantly increased compared with control cotton fabrics at Tmax (Residue: increase from 49.8 ± 1.3 to 69.9 ± 1.7%) and 750 °C (Residue: increase from 4.8 ± 0.4 to 13.7 ± 0.3%). It is concluded that the thermo-oxidative stability of samples coated with ASNDP is greatly improved.
The thermal stability of samples was studied in N2 atmosphere (Fig. 4c, d). Compared with the research in the air atmosphere, the thermal degradation of control cotton fabrics at high temperature is only completed in one step. It is precisely due to the thermal depolymerization of the trans-glycosylation reaction that control cotton fabrics exhibit Tonset and Tmax at 282 ± 4 °C and 367 ± 3 °C, respectively. Similar to the thermal degradation in the air, ASNDP plays an important role in generating Tonset and Tmax in advance, as well as in the formation of the char layer under Tmax (Residue: increase from 46.9 ± 1.1 to 67.7 ± 1.5%) and 750 °C (Residue: increase from 17.4 ± 0.6 to 42.3 ± 0.9%). It is speculated that the reason may be that ASNDP produced phosphoric acid compounds under high temperature environment, which can restrain the appearance of flammable levoglucosan and accelerate dehydration and carbonization of cellulose (Jiang et al. 2019), increasing the pyrolysis resistance of samples after coating.
An important parameter is the integral procedural decomposition temperature (IPDT), which is used to assess the overall inherent thermo-oxidative and thermal stability of uncoated and coated samples under high temperature environment, and its value is related to the volatile part of the samples (Fan et al. 2019; Park and Kim 2001). As depicted in Fig. 5, according to different regions partitioned by TG plot, the IPDT values of uncoated and coated cotton fabrics are calculated by the following formula:
where S1, S2 and S3 represent the areas of three different regions partitioned by TG plot, A* indicates the area ratio value between the total experimental curve and the total TG thermogram, K* represents the area ratio value between the total experimental curve and S1 area, Tf indicates the final experimental temperature (750 °C) and Ti represents the initial experimental temperature (40 °C).
As recorded in Table 2, compared with the uncoated sample, the IPDI value of the coated sample increased from 418.6 to 566.7 °C in the air test atmosphere. This shows that the thermal-oxidation stability of the coated cotton fabrics has been improved to a certain extent. Furthermore, it is obvious that the IPDI value of the coated cotton fabrics (1402.8 °C) is 792.7 °C higher than that of the original cotton (610.1 °C) in N2 test atmosphere, which confirms that ASNDP has extraordinary performance in enhancing the pyrolysis resistance of control cotton. In short, ASNDP endows cotton fabrics relatively good thermal and thermo-oxidative stability.
Combustion behavior of ASNDP-coated cotton fabrics
The cone calorimeter was applied to study the combustion characteristics of uncoated and coated samples. Figure 6 shows the digital photos of uncoated and coated cotton fabrics after the test. Obviously, the control cotton only retains a layer of transparent char layer (Fig. 6a), while the coated cotton remains a large amount of char layer (Fig. 6b) after burning. The detailed data and corresponding curves are shown in Table 3 and Fig. 7. As depicted in Fig. 7a, b and Table 3, the peak heat release rate (PHRR: reduce from 207.7 ± 0.6 to 65.0 ± 1.2 kW/m2) and total heat release (THR: reduce from 5.6 ± 0.3 to 3.7 ± 0.1 MJ/m2) of coated cotton are lower than those of uncoated cotton. It can be speculated that the phosphorus element produces phosphoric acid compounds during the combustion process to catalyze the dehydration of cotton fibers, thereby promoting the appearance of abundant char layer (Dong et al. 2019). At the same time, the ignition time (TTI) and time to peak heat release rate (TPHRR) of the coated cotton are also reduced to 5 ± 1 s and 14 ± 2 s (Table 3), respectively. The possible reason is that ASNDP changes the original radiation properties of cotton fabrics, or ASNDP promotes the premature combustion of cotton fabrics to form a char layer, which leads to the decrease of TTI and TPHRR values (Carosio et al. 2015). As we all know, the fire growth rate index (FIGRA) value has been applied to characterize the fire hazard of materials, and its value is calculated by the ratio of PHRR and TPHRR (Ling and Guo 2020). The FIGRA value of the coated cotton fabrics (4.6 kW/m2/s) is lower than that of the control cotton fabrics (6.5 kW/m2/s), which proves that ASNDP has the ability to reduce the harm to life in a fire. In addition, as presented in Fig. 7c, d and Table 3, the smoke produce rate (SPR) peak value and the total smoke production (TSP) of the coated cotton rise to 0.061 ± 0.002 m2/kg and 0.675 ± 0.013 m2/kg, respectively. The interpretations for the changes of SPR and TSP are that the ASNDP on cotton fabrics generates a lot of low-density volatile gases after burning, which prevent the diffusion of combustible gases into the cotton fibers to achieve flame retardant (Fang et al. 2016). This fact can also be characterized with using the CO2/CO ratio. The CO2/CO ratio (Table 3: reduce from 47.4 ± 0.2 to 14.2 ± 0.2) of coated cotton fabrics is reduced to a greater extent. The main reason is that the non-flammable gases emancipated from ASNDP reduce the amounts of free radicals (OH and H) propagating in the flame and prevent them from completely burning into CO2 (Zhang et al. 2020). As a consequence, the conclusion is that ASNDP has both gas phase and condensed phase flame retardant mechanism for cotton fabrics.
Surface morphology of ASNDP-coated cotton fabrics
The SEM was an important method to research the surface structure of materials, and the corresponding test results were shown in Fig. 8. It can be clearly noticed that the surface morphology of the control cotton fabrics is relatively smooth and retains their complete natural bending (Fig. 8a) (Qin et al. 2019). In contrast, the coated cotton fabrics are rough and small deposits of unreacted ASNDP can be seen on their outer layer (Fig. 8c), which proves that the flame retardant has been successfully coated on control cotton fabrics. After burning, the skeleton structure of the original cotton is thoroughly destroyed, and the initial integrity is also completely lost (Fig. 8b). In contrast, at low magnification, the coated samples after burning show obviously the fiber skeleton equivalent to that of the uncoated samples before burning (Fig. 8d). At high magnification, the fibers skeleton of the coated samples is slightly contracted after burning, which is caused by the polyphosphoric acid compounds accelerating the dehydration of the fibers to char layer (Pan et al. 2018). According to these results, this conclusion is consistent with TG analysis, ASNDP plays the effect of flame retardant cotton fabrics in the condensed phase.
Elemental analysis of ASNDP-coated cotton fabrics
The elemental composition of the uncoated and coated cotton fabrics surface was evaluated by EDS. The results obtained were described in detail in the combined Fig. 9. As summarized in Fig. 9a, the original cotton fabrics contain only the inherent C and O elements of natural cellulose. However, the coated cotton fabrics add P and N elements on the basis of the previous (Fig. 9b), which is the result of the chemical reaction between the original cotton fabrics and ASNDP. The more important discovery is that with the comparison of the element content between Fig. 9b, c, the flame retardant mechanism of ASNDP at the theoretical level can be obtained. The elemental contents of C and P before burning of coated cotton fabrics are 50.70% and 7.15%, and the corresponding values after burning are 78.32% and 4.68%, respectively. This reveals that P element is important for promoting the formation of char layer during the burning process of cotton fabrics (Alongi et al. 2011). The content of N element is also reduced from 8.79 to 0.45%, which is similar to the conclusion of the cone calorimetry test, that is, the volatile products containing nitrogen generated by the combustion of coated cotton fabrics play a role of gas phase flame retardation (Li et al. 2019b). Moreover, as presented in Fig. 9d, these elements are evenly arranged on the cotton fiber after combustion, indicating that the formed char skeleton has excellent uniformity. In summary, it is precisely because the coated cotton fabrics are a material containing P and N flame retardant elements, so that they possess outstanding flame retardancy.
FTIR analysis of char residue
The coated cotton fabrics were burned at ambient temperature (25 °C) to further research the flame-retardant mechanism of ASNDP in the condensed phase. Simultaneously, according to the results of TG analysis, the coated cotton fabrics were calcined at high temperature by changing different temperature gradients (200 °C, 300 °C, 400 °C, 500 °C, 600 °C) in a muffle furnace. The char layers formed after burning and heating were analyzed by FTIR, and the spectra acquired from the test results were shown in Fig. 10. It can be seen from the spectrum that compared with the control cotton fabrics, the coated cotton fabrics show the chemical characteristic peak of ASNDP at ambient temperature. The absorption peaks at 1574 cm−1, 1233 cm−1 and 764 cm−1 are attributed to P–O–C, P=O and P–C (He et al. 2018; Jin et al. 2017), respectively, which indicates that the –P=O(O−NH4+) groups of ASNDP have chemically reacted with –OH of cotton fabrics. After the coated cotton fabrics are burned at ambient temperature, the characteristic peaks of P–O–P and P–O–C are apparent in the spectrum, which emerge at 956 cm−1 and 1574 cm−1, respectively (Ling and Guo 2020; Ran et al. 2019). The speculated mechanism regarding flame retardancy is that the coated cotton fabrics can produce phosphorus-rich compounds during combustion, which react with cellulose in cotton fabrics to promote the formation of char. The char layers generated by the coated cotton fabrics at different heating temperatures demonstrate similar results of spectra analysis as the char layer obtained after burning at ambient temperature. Compared with the spectrum of coated cotton fabrics at ambient temperature, it can be seen that the sample has experienced preliminary thermal degradation under heating at 200 °C, which makes the intensity of many characteristic absorption peaks to be weakened. As the heating temperature rises to 300 °C, the char layer obtained from the coated cotton fabrics exhibits broad peaks of P–O–P and P–O–C around 956 cm−1 and 1574 cm−1, respectively (Ling and Guo 2020; Ran et al. 2019). This result strongly proves that the coated cotton fabrics begin to form phosphorus-rich compounds at 300 °C, and these compounds also undergo chemical reactions with cotton fibers. As the heating temperature rises to 400 °C and 500 °C, the spectra of the char layers obtained from the coated cotton fabrics shows that the width and intensity of the characteristic peaks of P–O–P and P–O–C are greatly enhanced. As the heating temperature rises to 600 °C, the P–O–P absorption peak of the obtained char layer disappears basically, while the P–O–C absorption peak still exists, which indicates that phosphorus-rich compounds have completely reacted with cotton fabrics at this temperature and all of them have transformed into phosphorus rich char layer. Corresponding to the results of TG and SEM tests, the coated cotton fabrics achieve the main flame retardant purpose in the condensed phase during the combustion or heating.
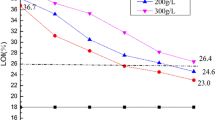
Washing stability of ASNDP-coated cotton fabrics
In order to determine the washing stability of cotton fabrics treated with 450 g/L ASNDP, the LOI values of the samples were measured after different washing cycles, and the results were presented in Fig. 11. As shown in Fig. 11, the LOI value of the coated cotton fabrics decreases from 29.5 ± 0.1 to 27.4 ± 0.2% after 5 washing cycles. The possible reason for this phenomenon is that the ASNDP which has not completely reacted with cotton fiber is removed from the fabric surface (Xu et al. 2020b). As the washing cycle increased to 10 times, the LOI value of the coated cotton fabrics continues to decrease to 26.6 ± 0.3%. When the washing cycle is increased to 20 times, the LOI value of the coated cotton fabric is 25.7 ± 0.2%. It can be seen that the LOI value difference of the coated samples is only 0.9% between 10 and 20 washing cycles.
Physical properties of ASNDP-coated cotton fabrics
The whiteness, bending rigidity and tensile strength of the cotton fabrics coated with 450 g/L ASNDP were determined and the results of these tests were recorded in Table 4. Compared with control cotton fabrics, the whiteness of coated cotton fabrics reduces from 80.7 ± 0.1 to 71.6 ± 0.2%, which is presumably caused by the color of ASNDP. Similarly, the bending rigidity and tensile strength of the coated cotton fabrics in the warp and weft directions have also decreased slightly. The possible reason is that the P–OH groups in ASNDP create a weakly acidic environment in the flame retardant solution of cotton fabrics (Xu et al. 2020a). On the one hand, the P–OH breaks the glycosidic bond of cellulose, which leads to the decrease in the tensile strength of coated cotton fabrics. On the other hand, the weakly acidic finishing solution is beneficial to bleach the textile size of cotton fabrics that has not been completely removed in the pre-treatment stage, which is a possible factor for the decrease in bending rigidity of cotton fabrics. In short, ASNDP improves the flame retardancy of cotton fabrics and has little effect on physical properties of the fabric.
Flame-retardant mechanism analysis of ASNDP-coated cotton fabrics
In order to profoundly comprehend the flame retardant pathway of ASNDP on control cotton fabrics, the schematic diagram was constructed in Fig. 12 to analyze the possible flame retardant mechanism. As summarized in Fig. 12a, the control cotton fabrics can burn violently in the case of contact with fire source and presence of combustion supporting gas (O2). The reason for the analysis is that the cotton fibers generate levoglucosan during combustion, which is a highly flammable chemical component and accelerates damage of fibers (Rosace et al. 2018). In comparison, as presented in Fig. 12b, the coated cotton fabrics produce extensive char layer after burning, which is exactly the macro performance of ASNDP to exert the fire resistance. Among them, the flame retardant mechanism is mainly summarized in the following reasons: the coated cotton fabrics generate phosphoric acid compounds at high temperatures, which include poly-phosphoric acid and poly-metaphosphoric acid (dehydration ability is stronger than the former), they promote the carbonization of cotton fabrics and constitute phosphorus-rich cross-linked product with P–O–C structure during burning (Fan et al. 2019; Nguyen et al. 2012); at high temperatures, a mixture of phosphorus degradation products with P–O–P structure and different condensation degrees is formed by the condensation reaction between phosphoric acid compounds, these high-viscous and glass-like degradation compounds with high phosphorus content can adhere to the surface of the cotton fabrics and act as a barrier to prevent the transfer of combustible gases and heat to the inside (Zhao et al. 2016); the N element in ASNDP releases a lot of low-density and non-flammable gases (NO, NH3, NO2) during combustion, which play the role of diluting the combustible gases on surface of the cotton fabrics and isolating oxygen (Sun et al. 2019). In conclusion, ASNDP is a high-efficiency flame retardant for cotton fabrics that can achieve flame-retardant purpose in both gas phase and condensed phase.
Conclusion
A novel phosphorus–nitrogen synergistic flame retardant (ASNDP) was successfully synthesized and was coated on cotton fabrics to investigate its flame retardant and thermal stability properties. The chemical structures of flame retardant were characterized by FTIR and NMR. The cotton fabrics coated with ASNDP displayed good flame resistance, which passed the vertical burning test standard and acquired low char length from 9.2 ± 0.4 to 5.6 ± 0.2 cm with 11.2 ± 0.2 to 17.4 ± 0.3% of weight gain. The results of cone calorimeter test indicated that the coated cotton fabrics had lower THR than the control cotton, and the FIGRA was also reduced to 4.6 kW/m2/s. The FTIR analysis of the coated cotton fabrics after burning and at different heating temperatures indicated that ASNDP achieved flame retardant effect in condensed phase. The TG analysis showed that ASNDP enhanced the thermo-oxidative and thermal stability of cotton fabrics at high temperature, and the residual amount retained by the coated samples at 750 °C under nitrogen atmosphere was 42.3 ± 0.9%. The XRD test proved that the crystal structure of the coated cotton fabrics was basically unchanged. The morphological structure and element composition of uncoated and coated cotton fabrics were analyzed by SEM and EDS, respectively. The results consistently showed that ASNDP has the ability to accelerate char-formation of cotton fabrics in the condensed phase and produced non-flammable gases in the gas phase to dilute and shield oxygen.
References
Alongi J, Malucelli G (2015) Cotton flame retardancy: state of the art and future perspectives. RSC Adv 5:24239–24263. https://doi.org/10.1039/C5RA01176K
Alongi J, Ciobanu M, Malucelli G (2011) Novel flame retardant finishing systems for cotton fabrics based on phosphorus-containing compounds and silica derived from sol–gel processes. Carbohydr Polym 85:599–608. https://doi.org/10.1016/j.carbpol.2011.03.024
Barbalini M, Bartoli M, Tagliaferro A, Malucelli G (2020) Phytic acid and biochar: an effective all bio-sourced flame retardant formulation for cotton fabrics. Polymers 12:811. https://doi.org/10.3390/polym12040811
Carosio F, Fontaine G, Alongi J, Bourbigot S (2015) Starch-based layer by layer assembly: efficient and sustainable approach to cotton fire protection. ACS Appl Mater Interfaces 7:12158–12167. https://doi.org/10.1021/acsami.5b02507
Castellano A, Colleoni C, Iacono G, Mezzi A, Plutino MR, Malucelli G, Rosace G (2019) Synthesis and characterization of a phosphorous/nitrogen based sol–gel coating as a novel halogen- and formaldehyde-free flame retardant finishing for cotton fabric. Polym Degrad Stab 162:148–159. https://doi.org/10.1016/j.polymdegradstab.2019.02.006
Cheng XW, Tang RC, Guan JP, Zhou SQ (2020) An eco-friendly and effective flame retardant coating for cotton fabric based on phytic acid doped silica sol approach. Prog Org Coat. https://doi.org/10.1016/j.porgcoat.2020.105539
Dong CH, Lu Z, Wang P, Zhu P, Li XC, Sui SY, Zhang L, Liu J (2017) Flammability and thermal properties of cotton fabrics modified with a novel flame retardant containing triazine and phosphorus components. Text Res J 87:1367–1376. https://doi.org/10.1177/0040517516652349
Dong CH, Sun L, Ma XB, Lu Z, He PS, Zhu P (2019) Synthesis of a novel linear alpha, omega-di (chloro phosphoramide) polydimethylsiloxane and its applications in improving flame-retardant and water-repellent properties of cotton fabrics. Polymers 11:1829. https://doi.org/10.3390/polym11111829
Fan MS, Feng N, Zhang YJ, Wang ZC, Qu MJ, Zhang GX (2019) Synergistic effects of aluminium hypophosphite on the flame retardancy and thermal degradation behaviours of a novel intumescent flame retardant thermoplastic vulcanisate composite. Plast Rubber Compos 48:270–280. https://doi.org/10.1080/14658011.2019.1606580
Fang F, Chen XX, Zhang X, Cheng C, Xiao DZ, Meng YD, Ding X, Zhang H, Tian XY (2016) Environmentally friendly assembly multilayer coating for flame retardant and antimicrobial cotton fabric. Prog Org Coat 90:258–266. https://doi.org/10.1016/j.porgcoat.2015.09.025
He PS, Chen XY, Zhu P, Liu J, Fan GD, Sui SY, Lu Z, Dong CH (2018) Preparation and flame retardancy of reactive flame retardant for cotton fabric. J Therm Anal Calorim 132:1771–1781. https://doi.org/10.1007/s10973-018-7057-6
Huang S, Feng YJ, Li SN, Zhou Y, Zhang FX, Zhang GX (2019) A novel high whiteness flame retardant for cotton. Polym Degrad Stab 164:157–166. https://doi.org/10.1016/j.polymdegradstab.2019.03.014
Jia YL, Lu Y, Zhang GX, Liang YJ, Zhang FX (2017) Facile synthesis of an eco-friendly nitrogen-phosphorus ammonium salt to enhance the durability and flame retardancy of cotton. J Mater Chem A 5:9970–9981. https://doi.org/10.1039/C7TA01106G
Jiang ZM, Li H, He YW, Liu Y, Dong CH, Zhu P (2019) Flame retardancy and thermal behavior of cotton fabrics based on a novel phosphorus-containing siloxane. Appl Surf Sci 479:765–775. https://doi.org/10.1016/j.apsusc.2019.02.159
Jin XD, Gu XY, Chen C, Tang WF, Li HF, Liu XD, Bourbigot S, Zhang ZW, Sun J, Zhang S (2017) The fire performance of polylactic acid containing a novel intumescent flame retardant and intercalated layered double hydroxides. J Mater Sci 52:12235–12250. https://doi.org/10.1007/s10853-017-1354-5
Laufer G, Kirkland C, Morgan AB, Grunlan JC (2012) Intumescent multilayer nanocoating, made with renewable polyelectrolytes, for flame-retardant cotton. Biomacromolecules 13:2843–2848. https://doi.org/10.1021/bm300873b
Lee DH, Jang J (2020) Synergistic flame-retardant finishing of cotton using dichlorotriazinyl phosphonate and triethanolamine. Fiber Polym 21:343–349. https://doi.org/10.1007/s12221-020-9442-6
Li SN, Zhong L, Huang S, Wang DF, Zhang FX, Zhang GX (2019a) A novel flame retardant with reactive ammonium phosphate groups and polymerizing ability for preparing durable flame retardant and stiff cotton fabric. Polym Degrad Stab 164:145–156. https://doi.org/10.1016/j.polymdegradstab.2019.04.009
Li SS, Lin XH, Liu Y, Li R, Ren XH, Huang TS (2019b) Phosphorus–nitrogen–silicon-based assembly multilayer coating for the preparation of flame retardant and antimicrobial cotton fabric. Cellulose 26:4213–4223. https://doi.org/10.1007/s10570-019-02373-5
Ling C, Guo LM (2020) Preparation of a flame-retardant coating based on solvent-free synthesis with high efficiency and durability on cotton fabric. Carbohydr Polym 230:115648. https://doi.org/10.1016/j.carbpol.2019.115648
Liu J, Dong CH, Zhang Z, Kong DZ, Sun H, Lu Z (2020) Multifunctional flame-retarded and hydrophobic cotton fabrics modified with a cyclic phosphorus/polysiloxane copolymer. Cellulose 27:3531–3549. https://doi.org/10.1007/s10570-020-03016-w
Nguyen TMD, Chang S, Condon B, Uchimiya M, Fortier C (2012) Development of an environmentally friendly halogen-free phosphorus–nitrogen bond flame retardant for cotton fabrics. Polym Adv Technol 23:1555–1563. https://doi.org/10.1002/pat.3029
Pan Y, Liu LX, Wang X, Song L, Hu Y (2018) Hypophosphorous acid cross-linked layer-by-layer assembly of green polyelectrolytes on polyester-cotton blend fabrics for durable flame-retardant treatment. Carbohydr Polym 201:1–8. https://doi.org/10.1016/j.carbpol.2018.08.044
Park SJ, Kim HC (2001) Thermal stability and toughening of epoxy resin with polysulfone resin. J Polym Sci 39:121–128. https://doi.org/10.1002/1099-0488(20010101)39:1%3c121:AID-POLB110%3e3.0.CO;2-N
Qin HL, Li XF, Zhang XL, Guo ZG (2019) Preparation and performance testing of superhydrophobic flame retardant cotton fabric. New J Chem 43:5839–5848. https://doi.org/10.1039/C9NJ00307J
Ran GW, Liu XD, Guo J, Sun J, Li HF, Gu XY, Zhang S (2019) Improving the flame retardancy and water resistance of polylactic acid by introducing polyborosiloxane microencapsulated ammonium polyphosphate. Compos Part B Eng 173:106772. https://doi.org/10.1016/j.compositesb.2019.04.033
Rosace G, Castellano A, Trovato V, Iacono G, Malucelli G (2018) Thermal and flame retardant behaviour of cotton fabrics treated with a novel nitrogen-containing carboxyl-functionalized organophosphorus system. Carbohydr Polym 196:348–358. https://doi.org/10.1016/j.carbpol.2018.05.012
Sun YF, Liu CY, Hong Y, Liu RP, Zhou XD (2019) Synthesis and application of self-crosslinking and flame retardant waterborne polyurethane as fabric coating agent. Prog Org Coat 137:105323. https://doi.org/10.1016/j.porgcoat.2019.105323
Thota S, Somisetti V, Kulkarni S, Kumar J, Nagarajan R, Mosurkal R (2020) Covalent functionalization of cellulose in cotton and a nylon-cotton blend with phytic acid for flame retardant properties. Cellulose 27:11–24. https://doi.org/10.1007/s10570-019-02801-6
van der Veen I, de Boer J (2012) Phosphorus flame retardants: properties, production, environmental occurrence, toxicity and analysis. Chemosphere 88:1119–1153. https://doi.org/10.1016/j.chemosphere.2012.03.067
Wan CY, Liu MS, Tian PX, Zhang GX, Zhang FX (2020) Renewable vitamin B5 reactive N-P flame retardant endows cotton with excellent fire resistance and durability. Cellulose 27:1745–1761. https://doi.org/10.1007/s10570-019-02886-z
Wang XY, Lu CQ, Chen CX (2014) Effect of chicken-feather protein-based flame retardant on flame retarding performance of cotton fabric. J Appl Polym Sci 131:40584. https://doi.org/10.1002/app.40584
Wei YX, Deng C, Wei WC, Chen H, Wang YZ (2019) Hybrid nanorods composed of titanium, silicon, and organophosphorus as additives for flame-retardant polycarbonate. ACS Appl Nano Mater 2:4859–4868. https://doi.org/10.1021/acsanm.9b00814
Xu F, Zhong L, Xu Y, Zhang C, Wang P, Zhang FX, Zhang GX (2019) Synthesis of three novel amino acids-based flame retardants with multiple reactive groups for cotton fabrics. Cellulose 26:7537–7552. https://doi.org/10.1007/s10570-019-02599-3
Xu DH, Gao ZY, Xu B, Ren H, Zhao XS, Zhang YN, Wang SJ, Jiang ZM, Zhu P (2020a) A facile and effective flame-retardant coating for cotton fabric with α-aminodiphosphonate siloxane. Polym Degrad Stab 180:109312. https://doi.org/10.1016/j.polymdegradstab.2020.109312
Xu DH, Wang SJ, Wang YM, Liu Y, Dong CH, Jiang ZM, Zhu P (2020b) Preparation and mechanism of flame-retardant cotton fabric with phosphoramidate siloxane polymer through multistep coating. Polymers 12:1538. https://doi.org/10.3390/polym12071538
Zhang Z, Dong CH, Liu J, Kong DZ, Sun L, Lu Z (2020) Preparation of a synergistic reactive flame retardant based on silicon, phosphorus and nitrogen and its application to cotton fabrics. Cellulose 27:1799–1815. https://doi.org/10.1007/s10570-019-02900-4
Zhao PH, Li XH, Zhang M, Liu SN, Liang WJ, Liu YQ (2014) Highly flame-retarding cotton fabrics with a novel phosphorus/nitrogen intumescent flame retardant. Korean J Chem Eng 31:1592–1597. https://doi.org/10.1007/s11814-014-0095-2
Zhao X, Gao S, Liu GS (2016) A THEIC-based polyphosphate melamine intumescent flame retardant and its flame retardancy properties for polylactide. J Anal Appl Pyrol 122:24–34. https://doi.org/10.1016/j.jaap.2016.10.029
Zhao B, Liu YT, Zhang CY, Liu DY, Li F, Liu YQ (2017) A novel phosphoramidate and its application on cotton fabrics: synthesis, flammability and thermal degradation. J Anal Appl Pyrol 125:109–116. https://doi.org/10.1016/j.jaap.2017.04.011
Zheng DD, Zhou JF, Wang Y, Zhang FX, Zhang GX (2018) A reactive flame retardant ammonium salt of diethylenetriaminepenta(methylene-phosphonic acid) for enhancing flame retardancy of cotton fabrics. Cellulose 25:787–797. https://doi.org/10.1007/s10570-017-1543-z
Zhu WJ, Yang MY, Huang H, Dai Z, Cheng BW, Hao SS (2020) A phytic acid-based chelating coordination embedding structure of phosphorus–boron–nitride synergistic flame retardant to enhance durability and flame retardancy of cotton. Cellulose 27:4817–4829. https://doi.org/10.1007/s10570-020-03063-3
Acknowledgments
We thankfully acknowledge the funding from the Natural Science Foundation of Shandong Province, China (Grant No. ZR2018MEM026) and the National Natural Science Foundation of China, China (Grant No. 51991354).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sun, L., Wang, H., Li, W. et al. Preparation, characterization and testing of flame retardant cotton cellulose material: flame retardancy, thermal stability and flame-retardant mechanism. Cellulose 28, 3789–3805 (2021). https://doi.org/10.1007/s10570-020-03632-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-020-03632-6